Abstract
In mammals, l-glutamine (Gln) can alter the glutamate-Gln cycle and consequently brain excitability. Here, we investigated in developing rats the effect of treatment with different doses of Gln on anxiety-like behavior, cortical spreading depression (CSD), and microglial activation expressed as Iba1-immunoreactivity. Wistar rats were suckled in litters with 9 and 15 pups (groups L 9 and L 15; respectively, normal size- and large size litters). From postnatal days (P) 7–27, the animals received Gln per gavage (250, 500 or 750 mg/kg/day), or vehicle (water), or no treatment (naive). At P28 and P30, we tested the animals, respectively, in the elevated plus maze and open field. At P30–35, we measured CSD parameters (velocity of propagation, amplitude, and duration). Fixative-perfused brains were processed for microglial immunolabeling with anti-IBA-1 antibodies to analyze cortical microglia. Rats treated with Gln presented an anxiolytic behavior and accelerated CSD propagation when compared to the water- and naive control groups. Furthermore, CSD velocity was higher (p < 0.001) in the L 15 compared to the L 9 condition. Gln treatment increased Iba1 immunolabeling both in the parietal cortex and CA1 hippocampus, indicating microglial activation. The Gln effect was dose-dependent for anxiety-like behavior and CSD in both litter sizes, and for microglial activation in the L 15 groups. Besides confirming previous electrophysiological findings (CSD acceleration after Gln), our data demonstrate for the first time a behavioral and microglial activation that is associated with early Gln treatment in developing animals, and that is possibly operated via changes in brain excitability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutamine (Gln) is found abundantly in the mammalian central nervous system, where it participates in a variety of metabolic pathways. In distinct types of neuronal cells, Gln influences the synthesis of the excitatory amino acids, glutamate (Glu) and aspartate, and the inhibitory amino acid, γ-amino butyric acid (GABA; Albrecht et al. 2011). A neuron-glia metabolic interaction, known as the glutamate-glutamine cycle, controls the extracellular concentration of Glu and enables the detoxification of ammonia that crosses freely from the blood across the blood–brain barrier (Daikhin and Yudkoff 2000). Enhanced Gln availability could alter the interactions in the glutamate–glutamine cycle and affect ammonia and Glu levels in the brain. Increased availability of Gln may also affect translocation of Gln into GABAergic neurons and GABA is taken up by astroglia (Holecek 2013).
In humans, Gln supplementation may be beneficial in preterm infants, especially in the reduction of infectious complications (Moe-Byrne et al. 2016). However, the potential effects of early Gln supplementation on brain development and subsequent neuron-glia organization and behavioral outcome have not yet been fully elucidated (De Keviet et al. 2012). In addition, few studies have so far evaluated the effect of Gln supplementation on the excitability-related balance between glutamatergic and GABAergic transmission (Lima et al. 2009). These authors reported in developing rats a facilitating effect of Gln (500 mg/kg/day over P7–P27) on the excitability-related phenomenon known as cortical spreading depression (CSD).
CSD is a fully reversible neural response, first described in the rabbit cortex, as a slowly propagating wave of depression of spontaneous neuronal activity produced by electrical, mechanical or chemical stimulation of one point on brain tissue, from which it spreads concentrically to remote cortical regions (Leão 1944). This phenomenon has since been demonstrated in a number of animal studies (Toriumi et al. 2016; Shatillo et al. 2015; Lopes-de-Morais et al. 2014; Guedes et al. 2013). CSD is facilitated under unfavorable lactation conditions (suckling in large litters; Guedes 2011), and this condition can also modulate the effect of other treatments (Guedes et al. 2013). Human studies have demonstrated the occurrence of this depolarizing phenomenon in the setting of a variety of pathological states, including migraine (Lauritzen and Strong 2016), cerebrovascular disorders (Nakamura et al. 1994), epilepsy (Dreier 2011), traumatic brain injury (Hartings et al. 2011), and neurosurgical procedures (Carlson et al. 2016).
Measuring CSD velocity of propagation along the cortical tissue is a reasonable and easy way of estimating the brain susceptibility to CSD. This has been experimentally characterized in our laboratory under conditions of environmental, pharmacological, and nutritional manipulations (Abadie-Guedes et al. 2016; Monteiro et al. 2015; Lopes-de-Morais et al. 2014), including treatment with other amino acids (Frazão et al. 2008). Recent studies have also shown possible relationships between alterations of CSD, and behavioral (Francisco and Guedes 2015) and microglial activation (Soares et al. 2015; Lima et al. 2013, 2014). Also, a relationship between litter size and microglia activation and behavior has been reported (Viana et al. 2013; Tu et al. 2012).
To test whether the CSD facilitating effect of early treatment with Gln is dose-dependent, we used three different doses of Gln during the brain development period. Furthermore, we investigated the Gln effect on anxiety-like behavior and Iba1-immunoreactivity of microglia. Considering that unfavorable lactation conditions can modulate both CSD and the brain effects of amino acids (Francisco and Guedes 2015), as well as microglia (Tu et al. 2012) and behavior (Viana et al. 2013), we analyzed the Gln effects on rats suckled in normal and large litters.
Materials and methods
Animals
The Wistar rat pups of this study (n = 117 males) were handled in accordance with the norms established by the National Institutes of Health Guide for Care and Use of Laboratory Animals (Bethesda, MD, USA). All experimental procedures were previously approved by the Institutional Ethics Committee for Animal Research of our University (Process no. 23076.010667/2012-84). Animals were housed in polypropylene cages (51 cm × 35.5 cm × 18.5 cm) in a room maintained at 22 ± 1 °C with a 12:12 h light:dark cycle (lights on at 6:00 a.m.). The newborn male and female rats, born from distinct dams, were pooled and assigned to be suckled under favorable or unfavorable conditions, represented, respectively, by normal size litters, with nine pups (L 9 groups; n = 65 males), and large size litters with 15 pups (L 15 groups; n = 52 males). On postnatal day (P) 25, both groups were weaned and switched to the maternal lab chow diet (Purina Ltd.) with 23% protein. Only the male pups (n = 117) were used in this study.
l-Glutamine treatment
From P7 to P27, three groups of male pups received per gavage three doses of Gln (Sigma, St. Louis, MO, USA): 250 mg/kg/day (Gln250 group; 14 L 9 and 13 L 15 pups), or 500 mg/kg/day (Gln500; 14 L 9 and 8 L 15 pups), or 750 mg/kg/day (Gln750; 15 L 9 and 11 L 15 pups). Two additional control groups received no gavage (naive group; 11 L 9 and 10 L 15 pups) or distilled water (vehicle; 11 L 9 and 10 L 15 pups). The gavage volume of the amino acid solutions or of distilled water ranged from 0.5 ml/day (in the second week of life) to 1.0 ml/day (in the third and fourth week of life).
Body weights
The rats were weighed at P7, P14, P21, and P30–35 (when the electrophysiological recordings were performed).
Elevated plus-maze test
The elevated plus-maze test was conducted on P28. The cross-shaped apparatus consisted of four arms (two closed arms and two open arms, each measuring 49 cm long × 10 cm wide) raised 55 cm above the floor. A central squared platform (10 × 10 cm wide) connected the open and closed arms. For each 5-min session, under dim light and in a sound-attenuated room, the rat was initially placed in the central platform facing an open arm. A video camera recorded the behavioral activity of the animal. The recorded activity was stored in a computer and subsequently analyzed with the software ANYmaze™ (version 4.99 m). After each test, the arms and the central platform were cleaned with a paper cloth soaked with 70% ethanol. The following parameters were analyzed: total distance traveled, number of entries into the open arms, and time spent in the open arms. We considered that the animal entered one open or one closed arm when its four paws entered the arm.
Open field test
On P30, the rats were placed in the center of a circular arena (diameter 100 cm and height 52 cm). The apparatus was placed in a room with a dim light and sound-attenuation. Rats were positioned in the center arena and their movements were captured using a digital camera for 5 min. Between each test, the open field was wiped with a paper cloth soaked with 70% ethanol. The recorded activity was stored in a computer and subsequently analyzed with the software ANYmaze™ (version 4.99 m). The following parameters were considered: total distance traveled, the number of entries in the central zone, and the time spent in central zone.
CSD recording
On the day of CSD recording (P30–35), the animal was anesthetized with a mixture of 1 g/kg urethane plus 40 mg/kg chloralose injected intraperitoneally. Three trephine holes were drilled on the right side of the skull, aligned in the frontal-to-occipital direction and parallel to the midline. One hole (2 mm in diameter) was positioned on the frontal bone and used to apply the stimulus (KCl) to elicit CSD. The other two holes (2 mm in diameter) were positioned on the parietal bone and used to record the propagating CSD wave. CSD was elicited at 20-min intervals by a 1-min application of a cotton ball (1–2 mm in diameter) soaked with 2% KCl solution (approximately 270 mM) to the anterior hole drilled at the frontal region. Rectal temperature was continuously monitored and maintained at 37 ± 1 °C by means of a heating blanket. The DC slow potential change accompanying CSD was recorded for 4 h using two Ag–AgCl agar–Ringer electrodes (one in each hole) against a common reference electrode of the same type, placed on the nasal bones. The CSD velocity of propagation was calculated from the time required for a CSD wave to pass the distance between the two cortical electrodes. In the two cortical recording places, the initial point of each DC-negative rising phase was used as the reference point to calculate the CSD velocities. In addition, amplitude and duration of the CSD waves were calculated, as previously reported (Mendes-da-Silva et al. 2014).
Immunohistochemistry
Rats treated with vehicle (n = 6, of which 3 L 9 and 3 L 15), Gln250 (n = 8, of which 4 L 9 and 4 L 15), or Gln500 (n = 8; 4 L 9 and 4 L 15) were perfused with 0.9% saline solution followed by 4% paraformaldehyde diluted in 0.1 M phosphate-buffered saline (pH 7.4). After being immersed in the fixative for 4 h, the brains were transferred to a 30% (w/v) sucrose solution for cryoprotection. Longitudinal serial sections (40-µm thickness) were obtained at −20 °C using a cryoslicer (Leica 1850). Sections were immunolabeled with a polyclonal antibody against ionized calcium-binding adapter molecule 1 (Iba-1) to detect microglia (1:3000; anti-Iba-1, #019-19741; Wako Pure Chemical Industries Ltd., Osaka, Japan). Free-floating sections were submitted to endogenous peroxidase blocking (2% H2O2 in 70% methanol for 10 min) and the sections incubated for 1 h in blocking buffer (BB) solution containing 0.05 M Tris-buffered saline (TBS; pH 7.4), 10% fetal calf serum, 3% bovine serum albumin, and 1% Triton X-100. The sections were then incubated overnight at 4 °C with rabbit anti-Iba-1 (1:1500 diluted in BB solution). After three washes with TBS + 1% Triton, sections were incubated at room temperature for 1 h with biotinylated anti-rabbit (1:500) secondary antibodies. Sections were then rinsed in TBS + 1% Triton and incubated with horseradish peroxidase streptavidin (1:500). The peroxidase reaction was visualized by incubating the sections in Tris buffer containing 0.5 mg/ml 3,3′-diaminobenzidine (DAB) and 0.33 µl/ml H2O2. Finally, the sections were mounted, dehydrated in graded alcohols, and coverslipped in Entellan® after xylene treatment. Densitometric analysis was performed on four parallel longitudinal sections for each animal. A Leica DMLS microscope coupled to a Samsung high-level color camera (model SHC-410NAD) was used to obtain digital images from brain sections. Images of the parietal cortex and CA1 hippocampus stained for Iba1 were obtained using a 20× microscope objective. In each section, photomicrographs of four fields within the parietal cortex (layer 2) and two fields of CA1 hippocampal region were analyzed, using the Image J software (National Institutes of Health, USA, version 1.46r), which is a worldwide used open tool for the analysis of scientific images (see Schneider et al. 2012 for a historical review). Care was taken to obtain the digital images using the same light intensity. Total immunoreactivity expressed as arbitrary units as well as the percentage of the area occupied by the Iba1-labeled cells were analyzed.
Statistics
Results in all groups are expressed as means ± standard deviations (SD). Data were compared between groups using ANOVA, including as factors lactation conditions (L 9 and L 15) and gavage treatment (naïve, vehicle, Gln250, Gln500, and Gln750) followed by a post hoc test (Holm–Sidak). A p value less than 0.05 was considered significant.
Results
Body weights
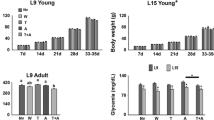
As shown in Fig. 1, L 15 animals presented with lower (p < 0.05) body weights, as compared with those of the L 9-groups. In the L 9 condition, the body weights of the control groups ranged from 15.4 ± 1.3 g at P7 to 107.8 ± 6.4 g at P30–35, whereas in the L 15 condition the weights ranged from 12.5 ± 1.0 to 74.3 ± 8.4 g. Treatment with Gln did not alter body weights of animals.
Body weight of male rats (30- to 35-days old) previously suckled in litters with 9 or 15 pups (respectively, L 9 and L 15 condition). Gln250, Gln500, and Gln750 are rats treated per gavage from postnatal days 7–27 with l-glutamine at the doses of 250, 500 and 750 mg/kg/day, respectively. Data are mean ± SD. Asterisk indicate significant difference between L 15 and the corresponding L 9 groups (p < 0.05; one-way ANOVA followed by the Holm–Sidak test)
Elevated plus-maze and open field tests
The effect of Gln treatment on the anxiety-like behavioral activity in the elevated plus-maze, and in the open field tests is shown in Fig. 2. Regarding the elevated plus maze, in the L 15 condition, Gln500- and Gln750 groups traveled higher distance than the controls and presented with longer time in the open arms than the L 15-Gln250, naive, and vehicle groups (p < 0.05).
Behavioral activity, in the elevated plus maze and open field apparatus, of young rats that were previously suckled in litters with 9 and 15 pups (respectively, L 9 and L 15 condition). Gln250, Gln500, and Gln750 are rats treated per gavage from postnatal days 7–27 with l-glutamine at the doses of 250, 500 and 750 mg/kg/day, respectively. Bars represent mean values ± standard deviation. Asterisk indicates values that are significantly different from naive and vehicle control groups. Plus sign significantly different from control groups and from Gln250. Double plus sign significantly different from the control and from the corresponding L 9 groups (p < 0.05; ANOVA followed by Holm–Sidak test)
In the open field, the Gln-treated animals of both L 9 and L 15 condition traveled a higher distance than the corresponding controls. In the L 15 condition, the Gln-treated animals entered more times in the central zone in comparison with the controls. The Gln500 and Gln750 groups also spent more time in the central zone.
CSD parameters
In all groups, topical application of 2% KCl for 1 min at the frontal cortex usually elicited a single CSD wave, which was recorded by the two electrodes located more posteriorly in the stimulated hemisphere. Figure 3 presents electrophysiological recordings on the cortical surface of five L 9 and five L 15 animals, illustrating the slow potential change accompanying CSD. The DC slow potential recordings confirmed the presence of CSD after KCl stimulation.
Slow potential changes (P) during cortical spreading depression (CSD), recorded at two cortical points (1 and 2), in ten developing rats, previously suckled in litters with 9 or 15 pups (respectively, L 9 and L 15 condition). The diagram of the skull shows the recording positions 1 and 2 from which the traces marked at the left with the same numbers were obtained. The position of the common reference electrode (R) on the nasal bones and the application point of the CSD-eliciting stimulus (KCl) are also shown. Gln250, Gln500, and Gln750 are rats treated per gavage with l-glutamine at doses of 250, 500, and 750 mg/kg/day, respectively. The vertical bars indicate 10 mV for P (negative upwards). CSD was elicited in the frontal cortex by chemical stimulation (a 1- to 2-mm diameter cotton ball soaked with 2% KCl) applied for 1 min on the intact dura mater, as indicated by the horizontal bars. The vertical dashed lines indicate the latency for a CSD wave to cross the inter-electrode distance. The latencies were shorter in the L 15 groups compared with the corresponding L 9 groups. In the groups treated with Gln, the latencies decreased when compared with the respective Naive and Vehicle controls. In both L 9 and L 15 condition, Gln500 and Gln750 groups displayed shorter latencies than the corresponding Gln250 rats
Regarding CSD velocity of propagation, ANOVA indicated intergroup differences [F(1, 106) = 316.466; p < 0.001], and post hoc (Holm–Sidak) test comparisons showed that the velocities were higher (p < 0.001) in the L 15 groups compared to the L 9 groups. Gln treatment significantly increased the CSD propagation velocities [F(4, 106) = 53.728; p < 0.001] compared with the Naive and Vehicle controls. In both lactation conditions, CSD velocity of the Gln250 animals (L 9 = 4.12 ± 0.13 mm/min; L 15 = 4.83 ± 0.21 mm/min) was higher than the controls (L 9 = 3.87 ± 0.11; L 15 = 4.52 ± 0.14 mm/min for the vehicle groups, and L 9 = 3.85 ± 0.13; L 15 = 4.61 ± 0.15 mm/min for the naive groups), but lower than the Gln500 (L 9 = 4.51 ± 0.14 mm/min; L 15 = 5.17 ± 0.27 mm/min) and Gln750 rats (L 9 = 4.52 ± 0.16; L 15 = 5.26 ± 0.46 mm/min). No difference was found between Gln500 and Gln750 groups in both L 9 and L 15 condition. The CSD velocities for all groups are shown in Fig. 4.
CSD velocity of young rats (30- to 35-days old) that were suckled in litters with 9 and 15 pups, resulting in two distinct lactation conditions (respectively, L 9 and L 15 groups). Gln250, Gln500, and Gln750 are rats treated per gavage with l-glutamine at the doses of 250, 500, and 750 mg/kg/day, respectively. Data are mean ± SD. Asterisk significantly different from the corresponding L 9 groups. Plus sign significantly different from the naive and vehicle control groups. Double plus sign significantly different from the Gln250 and control groups (p < 0.01; ANOVA followed by the Holm–Sidak test)
Data on amplitude and duration of the CSD negative slow potential change are shown in Table 1. ANOVA indicated a main effect of the treatment [F(4, 87) = 15.025; p < 0.001 for amplitude and F(4, 96) = 14.194; p < 0.001 for duration]. The Holm–Sidak test indicated that in L 9 condition all groups treated with Gln presented with larger amplitudes and lower durations when compared to vehicle- and naive controls. In this lactation condition (L 9), the Gln750 group had significantly higher amplitude than the Gln250 group. In L 15 condition, only the Gln750 group showed significantly higher amplitude and shorter duration when compared to the respective controls. The L 15 vehicle- and naive groups had a shorter duration when compared to the corresponding L 9.
Iba1 immunohistochemistry
Photomicrographs of Iba1-labeled cells in the parietal cortex and CA1 hippocampus are shown in Fig. 5a, b. The animals of the L 15 condition had greater immunoreactivity and higher percentage of marked area when compared to the corresponding L 9 condition (Fig. 5c, d). In both lactation conditions, Gln250 and Gln500 animals showed higher immunoreactivity compared to vehicle control, both in cortex and hippocampus. Unlike L 9 condition, the L 15 animals that were treated with Gln500 showed significantly greater immunoreactivity when compared to the Gln250 group. Regarding the percentage of labeled area, in the L 9 condition Gln250 and Gln500 exhibited higher percentage of labeled area when compared to control, while in L 15 condition only the Gln500 group was different from the control in the parietal cortex. In the hippocampus, only the Gln500 group of the L 9 condition was statistically different from the control group.
Low magnification photomicrographs of Iba1-labeled microglial cells in longitudinal sections of the parietal cortex (a) and CA1 hippocampus (b) of six young rats (with 30- to 35-days of life) suckled in litters with 9 and 15 pups, resulting in two distinct lactation conditions (respectively, L 9 and L 15 groups). Gln250 and Gln500 are rats treated per gavage with l-glutamine at the doses of 250 and 500 mg/kg/day, respectively. Scale bars 20 µm. The bar graphics show immunoreactivity expressed as arbitrary units and the percent area occupied by the Iba1-labeled cells of slices of the parietal cortex (c) and CA1 hippocampus (d) of vehicle control (n = 6; 3 L 9 and 3 L 15), Gln250 (n = 8; 4 L 9 and 4 L 15) and Gln500 rats (n = 8; 4 L 9 and 4 L 15). Data are expressed as mean ± SD. Asterisk p < 0.05 compared with L 9 condition. Plus sign p < 0.05 compared with vehicle control. Double plus sign p < 0.05 compared with the other two groups (ANOVA plus Holm–Sidak test)
Discussion
In this study, we confirmed our previous results (Lima et al. 2009), which indicated that treatment with Gln during the critical period of the nervous system development accelerates CSD, regardless of the lactation conditions. Furthermore, we could show, for the first time, changes in anxiety-like behavior and microglial immunoreactivity as a consequence of early treatment with Gln. In some instances, the Gln effects were significantly dose-dependent, and in other instances a non-significant dose-dependent tendency was observed.
Body weights were lower in the L 15 groups compared with the corresponding L 9 groups, confirming the effectiveness of increasing litter size in producing undernutrition, as previously indicated (Francisco and Guedes 2015; Rocha-de-Melo et al. 2006). In animal models, malnutrition during the critical period of brain development leads to a reduction in the number and/or size of brain cells, in myelin production and in the number of synapses, and changes in neurotransmitter systems (Morgane et al. 2002). Although some effects of malnutrition on the structure and brain metabolism are reversed by nutritional rehabilitation, other effects are not and continue with subsequent electrophysiological, behavioral, and cognitive changes (Levitsky and Strupp 1995; Penido et al. 2012). The treatment with Gln did not alter the animals’ body weight (Fig. 1). This is in line with previous reports on Gln treatment per gavage (Lima et al. 2009) and via subcutaneous injection (Ladd et al. 2010).
Our results of anxiety tests reveal a less anxious behavior in the L 15, but not L 9 groups treated with Gln as indicated by the longer permanence in the open arms in the elevated plus maze, and by a higher number of entries and more time spent in the central zone in the open field apparatus (Fig. 2). This lower anxiety-like behavior is in line with previous data on animals that were suckled in large litters (Clarke et al. 2013; Bulfin et al. 2011). In the L 9 groups, there was a non-significant tendency for an anxiolytic effect of Gln for all evaluated parameters, except for the time spent in the open arms of the Gln750 group. It is possible that Gln acts at the central nervous system as an anti-anxiety agent by acting as precursor of GABA. Previous studies have already demonstrated an increased GABA level in the brain of rats treated with Gln per gavage (Wang et al. 2007) or subcutaneously (Ladd et al. 2010). This finding could explain the Gln anxiolytic properties described here. An alternative interpretation of the behavioral data could include Gln-induced enhancement of locomotion, as suggested in humans (McCormack et al. 2015), and also as observed after taurine administration in rats (Francisco and Guedes 2015).
The higher CSD velocity from the L 15 animals confirms the facilitating action of early undernutrition, induced by suckling the pups in large litters. Concerning the mechanisms by which malnutrition facilitates CSD propagation, a larger volume of extracellular space in the brain hinders CSD elicitation and propagation (Mazel et al. 2002). Inadequate nutrient intake early in life increases cell-packing density and reduces the extracellular space, leading to facilitation of CSD. Another important factor that modulates CSD propagation is cortical myelination. Previous work has demonstrated an inverse correlation between the degree of cortical myelination and CSD propagation velocity (Merkler et al. 2009). Nutritional deficiency reduces brain myelination and increases CSD propagation velocity (De Luca et al. 1977; Rocha-de-Melo et al. 2006). Furthermore, malnutrition may impair glial function (Morgane et al. 1978) and caloric restriction may suppress induced microglial activation in non-cortical brain areas (Radler et al. 2015; Tu et al. 2012). Furthermore, CSD is facilitated by glial impairment (Largo et al. 1997) and induces potassium currents in microglia (Wendt et al. 2016). In addition, malnourished rats present increased levels of the enzyme glutamic acid decarboxylase (Díaz-Cintra et al. 2007). This condition, in association with reduced brain glutamate uptake (Feoli et al. 2006), enhances extracellular glutamate in the malnourished brain, which might contribute to CSD facilitation (Marrannes et al. 1988).
Our data clearly demonstrate that neonatal treatment with Gln dose-dependently accelerated the propagation velocity of CSD irrespective of the lactation condition (L 9 versus L 15). These results are coherent with a previous report (Lima et al. 2009), and reinforce the importance of Gln for the proper electrophysiological functioning of the brain, therefore suggesting that neonatal treatment with Gln can modulate, at least in part, the brain’s ability to propagate CSD. The vehicle group, which was treated per gavage with distilled water, presented CSD features similar to the naive control. In view of this observation, we are compelled to conclude that the assumed causal link between Gln treatment and the here described CSD changes is substantial, and cannot be attributed to the gavage stress.
Although in this study amino acid blood levels have not been monitored, it is reasonable to assume that the present long-term (3 weeks) neonatal Gln treatment might have caused an amino acid imbalance in plasma and tissues (Holecek 2011) and increased its brain concentration (Szpetnar et al. 2016). Thus, it is tempting to hypothesize that the increase in plasma Gln would impair the output of Gln from the astroglia to the bloodstream. The enhanced Gln availability could alter interactions in the glutamate–Gln cycle and affect ammonia and glutamate levels in the brain. This may occur in neurons by activation of the enzyme glutaminase and in astroglia by suppression of another enzyme, Gln synthetase. Enhanced availability of Gln may also affect translocation of Gln into GABAergic neurons and released GABA into astroglia (Holecek 2013). In fact, glutamate- and GABA-mediated mechanisms are important for the phenomenon of CSD (Holland et al. 2010; Haghir et al. 2009; Guedes et al. 1992; Marrannes et al. 1988), and glutamate to GABA imbalance may ultimately lead to a hyperexcitable brain (Becerra et al. 2016), which can help in explaining our findings. Another possibility that deserves future investigation would be the evaluation of the effects of Gln on the activation of astrocytes, which seems to be associated with microglia activation (Guan et al. 2016).
Under CSD accelerating and decelerating conditions, microglial immunolabeling has been found to be, respectively, enhanced (Lima et al. 2013, 2014) and reduced (Soares et al. 2015). These data suggest a direct relationship between CSD velocity of propagation and microglia activation. Recent evidence from others suggests that microglia is required for spreading depression (Pusic et al. 2014) and, conversely, CSD activates microglia (Takizawa et al. 2016) and NMDA receptors (Wendt et al. 2016). Our findings are in agreement with those of Lima et al. (2013, 2014), and Takizawa et al. (2016) as we found CSD acceleration and microglial activation in the Gln-treated animals, which suggests a positive modulating role for Gln in the microglial activation in the brain. All of the evidence notwithstanding, a deeper investigation on the possible directions of this relationship is still required.
Microglial cells are the resident immune cells of the CNS. With their highly dynamic processes, they continuously survey the microenvironment, thus being the first cells to be activated in response to injurious tissue demands. These cells are involved in inflammatory responses in the brain, as well as behavioral responses (Viana et al. 2013). It must be remarked that Iba1 immunolabeling is not a specific marker for microglia, as it also labels macrophages, which may infiltrate the CNS and may become involved in inflammatory responses in the brain, as well as behavioral responses (Wohleb et al. 2014).
Microglia help to prevent glutamate-related excitotoxicity by promoting glutamate uptake (Hanisch and Kettenmann 2007). Svoboda and Kerschbaum (2009) reported in vitro apoptosis of microglia as a result of the increase in Gln concentration, suggesting Gln toxicity. They concluded that hydrolysis of Gln and, accordingly, accumulation of ammonium in mitochondria induced the intrinsic pathway of apoptosis. In addition, recent evidence suggests that enhancement of Gln levels by glutaminase inhibition blocks glutamate release from stimulated microglia (Thomas et al. 2014). Data collectively support the Trojan horse hypothesis of Gln toxicity, which involves both astroglia and microglia cells (Albrecht and Norenberg 2006; Svoboda and Kerschbaum 2009).
In the Gln-treated groups, microglia activation has been observed in the parietal cortex and, to a lesser extent in the hippocampal CA1 area (Fig. 5), which suggests a regional difference in microglia activation. This is interesting, as suckling in large litters may increase microglia activation in the hippocampus (Viana et al. 2013) and overnutrition (suckling in litters with 4 pups) enhances microglia activation in hypothalamus and cerebellum (Tapia-González et al. 2011).
In conclusion, the results of this study suggest a brain effect of early Gln treatment, expressed as behavioral (anxiety), electrophysiological (CSD) and microglial alterations in developing rats. Regarding on how the Gln-induced anti-anxiety, pro CSD, and pro microglial activation are related to each other, and how unfavorable suckling conditions can modify this relationship, recent evidence from others indicate that microglia is required for CSD (Pusic et al. 2014), and suckling in large litters increases the brain GABA levels after Gln treatment (Ladd et al. 2010), and modulates CSD (Francisco and Guedes 2015), behavior, and microglia activation (Viana et al. 2013). Furthermore, the relevance of the CSD phenomenon for important neurological diseases has been well established along the last two decades (Gorji 2001; Dreier 2011; Torrente et al. 2014; Kramer et al. 2016). Most discussed mechanisms for this interplay might include Gln action on the GABA-glutamate balance (Holecek 2013; Wang et al. 2007). Future experiments are needed to further clarify the underlying mechanisms, as well as to explore the possibility of such Gln effects on the adult brain.
References
Abadie-Guedes R, Bezerra RS, Guedes RCA (2016) Alpha-tocopherol counteracts the effect of ethanol on cortical spreading depression in rats of various ages, with and without ethanol abstinence. Alcohol Clin Exp Res 40:728–733
Albrecht J, Norenberg MD (2006) Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 44:788–794
Albrecht J, Sidoryk-Węgrzynowicz M, Zielińska M, Aschner M (2011) Roles of glutamine in neurotransmission. Neuron Glia Biol 6(4):263–276
Becerra L, Veggeberg R, Prescot A, Jensen JE, Renshaw P, Scrivani S, Spierings EL, Burstein R, Borsook D (2016) A ‘complex’ of brain metabolites distinguish altered chemistry in the cingulate cortex of episodic migraine patients. Neuroimage Clin 11:588–594
Bulfin LJ, Clarke MA, Buller KM, Spencer SJ (2011) Anxiety and hypothalamic–pituitary–adrenal axis responses to psychological stress are attenuated in male rats made lean by large litter rearing. Psychoneuroendocrinology 36(7):1080–1091
Carlson AP, Shuttleworth CW, Mead B, Burlbaw B, Krasberg M, Yonas H (2016) Cortical spreading depression occurs during elective neurosurgical procedures. J Neurosurg 11:1–8
Clarke M, Cai G, Saleh S, Buller KM, Spencer SJ (2013) Being suckled in a large litter mitigates the effects of early-life stress on hypothalamic–pituitary–adrenal axis function in the male rat. J Neuroendocrinol 25(9):792–802
Daikhin Y, Yudkoff M (2000) Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr 130:1026S–1031S
De Kieviet JF, Oosterlaan J, Vermeulen RJ, Pouwels PJ, Lafeber HN, van Elburg RM (2012) Effects of glutamine on brain development in very preterm children at school age. Pediatrics 130:1121–1127
De Luca B, Cioffi LA, Bureš J (1977) Cortical and caudate spreading depression as an indicator of neural changes induced by early malnutrition in rats. Act Nerv Super 19:130–131
Díaz-Cintra S, González-Maciel A, Morales MA, Aguilar A, Cintra L, Prado-Alcalá RA (2007) Protein malnutrition differentially alters the number of glutamic acid decarboxylase-67 interneurons in dentate gyrus and CA1–3 subfields of the dorsal hippocampus. Exp Neurol 208:47–53
Dreier JP (2011) The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 17:439–447
Feoli AM, Siqueira IR, Almeida L, Tramontina AC, Vanzella C, Sbaraini S, Schweigert ID, Netto CA, Perry ML, Gonçalves CA (2006) Effects of protein malnutrition on oxidative status in rat brain. Nutrition 22:160–165
Francisco ES, Guedes RCA (2015) Neonatal taurine and alanine modulate anxiety-like behavior and decelerate cortical spreading depression in rats previously suckled under different litter sizes. Amino Acids 47:2437–2445
Frazão MF, Maia LMS, Guedes RCA (2008) Early malnutrition, but not age, modulates in the rat the l-arginine facilitating effect on cortical spreading depression. Neurosci Lett 447:26–30
Gorji A (2001) Spreading depression: a review of the clinical relevance. Brain Res Rev 38:33–60
Guan R, Wang T, Chen J, Luo W, Liu M (2016) The activation of microglia caused by lead and manganese co-exposure induces activation of astrocytes and decrease of glutamine synthetase activity. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 32:313–318
Guedes RCA (2011) Cortical spreading depression: a model for studying brain consequences of malnutrition. In: Preedy VR, Watson RR, Martin CR (eds) Handbook of behavior, food and nutrition. Springer, Berlin, pp 2343–2355
Guedes RCA, Cabral-Filho JE, Teodósio NR (1992) GABAergic mechanisms involved in cortical spreading depression in normal and early malnourished rats. In: Do-Carmo RJ (ed) Spreading depression. Experimental brain research series, vol 23. Springer, Berlin, pp 17–26
Guedes RCA, Rocha-de-Melo AP, Lima KR, Albuquerque JM, Francisco ES (2013) Early malnutrition attenuates the impairing action of naloxone on spreading depression in young rats. Nutr Neurosci 16:142–146
Haghir H, Kovac S, Speckmann EJ, Zilles K, Gorji A (2009) Patterns of neurotransmitter receptor distributions following cortical spreading depression. Neuroscience 163:1340–1352
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
Hartings JA, Watanabe T, Bullock MR, Okonkwo DO, Fabricius M, Woitzik J, Dreier JP, Puccio A, Shutter LA, Pahl C, Strong AJ (2011) Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain 134:1529–1540
Holecek M (2011) Adverse effects of chronic intake of glutamine-supplemented diet on amino acid concentrations and protein metabolism in rat: effect of short-term starvation. e-SPEN. Eur J Clin Nutr Metabol 6:e190–e196
Holecek M (2013) Side effects of long-term glutamine supplementation. Jpn J Parenter Enteral Nutr 37:607–616
Holland PR, Akerman S, Goadsby PJ (2010) Cortical spreading depression-associated cerebral blood flow changes induced by mechanical stimulation are modulated by AMPA and GABA receptors. Cephalalgia 30:519–527
Kramer DR, Fujii T, Ohiorhenuan I, Liu CY (2016) Cortical spreading depolarization: pathophysiology, implications, and future directions. J Clin Neurosci 24:22–27
Ladd FV, Ladd AA, Ribeiro AA, Costa SB, Coutinho BP, Feitosa GA, Andrade GM, Castro-Costa CM, Magalhães CE, Castro IC, Oliveira BB, Guerrant RL, Lima AA, Oriá RB (2010) Zinc and glutamine improve brain development in suckling mice subjected to early postnatal malnutrition. Nutrition 26:662–670
Largo C, Ibarz JM, Herreras O (1997) Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J Neurophysiol 78:295–307
Lauritzen M, Strong AJ (2016) ‘Spreading depression of Leão’ and its emerging relevance to acute brain injury in humans. J Cereb Blood Flow Metab. doi:10.1177/0271678X16657092
Leão AAP (1944) Spreading depression of activity in the cerebral cortex. J Neurophysiol 7:359–390
Levitsky DA, Strupp BJ (1995) Malnutrition and the brain: changing concepts, changing concerns. J Nutr 125:2212S–2220S
Lima DSC, Maia LMSS, Barboza EA, Duarte RA, Sousa LS, Guedes RCA (2009) l-Glutamine supplementation during the lactation period facilitates cortical spreading depression in well-nourished and early-malnourished rats. Life Sci 85:241–247
Lima CB, Soares GS, Vitor SM, Andrade-da-Costa BL, Castellano B, Guedes RCA (2013) Neonatal treatment with monosodium glutamate lastingly facilitates spreading depression in the rat cortex. Life Sci 17:388–392
Lima CB, Soares GS, Vitor SM, Andrade-da-Costa BL, Castellano B, Guedes RCA (2014) Spreading depression features and Iba1 immunoreactivity in the cerebral cortex of developing rats submitted to treadmill exercise after treatment with monosodium glutamate. Int J Dev Neurosci 33:98–105
Lopes-de-Morais AAC, Mendes-da-Silva RF, Santos EM, Guedes Guedes RCA (2014) Neonatal dexamethasone accelerates spreading depression in the rat, and antioxidant vitamins counteract this effect. Brain Res 1591:93–101
Marrannes R, Willems R, Prins E, Wauquier A (1988) Evidence for a role of the N-methyl-d-aspartate (NMDA) receptor in cortical spreading depression in the rat. Brain Res 457:226–240
Mazel T, Richter F, Vargová L, Syková E (2002) Changes in extracellular space volume and geometry induced by cortical spreading depression in immature and adult rats. Physiol Res 51(Suppl 1):85–93
McCormack WP, Hoffman JR, Pruna GJ, Jajtner AR, Townsend JR, Stout JR, Fragala MS, Fukuda DH (2015) Effects of l-alanyl-l-glutamine ingestion on one-hour run performance. J Am Coll Nutr 34(6):488–496
Mendes-da-Silva RF, Lopes-de-Morais AAC, Bandin-da-Silva ME, Cavalcanti GA, Rodrigues ARO, Andrade-da-Costa BLS, Guedes RCA (2014) Prooxidant versus antioxidant brain action of ascorbic acid in well-nourished and malnourished rats as a function of dose: a cortical spreading depression and malondialdehyde analysis. Neuropharmacology 86:155–160
Merkler D, Klinker F, Jürgens T, Glaser R, Paulus W, Brinkmann BG, Sereda MW, Stadelmann-Nessler C, Guedes RCA, Brück W, Liebetanz D (2009) Propagation of spreading depression inversely correlates with cortical myelin content. Ann Neurol 66:355–365
Moe-Byrne T, Brown JV, McGuire W (2016) Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst Rev. doi:10.1002/14651858.CD001457.pub5
Monteiro HMC, Barreto-Silva NL, Santos GE, Santos AS, Sousa MSB, Amâncio-dos-Santos A (2015) Physical exercise versus fluoxetine: antagonistic effects on cortical spreading depression in Wistar rats. Eur J Pharmac 762:49–54
Morgane PJ, Miller M, Kemper T, Stern W, Forbes W, Hall R, Bronzino J, Kissane J, Hawrylewicz E, Resnick O (1978) The effects of protein malnutrition on the developing nervous system in the rat. Neurosci Biobehav Rev 2:137–230
Morgane PJ, Mokler DJ, Galler JR (2002) Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev 26:471–483
Nakamura H, Strong AJ, Dohmen C, Sakowitz OW, Vollmar S, Sué M, Kracht L, Hashemi P, Bhatia R, Yoshimine T, Dreier JP, Dunn AK, Graf R Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain 133:1994–2006
Penido AB, Rezende GH, Abreu RV, Oliveira AC, Guidine PA, Pereira GS, Chianca DA Jr, Massensini AR, Moraes-Santos T, Moraes MF (2012) Malnutrition during central nervous system growth and development impairs permanently the subcortical auditory pathway. Nutr Neurosci 15:31–36
Pusic KM, Pusic AD, Kemme J, Kraig RP (2014) Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment. Glia 62(7):1176–1194
Radler ME, Wright BJ, Walker FR, Hale MW, Kent S (2014) Calorie restriction increases lipopolysaccharideinduced neuropeptide Y immunolabeling and reduces microglial cell area in the arcuate hypothalamic nucleus. Neuroscience 285:236–247
Rocha-de-Melo AP, Cavalcanti JB, Barros AS, Guedes RCA (2006) Manipulation of rat litter size during suckling influences cortical spreading depression after weaning and at adulthood. Nutr Neurosci 9:155–160
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Shatillo A, Salo RA, Giniatullin R, Gröhn OH (2015) Involvement of NMDA receptor subtypes in cortical spreading depression in rats assessed by fMRI. Neuropharmacology 93:164–170
Soares GS, Lima CB, Cavalcanti LC, Villacampa N, Castellano B, Guedes RC (2015) Brain effects of the lectin from Canavalia ensiformis in adult rats previously suckled in favorable and unfavorable conditions: a spreading depression and microglia immunolabeling study. Nutr Neurosci 18:307–315
Svoboda N, Kerschbaum HH (2009) l-Glutamine-induced apoptosis in microglia is mediated by mitochondrial dysfunction. Eur J Neurosci 30:196–206
Szpetnar M, Luchowska-Kocot D, Boguszewska-Czubara A, Kurzepa J (2016) The Influence of Manganese and Glutamine Intake on Antioxidants and Neurotransmitter Amino Acids Levels in Rats' Brain. Neurochem Res 41:2129–2139
Takizawa T, Shibata M, Kayama Y, Shimizu T, Toriumi H, Ebine T, Unekawa M, Koh A, Yoshimura A, Suzuki N (2016). High-mobility group box 1 is an important mediator of microglial activation induced by cortical spreading depression. J Cereb Blood Flow Metab (Epub ahead of print)
Tapia-González S, García-Segura LM, Tena-Sempere M, Frago LM, Castellano JM, Fuente-Martín E, García-Cáceres C, Argente J, Chowen JA (2011) Activation of microglia in specific hypothalamic nuclei and the cerebellum of adult rats exposed to neonatal overnutrition. J Neuroendocrinol 23(4):365–370
Thomas AG, O’Driscoll CM, Bressler J, Kaufmann WE, Rojas CJ, Slusher BS (2014) Small molecule glutaminase inhibitors block glutamate release from stimulated microglia. Biochem Biophys Res Commun 443:32–36. doi:10.1016/j.bbrc.2013.11.043
Toriumi H, Shimizu T, Ebine T, Takizawa T, Kayama Y, Koh A, Shibata M, Suzuki N (2016) Repetitive trigeminal nociceptive stimulation in rats increases their susceptibility to cortical spreading depression. Neurosci Res 106:74–78
Torrente D, Cabezas R, Avila MF, García-Segura LM, Barreto GE, Guedes RCA (2014) Cortical spreading depression in traumatic brain injuries: is there a role for astrocytes? Neurosci Lett 565:2–6
Tu Y-F, Lu P-J, Huang C-C, Ho C-J, Chou Y-P (2012) Moderate dietary restriction reduces p53-mediated neurovascular damage and microglia activation after hypoxic ischemia in neonatal brain. Stroke 43:491–498
Viana LC, Lima CM, Oliveira MA, Borges RP, Cardoso TT, Almeida IN, Diniz DG, Bento-Torres J, Pereira A, Batista-de-Oliveira M, Lopes AA, Silva RF, Abadie-Guedes R, Amâncio Dos Santos A, Lima DS, Vasconcelos PF, Cunningham C, Guedes RC, Picanço-Diniz CW (2013) Litter size, age-related memory impairments, and microglial changes in rat dentate gyrus: stereological analysis and three dimensional morphometry. Neuroscience 238:280–296
Wang L, Maher TJ, Wurtman RJ (2007) Oral l-glutamine increases GABA levels in striatal tissue and extracellular fluid. Faseb J 21:1227–1232
Wendt S, Wogram E, Korvers L, Kettenmann H (2016) Experimental cortical spreading depression induces NMDA receptor dependent potassium currents in microglia. J Neurosci 36(23):6165–6174
Wohleb ES, McKim DB, Sheridan JF, Godbout JP (2015) Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci 8:447. doi:10.3389/fnins.2014.00447.eCollection
Acknowledgements
The authors thank the following Brazilian agencies for financial support: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq No. 445101/2014-8), Instituto Brasileiro de Neurociências (IBN-Net/Finep No. 4191), and Capes (Edital 043/2013 Ciências Do Mar II). RCA Guedes is a Research Fellow from CNPq (No. 303636/2014-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: N. Singewald.
Rights and permissions
About this article
Cite this article
de Lima, D.S.C., Francisco, E.d., Lima, C.B. et al. Neonatal l-glutamine modulates anxiety-like behavior, cortical spreading depression, and microglial immunoreactivity: analysis in developing rats suckled on normal size- and large size litters. Amino Acids 49, 337–346 (2017). https://doi.org/10.1007/s00726-016-2365-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2365-2