Abstract
Fish generally have much higher requirements for dietary protein than mammals, and this long-standing puzzle remains unsolved. The present study was conducted with zebrafish (omnivores) and hybrid striped bass (HSB, carnivores) to test the hypothesis that AAs are oxidized at a higher rate than carbohydrates (e.g., glucose) and fatty acids (e.g., palmitate) to provide ATP for their tissues. Liver, proximal intestine, kidney, and skeletal muscle isolated from zebrafish and HSB were incubated at 28.5 °C (zebrafish) or 26 °C (HSB) for 2 h in oxygenated Krebs–Henseleit bicarbonate buffer (pH 7.4, with 5 mM d-glucose) containing 2 mM l-[U-14C]glutamine, l-[U-14C]glutamate, l-[U-14C]leucine, or l-[U-14C]palmitate, or a trace amount of d-[U-14C]glucose. In parallel experiments, tissues were incubated with a tracer and a mixture of unlabeled substrates [glutamine, glutamate, leucine, and palmitate (2 mM each) plus 5 mM d-glucose]. 14CO2 was collected to calculate the rates of substrate oxidation. In the presence of glucose or a mixture of substrates, the rates of oxidation of glutamate and ATP production from this AA by the proximal intestine, liver, and kidney of HSB were much higher than those for glucose and palmitate. This was also true for glutamate in the skeletal muscle and glutamine in the liver of both species, glutamine in the HSB kidney, and leucine in the zebrafish muscle, in the presence of a mixture of substrates. We conclude that glutamate plus glutamine plus leucine contribute to ~80% of ATP production in the liver, proximal intestine, kidney, and skeletal muscle of zebrafish and HSB. Our findings provide the first direct evidence that the major tissues of fish use AAs (mainly glutamate and glutamine) as primary energy sources instead of carbohydrates or lipids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dietary requirements of protein by fish range from 30 to 60% based on the species, age, size, and feeding habit (Ballantyne 2001; Wilson 2002), which were much greater than those for mammals and birds, such as swine (12–20%), chickens (14–22%), and dairy cows (10–18%) (NRC 2000, 2012; Kaushik and Seiliez 2010; Wu 2014). However, protein content or the composition of amino acids (AAs) in the whole body of the fish is similar to that of terrestrial animals, such as pigs, cows, and chickens (Latshaw and Bishop 2001; Lobley et al. 1980; Smits et al. 1988). Several reasons have been postulated to explain the high dietary protein requirement for fish. First, the basal energy needs of fish are less than those of terrestrial animals, due to its poikilothermic and ammoniotelic life mode (Kaushik and Seiliez 2010). Thus, the dietary content of lipids and starch is lower for fish, which results in a higher protein level in fish feeds. Second, the contribution from AAs toward the energy requirement may be high and the oxidation of AAs via the Krebs cycle helps to dispose of their carbon skeletons as CO2 and water (Weber and Haman 1996). Therefore, dietary protein contributes to not only the growth of fish (protein synthesis) but also their ATP production from AA catabolism.
Among the three types of major macronutrients (carbohydrates, protein, and lipids), most fish do not use carbohydrates (e.g., starch, glycogen, and simple sugars) as a major energy source (Cowey and Walton 1988). However, high rates of AA utilization in the whole body of fish have been observed (Jurss and Bastrop 1995; Li et al. 2009; van den Thillart 1986; Wilson 2002). There is a suggestion that 14–85% of the energy requirement of teleost fish is provided by AAs, depending on developmental stages (Van Waarde 1983). In the hepatocytes of fed and starved rainbow trout, the rates of oxidation of some AAs (alanine, serine, asparagine, and glycine) were relatively high, but the rates of oxidation of certain AAs (e.g., leucine and valine) and palmitate were low (French et al. 1981). Furthermore, AAs are the major metabolic fuels for marine fish embryos and yolk-sac larvae (Cowey and Walton 1988). Likewise, the oxidation of AAs as an entity may contribute to 50–70% of total energy needs in the marine fish embryos and yolk-sac larvae (Rønnestad et al. 1999; Rønnestad and Fyhn 2008). However, to the best of our knowledge, the use of individual AAs as metabolic fuels for specific tissues of teleosts is unknown.
Zebrafish (omnivores; Laale 1977) and hybrid striped bass (HSB, carnivores; Griffin et al. 1994) are two fish species with different dietary habits. They also differ in the gastrointestinal tract, as HSB have a stomach, while zebrafish do not. This study was conducted with the two fish species to test the hypothesis that AAs are oxidized at a higher rate than carbohydrates (e.g., glucose) and fatty acids (e.g., palmitate) to provide ATP for their tissues.
Materials and methods
Chemicals
The following radiolabeled chemicals were purchased from American Radiolabeled Chemicals (St. Louis, MO): d-[U-14C]glucose, l-[U-14C]glutamine, l-[U-14C]glutamate, l-[U-14C]leucine, l-[1-14C]leucine, and [U-14C]palmitic acid. Before use, 14C-labeled glutamine and leucine were purified using the Dowex AG1-X8 resin (acetate form, 200–400 mesh) (Self et al. 2004). 14C-labeled glutamate was purified by adding an equal volume of 1.5 M HClO4 and then neutralized by a half volume of 2 M K2CO3. Soluene was procured from Perkin-Elmer. The liquid scintillation cocktail for determining 14CO2 was made by dissolving 5 g of 2,5-diphenyloxazole and 0.2 g of 1,4-bis(5-phenyloxazol-2-yl) benzene into 1 L of a 1:1 mixture of toluene and 2-methoxyethanol. The sources of other chemicals, including fatty acid-free bovine serum albumin (BSA) and AAs, were as previously described (Hou et al. 2016a; Lenis et al. 2016). Before use, sodium palmitate (2.5 mM) was conjugated with 0.43 mM BSA in 150 mM NaCl. Briefly, 45 ml of 5.56 mM sodium palmitate solution (in 150 mM NaCl; preheated to 70 °C) was slowly added to 50 ml of 0.86 mM BSA solution (in 150 mM NaCl; preheated to 37 °C). The mixed solution (containing 2.5 mM palmitate) was stirred for 1 h at 37 °C and then adjusted to pH 7.4 and a final volume of 100 ml. After palmitate was conjugated with BSA, concentrated components (except NaCl) of Krebs–Henseleit bicarbonate (KHB) buffer were added to the solution to obtain 2 mM palmitate, physiological concentrations of minerals [119 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 25 mM NaHCO3 (Wu et al. 1994)], and 20 mM HEPES (pH 7.4; Wu 1997).
Animals
Wild-type young adult zebrafish (Danio rerio) were obtained from Aquariumfish.net. Juvenile HSB (Morone saxatilis ♀ X Morone chrysops ♂) were obtained from Keo Fish Farm (Keo, Arkansas, USA). Zebrafish and HSB were maintained in two separated water cycling systems at a temperature of 28.5 and 26 °C, respectively. Water was circulated through mechanical and biological filters and changed regularly (30–50% every 3 days). Air was supplied through air stones connected to air pumps, salinity was maintained at 1 ppt, and photoperiod was maintained at 14 h per day. Water quality parameters (pH, ammonia, nitrite, and nitrate) were monitored weekly and remained within acceptable limits. Fish were fed twice daily with commercial feeds in the morning and evening. All experimental procedures were approved by the Institutional Agricultural Animal Care and Use Committee of Texas A&M University.
Collection of tissues
On the day of tissue collection, zebrafish (~0.5 g) and HSB (~20 g) were dissected 4 and 6 h after feeding, respectively. For anesthesia, the fish were placed into water (pH 7.0) containing MS-222 (40 ppt) and an appropriate amount of NaHCO3. Thereafter, the liver, proximal intestine (2/3 of the whole intestine), kidney, and dorsal muscle (white muscle) samples were obtained. The proximal intestine was cut longitudinally and washed in phosphate-buffered saline to remove the remaining intestinal content, and then soft paper sheets (Kimtech) were used to dry water on the surface of the intestine. All tissues were sliced into small pieces.
Determination of substrate oxidation
Metabolic studies were conducted as previously described (Wu 1997), with some modifications. Briefly, each of the weighed tissue slices (15–40 mg) was incubated at 28.5 °C (zebrafish) or 26 °C (HSB) for 2 h in 1 ml of oxygenated (95% O2/5% CO2) KHB buffer (pH 7.4) containing 5 mM d-glucose, 1 nM insulin, and one of the following combinations of tracer and tracee: [U-14C]glucose, 2 mM glutamate + [U-14C]glutamate, 2 mM glutamine + [U-14C]glutamine, 2 mM leucine + [U-14C]leucine, 2 mM leucine + [1-14C]leucine, or 2 mM palmitate + [U-14C]palmitic acid. In parallel experiments, a tissue was incubated in the presence of a tracer plus a mixture of the unlabeled substrates [i.e., 5 mM glucose (physiological concentration in fish plasma), 2 mM each of glutamate, glutamine, leucine, and palmitate)]. The concentrations of AAs and palmitate were adopted to ensure that the substrates were not limiting for their oxidation in fish tissues. The specific radioactivity of each tracer in the incubation medium was approximately 2500 dpm/nmol. In all experiments, media containing the same components but no tissues were run as blanks, with six replicates for each radiolabeled substrate. Incubation was initiated by the addition of a tissue. After a 2-h incubation period, the reaction was terminated by the addition, through the rubber stopper, of 0.2 ml 1.5 M HClO4 into the incubation medium, followed by the addition, through the rubber stopper, of 0.2 ml Soluene into a microtube suspended within the tube to collect 14CO2 (Li et al. 2016). The second collection of 14CO2 for the oxidation of [1-14C]leucine was performed by the addition of 0.7 ml of 30% (v/v) H2O2 into the medium to decarboxylate [1-14C]ɑ-ketoisocaproate. 14C radioactivity was measured in the liquid scintillation cocktail using a Packard scintillation counter (Self et al. 2004). Based on the rates of 14CO2 production from a labeled substrate, the tissues used in our study were viable during a 2-h incubation period (data not shown).
Calculations and statistical analysis
Rates of oxidation of each substrate in tissues (CO2/mg tissue per h) were calculated as dpm of the 14CO2 produced by the tissue divided by the specific radioactivity of the substrate in the incubation medium. Rates of ATP production were calculated from the rates of CO2 production by multiplying the coefficient (ATP/CO2) according to the following equations:
The rates of ATP production from the oxidation of substrates into CO2 and H2O, expressed as mol ATP/mol substrate, were as follows: glutamate, 22.5; glutamine, 22.5; leucine, 34.5; palmitate, 106; and glucose, 30. It is assumed that ammonia is not converted into urea in the tissues of HSB and zebrafish. The coefficients of ATP production per mole of CO2 produced from the oxidation of substrates, expressed as mol ATP/mol CO2, were as follows: glutamate, 4.5; glutamine, 4.5; leucine, 5.75; palmitate, 6.625; and glucose, 5. Data were analyzed by one-way analysis of variance and the Student–Newman–Keuls multiple comparison test (Assaad et al. 2014). Log transformation of variables was performed when the variances of data were not homogenous among treatment groups, as assessed by the Levene’s test. Differences between values obtained in the presence or absence of a mixture of energy substrates were determined by the paired t-test. Probability values <0.05 were taken to indicate statistical significance.
Results
Oxidation of AAs, glucose, and palmitate in fish tissues
Data on the rates of CO2 production from the oxidation of different nutrients in HSB and zebrafish tissues are summarized in Tables 1 and 2, respectively. In the proximal intestine, liver, kidney, and skeletal muscle of HSB and in all zebrafish tissues studied except for the proximal intestine, the rate of CO2 production from [U-14C]glutamate oxidation was lower (P < 0.05) in the presence of a mixture of energy substrates, compared with the presence of 5 mM unlabeled glucose alone. In the proximal intestine, kidney, and skeletal muscle of both fish species, glutamate was the most oxidative among the tested nutrients under all the experimental conditions. The rate of CO2 production from [U-14C]glutamine oxidation was the highest in the liver and the second highest (after glutamate) in the kidney of HSB and zebrafish in the presence of 5 mM glucose or a mixture of substrates or in their proximal intestine in the presence of a mixture of substrates. The rate of CO2 production from [U-14C]leucine oxidation was the second highest in the skeletal muscle of both fish species, but was the lowest in the proximal intestine and liver of HSB and in the proximal intestine and kidney of zebrafish, when tissues were incubated with a mixture of substrates. The rate of hepatic CO2 production from [U-14C]leucine oxidation differed (P < 0.05) markedly between HSB and zebrafish.
The rate of CO2 production from [U-14C]palmitate oxidation was the lowest among the tested nutrients in the skeletal muscle of HSB and in the liver and skeletal muscle of zebrafish in the presence of 5 mM glucose or a mixture of substrates and could not be detected in HSB skeletal muscle incubated in the presence of a mixture of energy substrates. In the liver of both fish species, palmitate oxidation was limited under the experimental conditions. The rates of glucose oxidation differed (P < 0.05) between the two fish species. Specifically, in the presence of a mixture of energy substrates, the rate of CO2 production from [U-14C]glucose oxidation was much lower (P < 0.05) than that from glutamate or glutamine oxidation in the proximal intestine, liver, and kidney and was similar to that from leucine oxidation in the HSB skeletal muscle incubated in the presence of a mixture of substrates. Under the same experimental conditions, the rates of CO2 production from [U-14C]glucose oxidation in the proximal intestine and kidney were much higher (P < 0.05) in zebrafish than those in HSB, making glucose the second and third most oxidative substrate in the intestine and kidney, respectively.
ATP production from the oxidation of nutrients in fish tissues
Data on ATP production from the oxidation of nutrients by HSB and zebrafish tissues are summarized in Tables 3 and 4, respectively. Results of the comparison of ATP production from nutrients among tissues were generally similar to those for the rates of nutrient oxidation noted previously. In the presence of a mixture of energy substrates, the percentage of ATP produced from the oxidation of AAs (glutamate plus glutamine plus leucine) was 78.5, 89.1, 77.1, and 80.4%, and was 77.1, 80.7, 75.3, and 77.6% for the proximal intestine, liver, kidney, and skeletal muscle of HSB and zebrafish, respectively.
Comparisons of ATP production from nutrients among different tissues are summarized in Tables 5 and 6 for HSB and zebrafish, respectively. Kidneys from both fish species had the highest rate of ATP production per g tissue from glutamate, glutamine, glucose, palmitate, and leucine in the presence of 5 mM glucose or a mixture of energy substrates. The proximal intestine of HSB had the second highest rate of ATP production per g tissue for all nutrients. Based on tissue weights of 20-g juvenile HSB and 0.5-g zebrafish, the rates of ATP production from nutrient oxidation per tissue in the presence of a mixture of substrates are summarized in Table 7. Glutamate produced most ATP in the intestine, kidneys, and skeletal muscle of HSB and zebrafish, whereas glutamine was the most predominant metabolic fuel in the liver of both fish species.
Catabolism of [1-14C]leucine
Data on the catabolism of [1-14C]leucine by tissues of HSB and zebrafish are summarized in Table 8. ɑ-Ketoisocaproate (KIC) was a product of leucine transamination in their tissues. Leucine had the highest rate of net transamination in the kidney of both fish species, compared with their other tissues. The rates of net KIC release and the oxidative decarboxylation of leucine were also the highest in the kidney. Moreover, the rates of oxidative decarboxylation of leucine in the liver and proximal intestine of both fish species were much greater (P < 0.05) than the rates of net KIC release. This was also true for the kidney of zebrafish. The liver had a higher (P < 0.05) ratio of oxidative decarboxylation of leucine to net KIC release than the kidney in both fish species. The rates of net KIC release and the oxidative decarboxylation of leucine by the liver were greater (P < 0.05) in zebrafish than in HSB. The rates of oxidative decarboxylation of leucine and net KIC release were low in the skeletal muscle of both species. In all tissues of HSB and in the proximal intestine, liver and kidney of zebrafish, the rates of leucine transamination and oxidative decarboxylation were lower (P < 0.05) in the presence of a mixture of energy substrates than the presence of glucose.
Discussion
Animals exhibit tissue and species differences in nutrient metabolism (Wu 2017). Glutamate and glutamine are extensively oxidized by the small intestine of pigs and rats to generate a large amount of ATP (Wu 1998, 2010). In contrast, both glucose and fatty acids are the major metabolic fuels for the kidneys and skeletal muscle of mammals, and fatty acids are the primary source of energy in the liver of mammals (Jobgen et al. 2006). As noted previously, both omnivorous and carnivorous fish have a lower capacity to utilize dietary starch than omnivorous mammals and birds. There are reports that the skeletal muscle of an Antarctic teleost, Gobionotothm gibberifrons, actively oxidizes long-chain fatty acids to CO2 (Sidell et al. 1995), but the hepatocytes of fed rainbow trout have a limited ability to oxidize palmitate to CO2 (French et al. 1981). Polakof et al. (2010) have shown that the intestine of rainbow trout contains enzymes to metabolize glucose into lactate. At present, major sources of energy substrates for specific tissues in fish are unknown.
The use of radiolabeled nutrients provides an approach to identifying and quantifying their metabolic pathways in animal tissues (Wu 2013). This study determined, for the first time to our knowledge, the rates of oxidation of glutamate, glutamine, leucine, glucose, and palmitate individually or as a mixture of substrates in the proximal intestine, liver, kidney, and skeletal muscle of fish. Dietary glutamate, glutamine, and leucine are abundant in proteins of animal and plant origins, such as fish meal, poultry by-product meal, and soybean meal, which are widely used as protein sources for fish feed (Li et al. 2011). Moreover, it is well known that glutamate, glutamine, and their metabolites participate in multiple metabolic pathways, such as glutaminolysis, transamination, and the Krebs cycle (Wu 2017). Glutamine and glutamate are regulators of gene expression and cell signaling in mammals (Wu 2010). Relatively high activities of BCAA transaminase make the mammalian skeletal muscle the major site for initiating BCAA transamination in the body. Hence, a large amount of leucine (one of the BCAAs) is degraded by mammalian skeletal muscle to generate KIC. Based on the published studies involving mammals (Wu 2013), glutamate, glutamine, and leucine were chosen for the present investigation. For comparison, glucose and palmitate were used as the representatives for carbohydrates and lipids, respectively.
Glutamate and glutamine oxidation
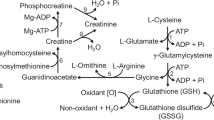
The rate of CO2 production from glutamate in the presence of a mixture of energy substrates varied greatly among tissues of fish. This AA was the most oxidative substrate in the proximal intestine, kidney, and skeletal muscle, and the second most oxidative substrate (after glutamine) in the liver of both HSB and zebrafish (Tables 1, 2). The proximal intestine, kidneys, skeletal muscle, and liver together comprised 45 and 47 % of the body weight in the juvenile HSB and young adult zebrafish, respectively. Our data indicate a quantitatively important role of glutamate oxidation in producing ATP in fish tissues. To generate ATP, the carbon backbone of glutamate is converted to α-ketoglutarate by glutamate dehydrogenase (GDH), glutamate-pyruvate transaminase, or glutamate-oxaloacetate transaminase. Both GDH and glutamate transaminases were found at much higher activities than fructose bisphosphatase in the livers of rainbow trout (French et al. 1981) and the sea bass (Enes et al. 2006). Moreover, Tng et al. (2008) reported that GDH activity in the liver and intestine of juvenile O. marmorata was increased by feeding. Likewise, GDH activity is highest in the kidneys of fish among digestive tissues and skeletal muscle (Christiansen and Klungs 1987). Many tissues (including the liver, intestine, kidney and muscle) of teleost and nonteleost fish possess a series of enzymes (including malic enzyme) to convert glutamate-derived α-KG into pyruvate (Chamberlin et al. 1991), which is subsequently oxidized to CO2 via pyruvate dehydrogenase and the Krebs cycle (Wu 2017). Therefore, a higher oxidative rate of glutamate over other nutrients in fish intestine, kidneys, and skeletal muscle may be due to higher activities of GDH plus glutamate transaminases than the enzymes that degrade glutamine, glucose, and palmitate. As a major energy substrate, glutamate is crucial for the growth, development, and health of fish. This finding supports the use of glutamate to improve intestinal morphology and function as well as whole-body growth in rainbow trout fed a soybean meal-based diet (Yoshida et al. 2016).
Glutamine was readily oxidized in the liver, proximal intestine, and kidneys of both HSB (Table 1) and zebrafish (Table 2), despite a lower rate of CO2 production from glutamine than glutamate by proximal intestine and kidneys in the presence of a mixture of energy substrates as noted previously. This is consistent with the report of glutamine oxidation in rat and chicken skeletal muscles (Wu et al. 1991). We found that there was a very low rate of glutamine oxidation in fish skeletal muscle incubated with a mixture of energy substrates (Tables 1, 2). This is in contrast to the report that mitochondria isolated from the lateral red muscle of teleost (Salvelinus namaycush) and nonteleost fish (Amia calva) fish actively oxidize glutamine (10 mM in the incubation medium) to CO2 (Chamberlin et al. 1991). Of note, glutamine was the most important source of ATP in the liver of both HSB and zebrafish (Table 4). Thus, glutamine can be actively taken up by the hepatocytes of fish. In certain mammalian cells (e.g., tumors), glutamine can contribute 30–50% of energy in the presence of physiological levels of glucose (Zielke et al. 1984).
Phosphate-activated glutaminase (a mitochondrial enzyme) plays a major role in initiating glutamine degradation in most mammalian tissues (including the small intestine, kidneys, and skeletal muscle (Wu 2013). This enzyme converts glutamine into glutamate and ammonia, and its activity is relatively high in the kidneys but very low in the white muscle of lake char fish (Chamberlin et al. 1991). There are also reports that ammonia is produced mainly in the liver mitochondrial matrix of ammoniotelic fishes (Ip and Chew 2010) and that glutamine degradation via glutaminase can account for 85% of the total ammonia excreted from some fish (Campbell et al. 1983). Based on the finding that more CO2 was produced from glutamine than glutamate in the liver, we suggest that the rate of the transport of glutamine by hepatocytes is higher than that of glutamate in fish and that the fish hepatocytes, like their mammalian counterparts, have a high glutaminase activity (Wu 2013). Because the rate of oxidation of glutamate by the small intestine is 2 to 4 times greater than that of glutamine in HSB and zebrafish, it is possible that phosphate-activated glutaminase limits the intestinal catabolism of glutamine. More research is warranted to test this hypothesis.
Fish tissues may interconvert glutamate and glutamine. When an incubation medium contained both of these two AAs, the intracellular specific radioactivity of [U-14C]glutamate or [U-14C]glutamine in a tissue may be affected by the presence of extracellular unlabeled glutamine or glutamate, respectively. We found that under the experimental conditions used (e.g., 2 mM glutamate and 2 mM glutamine in the incubation medium), the intracellular specific radioactivity of [U-14C]glutamate [measured as previously described (Wu et al. 1991)] in the presence of extracellular unlabeled glutamine was not affected in skeletal muscle, was about 6% lower in the small intestine and liver, and was about 10% lower in the kidneys for both zebrafish and HSB, when compared with the absence of glutamine. We also observed that the intracellular specific radioactivity of [U-14C]glutamine in the presence of extracellular unlabeled glutamate was not affected in the kidneys and small intestine, and was about 7% lower in the liver and skeletal muscle for both zebrafish and HSB, when compared with the absence of glutamate. Because there was little to only a small change in the intracellular specific radioactivity of [U-14C]glutamate or [U-14C]glutamine in the fish tissues, we concluded that the presence of both unlabeled glutamate and glutamine (2 mM each) in the incubation medium did not substantially underestimate the rates of oxidation of these two AAs to CO2.
Leucine oxidation
Both [1-14C]leucine and [U-14C]leucine have been employed to determine the metabolic pattern of leucine in cells and tissues of terrestrial animals (Lei et al. 2012, 2013; Wu and Thompson 1987). Much is known about inter-organ catabolism of BCAAs in mammals (Wu 2013). In their extrahepatic tissues, such as the small intestine and skeletal muscle, BCAAs undergo active transamination with α-ketoglutarate to form branched-chain α-ketoacids (BCKAs) and glutamate. The small intestine of pigs extracts 20–40% of dietary BCAAs in the first pass, thereby affecting the availability of these AAs for utilization by other organs (Hou et al. 2015, 2016b). In avian and mammalian skeletal muscles, BCAAs are used to synthesize glutamine and alanine, and these metabolic pathways are of nutritional and physiological significance (Wu 2013). In both the small intestine and skeletal muscle, the decarboxylation of BCKAs is limited due to a low activity of BCKA dehydrogenase; therefore, most of the BCKAs are released into the extracellular space (Wu 2013). In mammals, the liver and the kidneys play a major role in oxidizing BCKAs released from other tissues. Due to a low activity of hepatic BCAA transaminase, the mammalian liver has a limited capacity for degrading BCAAs (including leucine) to CO2 in comparison with the kidneys (Dawson et al. 1967; Wijayasinghe et al. 1983). Likewise, BCAA transaminase activity in the liver of lake trout is much lower than that in their kidneys and skeletal muscle (Hughes et al. 1983). Similar results were reported for the homogenates of tissues (e.g., kidney and skeletal muscle) from rainbow trout (Teigland and Klungsøyr 1983). Of interest, among the HSB and zebrafish tissues examined, the rates of net leucine transamination were highest in the kidney but lowest in skeletal muscle (Table 8). In all incubated tissues except for the zebrafish muscle, the rates of net leucine transamination were markedly inhibited by the presence of a mixture of energy substrates, which likely have a sparing effect on BCAA utilization by fish. Our finding that about 82, 70, 50, and 30% of the KIC produced from leucine was decarboxylated by the liver, proximal intestine, kidney, and skeletal muscle of HSB, respectively (Table 8) indicates a low activity of BCKA dehydrogenase in the muscle. Based on the rates of CO2 production from 14C-labeled substrates, our results showed that: (1) leucine oxidation produced more ATP in the kidneys than in other tissues of HSB and zebrafish; (2) leucine was a minor metabolic fuel in the intestine, liver, and kidney of HSB and zebrafish, as well as the skeletal muscle of HSB; and (3) leucine could contribute to about one-third of ATP production in zebrafish skeletal muscle where palmitate oxidation was not detectable in the presence of a mixture of energy substrate (Table 7). This illustrates a difference in AA metabolism between these two species.
Conclusion
Glutamate and glutamine were more actively oxidized in the proximal intestine, liver, and kidney of fish over the oxidation of glucose and palmitate. Glutamate provided more energy than glutamine in all the tissues except in the liver where glutamine served as the main emetabolic fuel. In the skeletal muscles of both HSB and zebrafish, glutamate was the preferred nutrient to generate ATP, followed by leucine and glucose in HSB or by leucine and glutamine in zebrafish. Together, glutamate plus glutamine plus leucine contributed to about 80% of ATP production in the fish tissues. Therefore, we suggest that AAs (primarily glutamate and glutamine) are the major metabolic fuels for the proximal intestine, liver, kidney, and skeletal muscle of HSB and zebrafish. These findings not only help solve the long-standing puzzle that fish have particularly high requirements for dietary protein, but also have important implications for formulating new, improved fish diets to provide optimal levels of protein, carbohydrate, and lipids.
Abbreviations
- AAs:
-
Amino acids
- BCAA:
-
Branched-chain amino acid
- BCKA:
-
Branched-chain α-ketoacid
- GDH:
-
Glutamate dehydrogenase
- KHB:
-
Krebs–Henseleit bicarbonate
- KIC:
-
ɑ-Ketoisocaproate
- HSB:
-
Hybrid striped bass
- NRC:
-
National Research Council
References
Assaad H, Zhou L, Carroll RJ, Wu G (2014) Rapid publication-ready MS-Word tables for one-way ANOVA. SpringerPlus 3:474
Ballantyne JS (2001) Amino acid metabolism. Fish Physiol 19:77–107
Campbell JW, Aster PL, Vorhaben JE (1983) Mitochondrial ammoniagenesis in liver of the channel catfish Ictalurus punctatus. Am J Physiol 244:R709–R717
Chamberlin ME, Glemet HC, Ballantyne JS (1991) Glutamine metabolism in a holostean (Amia calva) and teleost fish (Salvelinus namaycush). Am J Physiol 260:R159–R166
Christiansen DC, Klungs L (1987) Metabolic utilization of nutrients and the effects of insulin in fish. Comp Biochem Physiol B 88:701–711
Cowey CB, Walton MJ (1988) Studies on the uptake of (14C) amino acids derived from both dietary (14C) protein and dietary (14C) amino acids by rainbow trout, Salmo gairdneri Richardson. J Fish Biol 33:293–305
Dawson AG, Hird FJR, Morton DJ (1967) Oxidation of leucine by rat liver and kidney. Arch Biochem Biophys 122:426–433
Enes P, Panserat S, Kaushik S, Oliva-Teles A (2006) Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles. Comp Biochem Physiol A 143:89–96
French CJ, Mommsen TP, Hochachka PW (1981) Amino acid utilisation in isolated hepatocytes from rainbow trout. Eur J Biochem 113:311–317
Griffin ME, Wilson KA, Brown PB (1994) Dietary arginine requirement of juvenile hybrid striped bass. J Nutr 124:888–893
Hou Y, Yin Y, Wu G (2015) Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp Biol Med 240:997–1007
Hou YQ, Hu SD, Jia SC et al (2016a) Whole-body synthesis of l-homoarginine in pigs and rats supplemented with l-arginine. Amino Acids 48:993–1001
Hou YQ, Yao K, Yin YL, Wu G (2016b) Endogenous synthesis of amino acids limits growth, lactation and reproduction of animals. Adv Nutr 7:331–342
Hughes SG, Rumsey GL, Nesheim MC (1983) Branched-chain amino acid aminotransferase in the tissues of lake trout. Comp Biochem Physiol B 76:3–5
Ip YK, Chew SF (2010) Ammonia production, excretion, toxicity, and defense in fish: a review. Front Physiol 1:134
Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G (2006) Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17:571–588
Jurss K, Bastrop R (1995) Amino acid metabolism in fish. Biochem Mol Biol Fishes 4:159–189
Kaushik SJ, Seiliez I (2010) Protein and amino acid nutrition and metabolism in fish: current knowledge and future needs. Aquac Res 41:322–332
Laale HW (1977) The biology and use of zebrafish, Brachydanio rerio in fisheries research: a literature review. J Fish Biol 10:121–173
Latshaw JD, Bishop BL (2001) Estimating body weight and body composition of chickens by using noninvasive measurements. Poult Sci 80:868–873
Lei J, Feng DY, Zhang YL et al (2012) Regulation of leucine catabolism by metabolic fuels in mammary epithelial cells. Amino Acids 43:2179–2189
Lei J, Feng DY, Zhang YL et al (2013) Hormonal regulation of leucine catabolism in mammary epithelial cells. Amino Acids 45:531–541
Lenis YY, Wang XQ, Tang WJ et al (2016) Effects of agmatine on secretion of interferon tau and catecholamines and expression of genes related to production of polyamines by ovine trophectoderm cells. Amino Acids 48:2389–2399
Li P, Mai KS, Trushenski J, Wu G (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43–53
Li XL, Rezaei R, Li P, Wu G (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Li H, Meininger CJ, Bazer FW, Wu G (2016) Intracellular sources of ornithine for polyamine synthesis in endothelial cells. Amino Acids 48:2401–2410
Lobley GE, Milne V, Lovie JM et al (1980) Whole body and tissue protein synthesis in cattle. Br J Nutr 43:491–502
National Research Council (NRC) (2000) Nutrient requirements of beef cattle. National Academies Press, Wahsington DC
National Research Council (NRC) (2012) Nutrient requirements of swine. National Academies Press, Wahsington DC
Polakof S, Álvarez R, Soengas JL (2010) Gut glucose metabolism in rainbow trout: implications in glucose homeostasis and glucosensing capacity. Am J Physiol 299:R19–R32
Rønnestad I, Fyhn HJ (2008) Metabolic aspects of free amino acids in developing marine fish eggs and larvae. Rev Fish Sci 1:37–41
Rønnestad I, Thorsen A, Finn RN (1999) Fish larval nutrition: a review of recent advances in the roles of amino acids. Aquaculture 177:201–216
Self JT, Spencer TE, Johnson GA et al (2004) Glutamine synthesis in the developing porcine placenta. Biol Reprod 70:1444–1451
Sidell BD, Crockett EL, Driedzic WR (1995) Antarctic fish tissues preferentially catabolize monoenoic fatty acids. J exp Zool 271:73–81
Smits CHM, Moughan PJ, Smith WC (1988) Chemical whole-body composition of the 20 kg liveweight growing pig. New Zeal J Agric Res 31:155–157
Teigland M, Klungsøyr L (1983) Accumulation of α-ketoisocarproate from leucine in homogenates of tissues from rainbow trout (Salmo gairdnerii) and rat. An improved method for determination of branched chain keto acids. Comp Biochem Physiol B 75:703–705
Tng YYM, Wee NLJ, Ip YK, Chew SF (2008) Postprandial nitrogen metabolism and excretion in juvenile marble goby, Oxyeleotris marmorata (Bleeker, 1852). Aquaculture 284:260–267
Van Waarde A (1983) Aerobic and anaerobic ammonia production by fish. Comp Biochem Physiol B 74:675–684
van den Thillart G (1986) Energy metabolism of swimming trout (Salmo gairdneri)—oxidation rates of palmitate, glucose, lactate, alanine, leucine and glutamate. J Comp Physiol B 156:511–520
Weber JM, Haman F (1996) Pathways for metabolic fuels and oxygen in high performance fish. Comp Biochem Physiol A 113:33–38
Wijayasinghe MS, Milligan LP, Thompson JR (1983) In vitro degradation of leucine in muscle, adipose tissue, liver, and kidney of fed and starved sheep. Biosci Rep 3:1133 LP–1140 LP
Wilson RP (2002) Amino acids and proteins. In: Halver JE, Hardy RW (eds) Fish nutrition. Academic, New York, pp 143–179
Wu G (1997) Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol 272:G1382–G1390
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2010) Functional amino acids in growth, reproduction, and health. Adv Nutr 1:31–37
Wu G (2013) Amino acids: biochemistry and nutrition. CRC, Boca Raton
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol 5:34
Wu G (2017) Principles of Animal Nutrition. CRC Press, Boca Raton, Florida
Wu G, Thompson JR (1987) Ketone bodies inhibit leucine degradation in chick skeletal muscle. Int J Biochem 19:937–943
Wu G, Thompson JR, Baracos VE (1991) Glutamine metabolism in skeletal muscle from the broiler chick (Gallus domesticus) and the laboratory rat (Rattus norvegicus). Biochem J 274:769–774
Wu G, Knabe DA, Flynn NE (1994) Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299:115–121
Yoshida C, Maekawa M, Bannai M, Yamamoto T (2016) Glutamate promotes nucleotide synthesis in the gut and improves availability of soybean meal feed in rainbow trout. Springerplus 5:1021
Zielke HR, Zielke CL, Ozand PT (1984) Glutamine: a major energy source for cultured mammalian cells. Fed Proc 43:121–125
Acknowledgements
This research was supported by Texas A&M AgriLife Research (H-8200) and Guangdong Yuehai Feeds Group Co., Ltd. (Zhanjiang, China). We thank research assistants in our laboratory for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Additional information
Handling Editors: C.-A.A. Hu, Y. Yin, Y. Hou, G. Wu, Y. Teng.
Rights and permissions
About this article
Cite this article
Jia, S., Li, X., Zheng, S. et al. Amino acids are major energy substrates for tissues of hybrid striped bass and zebrafish. Amino Acids 49, 2053–2063 (2017). https://doi.org/10.1007/s00726-017-2481-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2481-7