Abstract
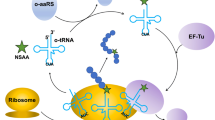
The genetic incorporation of unnatural amino acids (UAAs) into proteins has been a useful tool for protein engineering. However, most UAAs are expensive, and the method requires a high concentration of UAAs, which has been a drawback of the technology, especially for large-scale applications. To address this problem, a method to recycle cultured UAAs was developed. The method is based on recycling a culture medium containing the UAA, in which some of essential nutrients were resupplemented after each culture cycle, and induction of protein expression was controlled with glucose. Under optimal conditions, five UAAs were recycled for up to seven rounds of expression without a decrease in expression level, cell density, or incorporation fidelity. This method can generally be applied to other UAAs; therefore, it is useful for reducing the cost of UAAs for genetic incorporation and helpful for expanding the use of the technology to industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genetic incorporation of unnatural amino acids (UAAs) has been a powerful tool that allows for the introduction of biochemically interesting functional groups into proteins (Chin 2014; Liu and Schultz 2010; Wang and Schultz 2005). To date, over 100 UAAs have been incorporated into various proteins, and the UAAs have been used to conjugate biochemical probes to a defined site on the proteins (Chin et al. 2002; Lang et al. 2012a; Lee et al. 2013; Plass et al. 2011; Seitchik et al. 2012), control post-translational modifications in cells (Lemke et al. 2007), probe structural changes in the proteins (Lee et al. 2009b; Schmidt et al. 2014), confer a new function on the proteins (Drienovska et al. 2015; Lee and Schultz 2008; Lee et al. 2009a), and image protein localization in live cells (Chatterjee et al. 2013). This method has several advantages over other techniques for protein modification, including technical simplicity, high protein yield, and no limitation in target protein selection (Chin 2014; Liu and Schultz 2010; Wang and Schultz 2005). These advantages are based on the use of endogenous materials for the biosynthesis of mutant proteins containing a UAA, except for an evolved aminoacyl-tRNA (aatRNA)/aa-tRNA synthetase (aaRS) pair and a UAA.
While an aatRNA/aaRS pair can be provided by the expression of the corresponding genes in the host cell, the UAA must be provided in the growth medium. A high concentration of UAAs (typically 1 mM or higher) is required for sufficient incorporation efficiency for most UAAs. Although many UAAs incorporated into proteins with the genetic method are commercially available, they are relatively expensive because they have at least one stereogenic center and multiple reactive functionalities. In addition, many biochemically interesting UAAs are not commercially available and have to be chemically synthesized, which makes them even more expensive. To address this issue, the genetic incorporation of UAAs biosynthesized within the host cell was reported, in which four phenylalanine derivatives were biosynthesized by an aminotransferase from their corresponding α-keto acids and incorporated directly into a protein in the host cell (Jung et al. 2014). Although the work presented a direction to resolve the issues, it is applicable to only a limited number of UAAs. In this study, we report a method by which UAAs can be recycled for multiple rounds of expression of a target protein. Using this method, five UAAs were recycled and genetically incorporated multiple times without a decrease in incorporation efficiency or protein yield.
Materials and methods
All chemicals and DNA oligomers were obtained from commercial sources and used without further purification. Electron spray ionization mass spectroscopy (ESI–MS) was performed using the Ultra High Resolution ESI quadrupole time-of-flight (Q-TOF) MS/MS Compact System.
Expression of mutant proteins in a pBAD expression system
The wild-type maltose binding protein (MBP) gene was amplified from a commercial vector containing the gene and then inserted into the pBAD/Myc-His vector (Invitrogen) to generate pBAD-MBP. Site-directed mutagenesis was used to introduce the Y167TAG mutation into the MBP gene. To express the MBP mutant containing a UAA at the 167 position, Escherichia coli DH10β cells were transformed with pBAD-MBP-Y167TAG and a pEvol plasmid (Young et al. 2010) containing the corresponding aa-tRNA/aaRS pair genes. Cells were amplified in lysogeny broth (LB) supplemented with ampicillin (100 μg/mL) and chloramphenicol (35 μg/mL). A starter culture (0.2 mL) was used to inoculate 100 mL R-medium (50 mM Na2HPO4, 50 mM KH2PO4, 25 mM (NH4)2SO4, 2 mM MgSO4, 0.1 % trace metals, 0.5 % glycerol, 0.05 % glucose, 0.2 % arabinose, and 5 % amino acids) (Table 1) supplemented with ampicillin (100 μg/mL), chloramphenicol (35 μg/mL), and 1 mM UAA. After 24-h incubation at 37 °C, cell density was measured using a spectrophotometer (600 nm). Cells were then harvested by centrifugation and the supernatant was collected. A new culture medium was prepared by adding an optimized amount of each component (10 mM Na2HPO4, 10 mM KH2PO4, 5 mM (NH4)2SO4, 0.4 mM MgSO4, 0.01 % trace metals, 0.25 % glycerol, 0.05 % glucose, 0.02 % arabinose, and 5 % amino acids) and filtering the mixture for sterilization. The starter culture was added to the new medium, and the culture was incubated for 24 h at 37 °C. The same procedure was then repeated for the next culture.
Expression of mutant proteins in a pET expression system
pET20b-MBP-Y167TAG was constructed as described for the pBAD expression system. E. coli BL21 (DE3) cells were transformed with pET20b-MBP-Y167TAG and the pEvol plasmid used for the pBAD expression system. The same expression conditions were used as described in the pBAD expression system, except that 0.2 % lactose was used instead of 0.2 % arabinose.
Analysis of whole cell lysates
After each culture, a small portion (500 μL) of the culture was taken and centrifuged, and the cell pellets were frozen. The frozen cells were thawed on ice and resuspended with Bugbuster (50 μL; Novagen) containing benzonase (Sigma-Aldrich), and the mixture was incubated for 1 h at room temperature. Cell debris was removed by centrifugation and the supernatant was analyzed by SDS-PAGE.
Amylose resin purification
Amylose resin purification was performed according to the manufacturer’s protocol (New England Biolabs, Inc.). Cells (from a 100 mL culture) were harvested by centrifugation, resuspended in 10 mL column buffer (pH 7.4) containing 20 mM Tris–HCl, 200 mM NaCl, and 1 mM EDTA, and sonicated. Cell debris was removed by centrifugation, and the supernatant was incubated with 500 μL amylose resin (New England Biolabs) in a polypropylene column (Qiagen) for 1 h at 4 °C. The resin was washed three times with 5 mL column buffer, and the target protein was eluted with 5 mL elution buffer (pH 7.4) containing 20 mM Tris–HCl, 200 mM NaCl, 1 mM EDTA, and 10 mM maltose.
Results and discussion
A straightforward approach recycling a cultured UAA is to isolate the UAA from the culture medium. Purification by high-performance liquid chromatography (HPLC) would be a suitable method for isolation of the UAA used. However, considering the complex composition of the culture medium, the process would be labor-intensive, and the purity and yield of the recovered UAA would be unsatisfactory. Alternatively, the UAA in the culture medium could be reused without isolation. To do this, two issues would need to be addressed: (1) inducers such as isopropyl β-D-1-thiogalactopyranoside (IPTG) and L-arabinose which are typically used to promote high levels of protein expression in bacterial cells need to be removed for recycled expression, because cells would otherwise not grow to the desired cell density; (2) essential nutrients should be resupplied at appropriate concentrations.
It was expected that the first issue could not be solved by removing the inducer molecules from the culture medium. Instead, induction could be prevented until the cell density reaches a sufficient level. Early induction of protein expression can be prevented using glucose as a carbon source (Grossman et al. 1998). In the presence of glucose, the lac and araBAD promoters are blocked by catabolite repression, even if lactose or arabinose is present in the medium. Indeed, this concept had been applied to develop an auto-inducing medium for high-throughput screening of a large number of protein expressions, in which several defined media designed for various applications were tested (Studier 2005). Therefore, the first issue would be solved using glucose in a defined medium, which would delay the induction timing.
The previous report (Studier 2005) on the development of auto-inducing media mentioned above provided information that addresses the second issue. One of the media used in the report was chosen for this study with minor modifications, and the composition is given in Table 1. The medium (R-medium) contains glycerol as a major carbon source, and sodium hydrogen phosphate and potassium dihydrogen phosphate as sources of sodium, potassium, and phosphate ions. The phosphate buffer system is used to prevent the culture medium from becoming acidic, as glucose metabolism generates acids, which makes the culture medium acidic as bacterial cells grow. Ammonium sulfate is used to supply nitrogen and sulfur, and magnesium sulfate to supply magnesium ion. The metal mixture and 18 amino acids are used to facilitate cell growth to a high density. Glucose is used to delay induction of protein expression, as mentioned above. To reuse a culture medium, each component needs to be resupplied after each cycle, and the amount of the components would be determined experimentally to maximize protein expression.
Initially, R-medium was tested for the genetic incorporation of a UAA into a protein. p-Azido-l-phenylalanine (AF) (Fig. 1) was used for this experiment because this amino acid is one of the most extensively used UAAs (Chin et al. 2002). The amino acid was incorporated into MBP at position 167 (Tyr167), by substituting an amber codon (TAG) for Tyr167 in the corresponding gene, and expression was achieved with a pBAD expression system. Because the medium contains a higher concentration of phenylalanine than typical culture media, such as LB, a significant amount of phenylalanine incorporation was observed in the absence of AF (data not shown). To solve this problem, R-medium containing 17 amino acids (Table 1) was used for the genetic incorporation of AF. Whole cell lysates were analyzed by SDS-PAGE, and the target protein was expressed only in the presence of AF (Figure S1). In addition, the expression level in the defined medium was comparable to that in LB.
Next, experiments to recycle a cultured UAA were carried out by reusing the cultured medium containing the UAA. After the first culture, cells were harvested by centrifugation, and the supernatant was collected. A new culture medium was prepared by adding the same amount of each component (Supplement 1 in Table 1) as that used for the initial medium preparation except the UAA, and the medium was filtered for sterilization. A small decrease in the culture volume was observed after the first culture, due to evaporation during incubation, which could be compensated for by the volume of the added components. A starter culture in LB medium (2 % of the total culture volume) was added to the new medium, and the culture was grown for 24 h at 37 °C. The same procedure was repeated several times, and the expression levels of the target protein were analyzed (Fig. 2). A significant reduction in the expression level and cell density was observed from the fifth round of expression. The high ionic strength generated by the ionic components added at each recycling process likely caused the reduction. Although the pH reduction was expected because of the consumption of glucose by bacterial cells, the reduction was not observed because the addition of sodium hydrogen phosphate and potassium dihydrogen phosphate buffered the pH change.
Expression of MBP-Y167AF with the genetic incorporation of recycled AF in R-medium supplemented as per supplement condition 1 (Table 1) for recycling. Whole cell lysates were analyzed. aCell density relative to the first reuse cycle
A complete supplement of all medium components at each recycle process caused significant toxicity for cell growth, but the supplement was not toxic up to the fourth round of expression, which meant partial supplementation or selective supplementation of some components would solve this issue. To examine the effect of partial supplementation, 10–20 % of the initial amount of each component was added after each culture, and glucose, which was consumed completely every culture, was supplemented by 100 % (Table 1, Supplement 2). A slight reduction in pH was observed, and the pH change was adjusted by adding sodium hydroxide to the medium. Cell density and target protein expression were analyzed after several cycles, as done previously, and both decreased significantly after the fourth round of expression (Figure S2). It was expected that the reduction in cell density and protein expression was caused by the insufficient supplementation of amino acids and/or L-arabinose. Subsequent studies were carried out by varying the supplemented amount of the two components, to determine the cause of the problem. The increase in the amount of L-arabinose (Table 1, Supplement 3) did not improve the cell density or protein expression (data now shown). However, the increase in the percentage of amino acids with constant supplementation of L-arabinose (Table 1, Supplement 4) maintained the initial levels of cell density and protein expression (Fig. 3). For seven expression cycles, no decrease in cell density or target protein expression was observed. For the seventh expression cycle, the target protein was purified and analyzed by ESI–MS (Fig. 4), and the data showed the incorporation of AF without incorporation of any natural amino acids. These results show that AF can be reused for genetic incorporation into proteins by recycling the culture medium containing the amino acid without a decrease in efficiency of incorporation or protein yield. The same experiment was performed with a pET expression system to test if the method is applicable to a different expression system. In this case, lactose was used as an inducer because IPTG induction was not effectively suppressed by glucose, resulting in a significant reduction in cell density (data not shown). Protein expression with the recycled UAAs was similar to the expression with the pBAD expression system, showing that the method is applicable to both expression systems (Fig. 5).
Expression of MBP-Y167AF by the genetic incorporation of recycled AF in R-medium supplemented as per supplement condition 4 (Table 1) for recycling. Whole cell lysates were analyzed. aCell density relative to the first reuse cycle
SDS-PAGE and ESI–MS analyses of purified MBP-Y167AF from the seventh culture. a SDS-PAGE analysis of purified WT MBP and MBP-Y167AF; b ESI–MS results for WT MBP and c MBP-Y167AF; Insets the deconvoluted spectra: expected mass difference between WT MBP and MBP-Y167AF, 25 Da; observed mass difference, 26 Da
Expression of MBP-Y167AF by the genetic incorporation of recycled AF with the pET expression system. All experimental conditions are the same as those used for Fig. 3. Whole cell lysates were analyzed. aCell density relative to the first reuse cycle
To determine whether the UAA recycling method could be applied to other UAAs, four additional UAAs were tested (Fig. 1). 2,2′-Bipyridin-5-yl-l-alanine (BPA) (Xie et al. 2007) and (2S)-2-amino-4-(7-hydroxycoumarin-4-yl)butanoic acid (CMA) (Wang et al. 2006) were genetically incorporated using Methanococcus jannaschii tyrosyl-tRNA/aaRS, and N 6-benzyloxycarbonyl-l-lysine (CBZK) (Yanagisawa et al. 2008) and N 6-[(1R,8S,9R)-bicyclo[6.1.0]non-4-yn-9-ylmethoxy]carbonyl-l-lysine (BCNK) (Lang et al. 2012b) were genetically incorporated using Methanosarcina mazei and Methanosarcina bakeri pyrrolysyl-tRNAs/aaRSs. The same protocol was used to express MBP by amber stop codon suppression at position 167. For three expression cycles, no decrease in protein expression or cell density was observed (Fig. 6). The differences in the expression level for each UAA were due to the intrinsic differences in the incorporation efficiency of each aaRS, and not due to any problem in the recycling method. Although the four UAAs were not extensively tested, as in the case of AF, it was expected that there would be no significant differences in their expression with increase in the cycles. These results show that the recycled UAAs can be efficiently incorporated into proteins without a decrease in the expression level or cell density.
Expression of MBP-Y167AF by the genetic incorporation of BPA, CBZK, CMA, or BCNK which were recycled by reusing R-medium with optimal supplementation (supplement 4 in Table 1). For each expression cycle, the corresponding aatRNA/aaRS pairs for each UAA were used. Whole cell lysates were analyzed. aCell density relative to the first reuse cycle
Conclusions
One of the important limitations in the genetic incorporation of UAAs is the high cost of the UAAs. To alleviate this limitation, a method to recycle cultured UAAs was developed, in which induction of protein expression was delayed using glucose. Some of essential nutrients were supplemented after each culture cycle, and under optimal conditions, five UAAs were used for up to seven expression cycles without a decrease in protein expression, cell density, or incorporation fidelity. Because this method can generally be applied to other UAAs, it is useful for reducing the cost of UAAs, especially for processes requiring a large culture volume, and helps expand the use of the technology to industrial applications.
References
Chatterjee A, Guo J, Lee HS, Schultz PG (2013) A genetically encoded fluorescent probe in mammalian cells. J Am Chem Soc 135:12540–12543
Chin JW (2014) Expanding and reprogramming the genetic code of cells and animals. Annu Rev Biochem 83:379–408
Chin JW, Santoro SW, Martin AB, King DS, Wang L, Schultz PG (2002) Addition of p-azido-l-phenylalanine to the genetic code of Escherichia coli. J Am Chem Soc 124:9026–9027
Drienovska I, Rioz-Martinez A, Draksharapu A, Roelfes G (2015) Novel artificial metalloenzymes by in vivo incorporation of metal-binding unnatural amino acids. Chem Sci 6:770–776
Grossman TH, Kawasaki ES, Punreddy SR, Osburne MS (1998) Spontaneous cAMP-dependent derepression of gene expression in stationary phase plays a role in recombinant expression instability. Gene 209:95–103
Jung JE, Lee SY, Park H, Cha H, Ko W, Sachin K, Kim DW, Chi DY, Lee HS (2014) Genetic incorporation of unnatural amino acids biosynthesized from α-keto acids by an aminotransferase. Chem Sci 5:1881–1885
Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A, Chin JW (2012a) Genetically encoded norbornene directs site-specific cellular protein labelling via a rapid bioorthogonal reaction. Nat Chem 4:298–304
Lang K, Davis L, Wallace S, Mahesh M, Cox DJ, Blackman ML, Fox JM, Chin JW (2012b) Genetic encoding of bicyclononynes and trans-cyclooctenes for site-specific protein labeling in vitro and in live mammalian cells via rapid fluorogenic diels–alder reactions. J Am Chem Soc 134:10317–10320
Lee HS, Schultz PG (2008) Biosynthesis of a site-specific DNA cleaving protein. J Am Chem Soc 130:13194–13195
Lee HS, Spraggon G, Schultz PG, Wang F (2009a) Genetic incorporation of a metal-ion chelating amino acid into proteins as a biophysical probe. J Am Chem Soc 131:2481–2483
Lee HS, Guo J, Lemke EA, Dimla RD, Schultz PG (2009b) Genetic incorporation of a small, environmentally sensitive, fluorescent probe into proteins in Saccharomyces cerevisiae. J Am Chem Soc 131:12921–12923
Lee YJ, Wu B, Raymond JE, Zeng Y, Fang X, Wooley KL, Liu WR (2013) A genetically encoded acrylamide functionality. ACS Chem Biol 8:1664–1670
Lemke EA, Summerer D, Geierstanger BH, Brittain SM, Schultz PG (2007) Control of protein phosphorylation with a genetically encoded photocaged amino acid. Nat Chem Biol 3:769–772
Liu CC, Schultz PG (2010) Adding new chemistries to the genetic code. Annu Rev Biochem 79:413–444
Plass T, Milles S, Koehler C, Schultz C, Lemke EA (2011) Genetically encoded copper-free click chemistry. Angew Chem Int Ed 50:3878–3881
Schmidt MJ, Borbas J, Drescher M, Summerer D (2014) A genetically encoded spin label for electron paramagnetic resonance distance measurements. J Am Chem Soc 136:1238–1241
Seitchik JL, Peeler JC, Taylor MT, Blackman ML, Rhoads TW, Cooley RB, Refakis C, Fox JM, Mehl RA (2012) Genetically encoded tetrazine amino acid directs rapid site-specific in vivo bioorthogonal ligation with trans-cyclooctenes. J Am Chem Soc 134:2898–2901
Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234
Wang L, Schultz PG (2005) Expanding the genetic code. Angew Chem Int Ed 44:34–66
Wang J, Xie J, Schultz PG (2006) A genetically encoded fluorescent amino acid. J Am Chem Soc 128:8738–8739
Xie J, Liu W, Schultz PG (2007) A genetically encoded bidentate, metal-binding amino acid. Angew Chem Int Ed 46:9239–9242
Yanagisawa T, Ishii R, Fukunaga R, Kobayashi T, Sakamoto K, Yokoyama S (2008) Multistep engineering of pyrrolysyl-tRNA synthetase to genetically encode N(epsilon)-(o-azidobenzyloxycarbonyl) lysine for site-specific protein modification. Chem Biol 24:1187–1197
Young TS, Ahmad I, Yin JA, Schultz PG (2010) An enhanced system for unnatural amino acid mutagenesis in E. coli. J Mol Biol 395:361–374
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014003870).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: S. Murch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ko, W., Kim, S., Jo, K. et al. Genetic incorporation of recycled unnatural amino acids. Amino Acids 48, 357–363 (2016). https://doi.org/10.1007/s00726-015-2087-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2087-x