Abstract
Mitochondria, once merely considered as the “powerhouse” of cells, as they generate more than 90 % of cellular ATP, are now known to play a central role in many metabolic processes, including oxidative stress and apoptosis. More than 40 known human diseases are the result of excessive production of reactive oxygen species (ROS), bioenergetic collapse and dysregulated apoptosis. Mitochondria are the main source of ROS in cells, due to the activity of the respiratory chain. In normal physiological conditions, ROS generation is limited by the anti-oxidant enzymatic systems in mitochondria. However, disregulation of the activity of these enzymes or interaction of respiratory complexes with mitochondriotropic agents may lead to a rise in ROS concentrations, resulting in oxidative stress, mitochondrial permeability transition (MPT) induction and triggering of the apoptotic pathway. ROS concentration is also increased by the activity of amine oxidases located inside and outside mitochondria, with oxidation of biogenic amines and polyamines. However, it should also be recalled that, depending on its concentration, the polyamine spermine can also protect against stress caused by ROS scavenging. In higher organisms, cell signaling pathways are the main regulators in energy production, since they act at the level of mitochondrial oxidative phosphorylation and participate in the induction of the MPT. Thus, respiratory complexes, ATP synthase and transition pore components are the targets of tyrosine kinases and phosphatases. Increased ROS may also regulate the tyrosine phosphorylation of target proteins by activating Src kinases or phosphatases, preventing or inducing a number of pathological states.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mitochondria and energy demand by cells
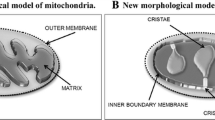
Mitochondria are cytoplasmic organelles forming an integral part of all eukaryotics. They are considered the “powerhouse” of cells, as they convert organic materials into energy. Their two-membrane constitution allows them to build a particularly complex structure, which includes: (1) an outer membrane, surrounding the inner compartments and exhibiting non-specific permeability to metabolites involved in mitochondrial bioenergetics; in particular, the outer membrane contains the enzyme monoamine oxidase (MAO); (2) a small compartment, the intermembrane space, which separates the outer membrane from the inner one; it contains the adenine nucleotide-balancing enzyme, adenylate kinase, and several pro-apoptotic factors; (iii) the inner membrane, containing the protein complexes responsible for energy transduction, with many deep invaginations called cristae (Ernster and Kuylenstierna 1969). The inner membrane surrounds the inner matrix space, rich in soluble proteins and containing, as generally reported, mitochondrial DNA encoding 13 proteins, all of which are located in the inner membrane and form part of the oxidative phosphorylation machinery (Wallace 1997). There is also a recently discovered fourteenth protein, the peptide humanin, which is involved in protection against stress (Guo et al. 2003; Yen et al. 2013). The matrix space also contains enzymes of the Krebs cycle, among many others. Both these membranes come together at particular contact sites and fuse, forming gap junctions. In liver, each mitochondrion contains 100–200 gap junctions, with a diameter of about 20 nm (Hackenbrock 1968).
The inner and outer membranes differ greatly in permeability. The inner membrane is impermeable to all solutes, even the smallest ion, the proton, with the major exception of oxygen, which is soluble in lipid bilayers. Mono- or bi-directional transport of metabolites occurs only via specific transporters and exchangers. Thus, a large number of proteins are involved, including adenine nucleotide translocase (AdNT), which exchanges ATP for ADP (Klingenberg 1979; Klingenberg et al. 1995), the phosphate transporter (Fonyo et al. 1975; Wohlrab 2005), di- and tri-carboxylate transporters and the carnitine shuttle (Palmieri and Klingenberg 1979; LaNoue and Schoolwerth 1979), the polyamine transporter (Toninello et al. 1988, 1992), and the Ca2+ uniporter (Carafoli and Crompton 1978; Nicholls and Crompton 1980), etc. All these transporters catalyze the uptake and release of substrates and reaction products involved in oxidative phosphorylation. This process uses electrons predominantly delivered by NADH and also FADH2, derived from macromolecule metabolism or the energy stores of the organism. These electrons flow along the electron transport chain to the final electron acceptor, oxygen, which is reduced to H2O. The electron transport chain is composed of a sequence of respiratory complexes including NADH-ubiquinone reductase (complex I), succinate dehydrogenase (complex II), ubiquinol cytochrome c reductase, also called bc1 complex (complex III), and cytochrome c oxidase (complex IV). Among these complexes, ubiquinone accepts electrons from complexes I and II and transfers them to complex III, cytochrome c accepts electrons from complex III and transfers them to complex IV (Slater 1953). Electron transport is coupled to the proton pumping activity of complexes I, III and IV, extruding protons and generating the electrochemical gradient, Δμ +H , across the inner membrane, also called the proton motive force. Δμ +H is used by ATP-synthase (complex V) to synthesize ATP from ADP and phosphate, coupled to a reversal flow of protons from the intermembrane space to the matrix. Mitochondrial-synthesized ATP represents the main energy supply for all cell processes, providing more than 15 times the ATP produced by anaerobic glycolysis (Alberts et al. 2002). The Nobel Prize winner Peter Mitchell, with his chemiosmotic hypothesis, was the first to propose that an electrochemical gradient, generated by the proton pumps coupled to the electron flux through the electron transport chain, is the driving force synthesizing ATP by ATP-synthase (Mitchell 1961). This statement represents the “central dogma” of Mitchell’s chemiosmotic theory, universally accepted by the biochemical scientific community and one of the main scientific accomplishments of the 20th century.
Energy homeostasis is one of the main determinants for life in all higher organisms. It has been calculated that a person on average uses 100 kcal/h at rest, corresponding to the production of about 65 kg of ATP/day (Rich 2003). During normal daily exercise, these values may increase many times. Unlike many unicellular organisms, which have evolved devices allowing them to live and grow in anaerobiosis, animals depend entirely on cell respiration. This is because of the presence in the human body of approximately, 1014 cells which, although exhibiting very high density, are highly specialized and energy-expensive. These characteristics require efficient supplies of fuel and oxygen and the removal of carbon dioxide through blood circulation. Thus, the supply of constant energy through glycolysis alone is not feasible in healthy animals: cell respiration must be carried out by mitochondria. The energy demand of cells ranges widely, depending on their function and activity. This means that all higher organisms must make adjustments in energy production to physiological demand. Britton Chance was the first to propose a mechanism for controlling oxidative phosphorylation, called respiratory control (Chance and Williams 1955), which could be measured by calculating the ratio between the rate of oxygen consumption in respiratory state 3 and that in state 4 (the respiratory control index). State 3 is the metabolic state during ATP synthesis, that is, in the presence of ADP and phosphate. State 4 is the resting condition without ATP synthesis, that is, in the absence of added ADP. Another parameter to assess respiratory control is the P/O ratio, or the ratio between the amount of ATP synthesized and oxygen consumed. These parameters, evaluated in isolated mitochondria, show that oxidative phosphorylation is limited by the availability of ADP and phosphate (substrates for ATP synthesis). In other words, oxidative phosphorylation increases when ATP, used for cell requirements, regenerates ADP and phosphate. It should be noted that ATP production by mitochondria is also regulated by several enzymes of the Krebs cycle which, in turn, are controlled by matrix Ca2+ concentration. In this regard, many cell processes are involved in the regulation of Ca2+ fluxes across the plasma membrane, endoplasmic reticulum and mitochondrial membrane, to maintain intracellular Ca2+ homeostasis. This concerted activity among all the Ca2+ transport systems is essential for energy transduction and cell life. Several qualified reviews have described Ca2+ transport and its pathophysiological role in mammalian mitochondria (Carafoli 1982, 2003; Gunter et al. 2000; Rizzuto et al. 2000; Gunter and Gunter 2001; Pfeiffer et al. 2001; Salvi and Toninello 2004; Harrington and Murphy 2015).
Mitochondrial Ca2+ homeostasis is maintained by the activity of the electrophoretic uniporter sensitive to membrane potential, driving its uptake (Rottenberg and Scarpa 1974) and by the Na+-dependent and Na+-independent electroneutral exchangers responsive for its efflux (Crompton et al. 1976; Puskin et al. 1976). Another mechanism capable of sequestering significant amounts of Ca2+ from cytosolic Ca2+, in rapid pulses, called RaM, has also been identified (Sparagna et al. 1995).
Intracellular Ca2+ homeostasis is also controlled by interaction between the endoplasmic reticulum (ER), which fine-tunes cytoplasmic Ca2+ concentrations, and the mitochondrion, the major Ca2+ buffering organelle in cells. The physical ER-mitochondrion interaction is called the mitochondria-associated ER membrane (MAM), the interface between the organelles. MAM plays a pivotal role in several cell functions, including Ca2+ signaling, lipid transport, energy metabolism and cell survival, and is in fact vital for regulating Ca2+ levels in the mitochondrial matrix. The supply of Ca2+ to mitochondria, as mentioned above, is crucial for matching ATP production by the Krebs cycle with ATP demand (Hayashi et al. 2009).
A receptor called sigma-1 receptor (Sig-1R) has been identified in the MAM and regulates the stability of inositol 1,4,5-triphosphate (IP3) receptors to ensure proper Ca2+ signaling between the ER and the mitochondrion (Hayashi and Su 2007). Sig-1R also functions against oxidative stress in cells, as evidenced by metabolic and proteomic studies (Pal et al. 2012). Ca2+ concentration in the mitochondrial matrix is also sensitive to the presence of natural polyamines, in particular spermine. In the presence of polyamines, mitochondria can enhance Ca2+ accumulation, due to activation of the saturable systems of Ca2+ uptake. In addition, polyamines can buffer extra mitochondrial Ca2+ concentrations to levels similar to those in the cytosol of resting cells (Salvi and Toninello 2004). The mechanism involved is due to a rise in the affinity of the Ca2+ transport system, induced by polyamines, most probably exhibiting allosteric behavior. The regulatory site of this mechanism is the S1 binding site of polyamines (Dalla Via et al. 1996, 1999) operating in physiological conditions and located in the energy well between the two peaks present in the energy profile identified for spermine transport (Toninello et al. 2000).
As well as the above-mentioned control mechanisms of mitochondrial bioenergetics, several other levels of control exist, involving the tissue-specific expression of isozymes, allosteric control and cell signaling (Hüttemann et al. 2007). Unlike the first two mechanisms, which are involved in intracellular responses, cell signaling is mainly implicated in higher-order communications between cells and organs. Within the cell there are sequences of enzymes such as kinases and phosphatases, involved in the above process, which add or remove phosphate groups to or from their targets. As pointed out by the above authors, the traditional opinion regarding the presence of linear cascades in signal transduction must be considered an over-simplification, as much cross-talk goes on among the various signaling pathways. This network of signals may hinder the interpretation of experimental results, explaining some reported contrasting results. Mitochondria have come back again as several phosphorylated proteins, kinases and phosphatases have been identified in their inner compartments, suggesting that these organelles play an important role in signaling pathways (Salvi et al. 2002a, b, 2004, 2005a, b, 2007a, b; Horbinski and Chu 2005; Pagliarini and Dixon 2006; Vogt et al. 2007; Tibaldi et al. 2008; Lewandrowski et al. 2008) (see “Tyrosine phosphorylation, ROS, polyamines and mitochondria”).
Mitochondria and ROS (oxidant generation by mitochondria)
Mitochondria are the most important source of reactive oxygen species (ROS) in most types of mammalian cells (Andreyev et al. 2005; Turrens 2003; Balaban et al. 2005; Chance et al. 1979; Cadenas and Davies 2000; Raha and Robinson 2000; Adam-Vizi and Chinopoulos 2006; Muller 2000). ROS generation contributes to severe mitochondrial damage in a range of pathologies, but it is also involved in redox signaling from organelles to other cell compartments (Balaban et al. 2005; Chance et al. 1979; Cadenas and Davies 2000; Raha and Robinson 2000; Adam-Vizi and Chinopoulos 2006; Muller 2000; Dröge 2002).
More than 95 % of inhaled oxygen undergoes tetravalent reduction, to produce H2O by the last reaction of the respiratory chain catalyzed by cytochrome c oxidase (complex IV of the chain):
This terminal reaction allows continued electron transport along the chain, together with the proton pumping which generates Δμ +H and consequently ATP synthesis. Blocking the electron flux through the respiratory complexes results in dissipation of the proton motive force and a consequent halt in ATP production. This fact shows that one of the peculiar roles of oxygen in all aerobic organisms is to act as a dump for electrons, to maintain the coupling between mitochondrial respiration and ATP synthesis. During two-electron transport along the respiratory chain, there are also alternating one-electron redox reactions, which predispose each transporter to become involved in side-reactions with molecular oxygen.
Mammalian complex I is the entry point for electrons coming from NADH and entering the respiratory chain. The FMN cofactor acts as electron acceptor and transfers the electrons along a chain of iron–sulfur (FeS) centers to the ubiquinone pool. It should be recalled that mitochondria contain a putative ubiquinone at the level of complex I and a large pool of ubiquinone and ubiquinol in the bulk of the membrane but interacting with the components of the bc1-complex (complex III). In both cases, ubiquinone cycles between the quinone (completely oxidized) to the semiquinone (one-electron reduction product) and to quinol (fully reduced by two electrons), and vice versa. The semiquinone radical may transfer its unpaired electron to oxygen directly, thus generating superoxide anion O ·−2 instead of the next electron transporter in the chain. Another mechanism by means of which complex I produces large amounts of O ·−2 occurs during reverse electron transport, when electron supply reduces the ubiquinone pool: in the presence of high electrochemical gradients, this forces electrons back from ubiquinol into complex I and reduces NAD+ to NADH at the FMN site (Adam-Vizi and Chinopoulos 2006; Kudin et al. 2004; Votyakova and Reynolds 2001; Liu et al. 2002).
Similar side-reactions with molecular oxygen may take place in other segments of the respiratory chain with the generation of the toxic O ·−2 . In particular, several iron–sulfur clusters within the respiratory complexes are also involved. On the basis of these considerations, it is commonly held, as above stated, that mitochondria are the main intracellular source of oxygen radicals in physiological conditions. Thus, it is estimated that 1–4 % of the total oxygen in mitochondria is constitutively converted to superoxide anion. In humans, mean values in the range of 190–355 mmol of O ·−2 are produced per day from mitochondrial respiration. There is ongoing debate regarding the exact sites of the production of O ·−2 . In a review, Murphy (2009) reported the principles which govern O ·−2 production in the matrix of mammalian mitochondria. His observations showed that the O ·−2 flux is related to the concentration of potential electron donors, the local concentration of O2, and the rate constant for reactions among them. Mitochondria have two modes of operation in producing significant O ·−2 concentrations. The first takes place at the level of Complex I, when mitochondria are not synthesizing ATP and consequently have a high Δμ +H , very low oxygen consumption, and a greatly reduced CoQ pool. The second mode occurs when there is a high NADH/NAD+ ratio in the matrix (see also the description above). Instead, when mitochondria are involved in ATP synthesis and have both lower Δµ +H and NADH/NAD+ ratio and low respiration, the extent of O ·−2 generation is much lower (for a complete explanation, see Murphy 2009). However, the authors emphasize that all these parameters, together with the CoQH2/CoQ ratio and local O2 concentrations, vary considerably and are very difficult to measure in vivo. Consequently, it is not possible to determine O ·−2 production in in situ mitochondria by applying O ·−2 production rates in isolated mitochondria. Such extrapolations reported in the literature are considered misleading (Murphy 2009).
The pioneering works of Chance et al. and others (Chance et al. 1979; Loschen et al. 1971; Boveris and Chance 1973) reported that isolated mitochondria can produce H2O2 from the dismutation of superoxide anion generated within mitochondria (Loschen et al. 1974; Forman and Kennedy 1974; Winterbourn et al. 1978). This reaction is catalyzed by mitochondrial superoxide dismutase (MnSOD), which has a rate constant for O ·−2 dismutation of the order of 109 M−1 s−1 (Turrens 2003; Cadenas and Davies 2000). The spontaneous dismutation of O ·−2 has a rate constant of 106 M−1 s−1. However, in the absence of MnSOD, the production of O ·−2 decreases by allowing the reverse reaction between superoxide and the electron donor (Winterbourn et al. 1978; Schafer and Buettner 2001). Another ROS, the hydroxyl radical (OH·) is generated in mitochondria by the interaction of H2O2 with Fe2+ of the iron–sulfur proteins in the respiratory chain (Fenton reaction). OH· can also be produced by the Haber–Weiss reaction between superoxide anion and hydrogen peroxide. The hydroxyl radical is the most toxic of the ROS, due to its very high reactivity. Its half-life, measured in nano-seconds, is determined by diffusion time, i.e., the time necessary for OH· to interact with the target molecule (see also below). The hydroxyl radical reacts with the biomolecules mainly by means of hydrogen addition or subtraction, during which characteristic products are generated, described as biological markers of oxidative stress. H2O2 produced by the dismutation of O ·−2 , catalyzed by MnSOD, may diffuse out of mitochondria (Boveris 1984; Barja 1999). It has in fact been demonstrated that O ·−2 is released from mitochondria through the voltage-dependent anion channel (Han et al. 2003). These authors proposed that O ·−2 released into the cytoplasm plays an important role in cell signaling. Cytoplasmic aconitase and other enzymes may also be targets of O ·−2 . Mitochondria can also produce H2O2 by oxidative deamination of biogenic amines by MAOs, a family of FAD-containing enzymes present on the outer membrane of mitochondria (Hauptmann et al. 1996). MAO was discovered in 1928 in liver and was named tyramine oxidase (Hare 1928). In humans, there are two types of MAO: MAO-A and MAO-B. The former is found in neurons, astroglia, liver, gastrointestinal tract, and placenta, and is particularly important in the catabolism of monoamines in food. In detail, MAO-A catabolizes serotonin, norepinephrine, epinephrine and dopamine. MAO-B is mainly found in blood platelets, but also in neurons and astroglia, and oxidizes phenylethylamine and dopamine. Thus, both MAOs are involved in the inactivation of monoaminergic neurotransmitters. Due to this primary role, alterations in their activity are thought to be responsible for several neurological disorders. In this regard, MAO inhibitors are considered to be one of the most important classes of drugs for therapeutic interventions, especially in neurological pathologies such as Parkinson’s and Alzheimer’s diseases (La Regina et al. 2007; Valente et al. 2011). In conclusion, MAO activities represent an additional source of H2O2, not linked to respiration and associated with direct two-electron reduction of O2 to H2O2. It should be noted that the compartmentalization of the source of O ·−2 and H2O2 is functionally associated with and coordinated by the endogenous safety system composed of MnSOD, catalase and glutathione peroxidases, the activity of which leads to regulation of intramitochondrial levels of the above ROS in aerobic organisms (Chance et al. 1979; Naqui et al. 1986). External ROS produced by MAOs can be scavenged by the systems present in the cytoplasm.
The metabolic state of mitochondria determines the physiological rate of ROS production, associated with the respiratory chain. In particular, respiratory state 4 is characterized by no availability of ADP and slow oxygen consumption. Thus, as also mentioned above, this resting state is associated with a relatively fast rate of superoxide and hydrogen peroxide production, most probably due to the high reduction level of the respiratory chain components. Instead, respiratory state 3, with very high oxygen uptake and high ADP, has a slow rate of ROS production, caused by the highly oxidized level of the respiratory chain carriers. Last, in anoxic state 5, with limited O2 supply and block of respiration, generation of O ·−2 and H2O2 does not occur (Loschen et al. 1974; Boveris et al. 1999). Besides the particular metabolic state of mitochondria, ROS generation can be modulated by another physiologically important reactive species, nitric oxide (·NO). ·NO is a highly diffusible free radical, formed by conversion of arginine to citrulline in mitochondria and catalyzed by a specific form of mitochondrial nitric oxide synthase in certain tissues such as liver, brain, heart and thymus (Navarro and Boveris 2008; Alvarez et al. 2003; Haynes et al. 2004). ·NO reversibly inhibits mitochondrial cytochrome c oxidase and electron flux along the respiratory chain, preventing the oxidation of ubiquinol to ubisemiquinone radical, with subsequent autoxidation, leading to increased production of superoxide anion (Palacios-Callender et al. 2004). This effect has been proposed as the primary regulatory role of ·NO in mitochondria (Brown and Borutaite 2004). ·NO does maintain relatively higher respiration in very low concentrations of O2, i.e., physiological levels. This regulatory property is particularly important in hypoxic conditions (Palacios-Callender et al. 2004). It should also be noted that ·NO can react in mitochondria with O ·−2 , producing peroxynitrite (ONOO−) in a very rapid reaction (Szabó et al. 2007; Packer et al. 1996).
When examining the production of ROS and RNS (reactive nitrogen species) by mitochondria, it is interesting to take into account the diffusion radius and compartmentalization of the different types of radicals, to evaluate their capacity of reacting with targets. It has been reported that oxidants may have different effects, depending on where they are produced. Thus, species like O ·−2 , with low permeability, are largely restricted to reactions in the compartment in which they are generated, unless there is no channel for their export (Han et al. 2003). Instead, hydrogen peroxide is membrane-permeable and exhibits a diffusion distance for each activity of 1.5 mm, whereas peroxynitrite has a diffusion distance of 50 μM. About OH· diffusion, it should be recalled that in mitochondria it is generated from H2O2 in the presence of Fe2+ and exhibits a rate constant, for reacting with a substrate, e.g., GSH, of 1 × 1010 M−1 s−1 (for a review, see Winterbourn 2008). This means that OH· can be produced far from the site of H2O2 generation and exhibits an instantaneous reaction rate.
As well as mitochondria, ROS are also generated in multiple compartments by several enzymes within cells. Contributions come from proteins in the plasma membrane, examples being the family of NADH oxidases (Lambeth 2004), lipid metabolism in peroxisomes, and the activity of various cytosolic enzymes such as cyclooxygenase. The endoplasmic reticulum can also produce ROS. Accumulation of misfolded proteins due to ER stress can release Ca2+ from the ER lumen and activate its chaperone machinery (Zhang and Kaufman 2008), producing ROS. This is because correct folding of proteins is an energy-dependent process requiring oxidizing conditions and involving the transfer of reducing equivalents to molecular oxygen (Cuozzo and Kaiser 1999). In addition, Ca2+ released from ER is taken up into the mitochondrial matrix, causing collapse of electrical potential, disruption of electron transport, and a further increase in ROS generation (Görlach et al. 2006). Biological membranes are highly sensitive to damage by free radicals, particularly those rich in polyunsaturated fatty acids, and ROS damage their integrity and functions by compromising the capacity of cells to maintain the ionic gradient and affecting the asymmetric distribution of phospholipids. Lipid peroxides formed by the above reactions are degraded to form malondialdehyde (MDA) and hydroxynonenal (HNE). These compounds react with proteins by forming cross-linkages and chemical adducts, known as final products of advanced lipoperoxidation (ALE). Both MDA and HNE adducts with lysine residues have been identified in the lipoproteins of the vascular wall in atherosclerosis and neuronal plates in Alzheimer’s disease, supporting the implication of oxidative damage in these diseases (Aliev et al. 2002). Hydroxyl radicals may be involved in reactions with phenylalanine, tyrosine and nucleic acids by forming characteristic hydroxylated derivatives. The levels of other ROS and RNS can be assessed by measuring levels of specific markers of oxidative stress (specific oxidation products).
In particular, nitrotyrosine and ALE are present in elevated amounts in atherosclerotic plates and Alzheimer’s disease.
ROS also reacts with carbohydrates to form dicarbonyl compounds which, in turn, react with proteins, forming cross-linkages and adducts, known as glycoxidation products. These compounds are found in large quantities in tissue proteins in diabetes, as a result of hyperglycemia and oxidative stress (Kuroki et al. 2003). The increase in the chemical modifications of proteins by the final products of lipoperoxidation and glycoxidation is implied in the development of vascular, renal and retinal complications.
It is to underline that, H2O2 produced within the mitochondrial matrix can interact with peroxiredoxins, catalase and glutathione peroxidase thus underlying a scavenging effect (Rhee et al. 2001; Salvi et al. 2007a, b; Radi et al. 1991; Imai and Nakagawa 2003).
Mitochondria and apoptosis
All the above observations open up new insight on mitochondrial functions, so that, besides being considered as the control centers of energy production, mitochondria are also well-known as the controllers of several other cell activities. They play pivotal roles in apoptosis, necrosis and many other metabolic processes, and are also involved in many human diseases (Di Mauro and Schon 2003; Newmeyer and Ferguson-Miller 2003; Voet and Voet 2004) (see also “Diseases linked to mitochondrial dysfunctions”). These functions account for a large number of agents operating as means of communication to and from mitochondria such as ions, metabolites, hormones, transcription factors, etc. (Butow and Avadhani 2004; Goldenthal and Marín-García 2004; MacDonald et al. 2005). Mitochondria thus behave as centers capable of receiving and integrating cellular signals and transmitting them to other cellular compartments. Apoptosis, or programmed cell death, is a natural process, essential for the physiological development, homeostasis and survival of most multicellular organisms (Schwartzman and Cidlowski 1993) and also particularly important for its function in removing damaged, infected or potentially tumoral cells. Apoptosis is clearly distinct from necrosis. The intact state of the cell membrane is severely compromised or damaged in necrosis, resulting in cell lysis. Necrosis generally occurs in a group of cells or at particular loci of tissues, whereas apoptosis may occur in single cells. During apoptosis, a cell undergoes a series of events, activated by a genetic program, ending in DNA fragmentation and the formation of apoptotic bodies. Despite this, the plasma membrane of early apoptotic cells and bodies remains intact, without losing endogenous materials and thus preventing local tissue damage. Apoptosis may be triggered by a large number of effectors including, as mentioned above, inducers of oxidative stress. ROS generated during normal electron flux are generally scavenged by the anti-oxidant safety systems present in mitochondria. However, the action of these systems may be ineffective, particularly when exogenous mitochondriotropic agents interact with the components of respiratory complexes, resulting in the production of large quantities of ROS. In such cases, ROS may induce oxidative stress, with damage to mitochondrial structures and metabolites.
Oxidative stress may also affect critical thiol groups present on AdNT, with formation of disulphide bridges and induction of the mitochondrial permeability transition (MPT), a phenomenon closely correlated with intrinsic apoptosis. ROS generation may be involved in other mechanisms, related in various ways to apoptosis. As noted above, mitochondria possess a sophisticated network of signals involving tyrosine kinase and phosphatase activity (Salvi et al. 2002a, b, 2004, 2005a, b, 2007a, b; Horbinski and Chu 2005; Pagliarini and Dixon 2006; Vogt et al. 2007; Tibaldi et al. 2008; Lewandrowski et al. 2008). Several studies have shown that these signals are correlated with triggering of pro-apoptotic pathways. In this case, ROS would have the function of modulating the tyrosine phosphorylation involved in these pathways (see “Tyrosine phosphorylation, ROS, polyamines and mitochondria”). In conclusion, besides having damaging effects, when they are present in mitochondria in not excessive concentrations, ROS may have a dual effect in prompting apoptosis, by activating the intrinsic pathway, and regulating apoptotic signal pathways.
In any case, all the stimuli leading to activation of the pro-apoptotic process appear to converge towards a highly conserved sequence of events, as the morphological and biochemical characteristics of that process are maintained during evolution. Activation of the caspase cascade plays a prominent role in this program (Salvesen and Dixit 1999). Kannan and Jain (2000) noted that, although the initial signal for programmed cell death may be of different origin, its phenotype is uniformly similar and highly conserved during evolution. In other words, multiple signal pathways may converge upstream of a common mechanism of events, resulting in predisposing cells to apoptosis (Schwartzman and Cidlowski 1993; Thompson 1995; Kerr et al. 1972, 1994; Duke et al. 1996). The fact that mitochondria play a central role in apoptosis is now accepted by almost all researchers in the field (Zamzami et al. 1995, 1996; Green and Reed 1988; Susin et al. 1998). There is also the general opinion that mitochondria-mediated apoptosis has wide-ranging implications in cancer research, senescence, and the death of an organism. However, it is also important to evaluate the intensity of the apoptotic process, as slight or excessive apoptosis may lead to different biological consequences (Du et al. 1997). In particular, slight apoptosis is responsible for rheumatoid arthritis and cancer, whereas ischemic heart diseases, AIDS and neurodegenerative diseases are the results of excessive apoptosis (Kerr et al. 1972, 1994; Thompson 1995; Duke et al. 1996; Golstein 1998).
Diseases linked to mitochondrial dysfunctions
As introduced in “Mitochondria and apoptosis”, a growing number of mitochondrial pathologies are attributed to mitochondrial dysfunctions. Most of them, known as encephalomyopathies, affect the brain and skeletal muscles, the organs requiring large supplies of energy. In this regard, several myopathies are the result of defects in enzymes such as pyruvate dehydrogenase and carnitine acyl transferase (CoA), in respiratory chain activity (particularly complex I, complex III and ubiquinone) or phosphorylation defects. Many encephalomyopathies are due to single macrodeletions or point mutations in mtDNA: examples are Kearns–Sayre syndrome (KSS) (Kearns and Sayre 1958), mitochondrial encephalomyopathy, lactic acidosis, stroke-like syndrome (Pavlakis et al. 1984), myoclonic epilepsy with ragged red fibers (Fukuhara et al. 1980), and Leber’s hereditary optic neuropathy (Leber 1871). As noted above, the field of mitochondrial-associated pathologies has grown exponentially and large numbers of mtDNA mutations, mainly due to ROS activity, have been identified in corresponding clinical phenotypes (Schon et al. 2012). An exhaustive review has recently been published regarding mtDNA defects and their effects on the pathophysiological mechanisms underlying pathologies associated with the central and peripheral nervous systems (Carelli and Chan 2014).
Other very interesting systematic reviews have recently been published, covering such fields as cardiac electrical diseases in KSS and mitochondrial cytopathy (Kabunga et al. 2014), mtDNA aberrations and pathophysiological implications in hematopoietic diseases, chronic inflammatory diseases and cancers (Kim et al. 2015), and the role of mitochondrial oxidative stress on the pathogenesis of target organ damage in hypertension (Rubattu et al. 2014).
In several pathologies such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis, in which defective mitochondria are involved, one still unresolved question is whether mitochondrial dysfunction is a primary cause or a secondary consequence of another essential deficiency within specific neuronal tissues (Scheffler 2008). There is strong emerging consensus by researchers in the field that mitochondrial energy metabolism, oxidative stress, ROS production, apoptosis and neurodegeneration are all part of an intricate picture, in which it will be necessary to discover the definitive cause-and-effect relationship leading to a particular pathology. The problem raised by the above observations and discussions takes into account the possibility that specific mtDNA haplotypes predisposing toward or increasing the risk of triggering a neurodegenerative disease act in combination with nuclear mutations.
Mitochondria as targets of “mitochondrial medicine”
As already noted, ROS and RNS are closely related to degenerative diseases involving neuron death, including ischemic and hemorrhagic stroke, degenerative cardiac myocyte death, Alzheimer’s disease, Parkinson’s disease and cancer. The removal of the scavenging effects of these compounds by the mitochondrial safety systems results in structural alterations of organelles, with activation of the cytosolic stress pathway and DNA damage. In particular, membrane cardiolipin is affected, thereby triggering the release of cytochrome c, AIF and Smac–Diablo, and activates the intrinsic apoptotic pathway. In addition, neurodegenerative diseases often arise from massive death of neural cells (Mattson 2000), whereas malignant cell transformation should be facilitated by the inhibition of apoptosis. The demonstration that mitochondria play a central role in many pathologies, together with emerging interest in mitochondrial diseases, has led to the creation of a new research field, to identify new pharmacological tools to block (in neurodegenerative diseases) or activate (in cancer) cell death processes. Many new approaches and contexts are involved, as well as renewed interest in the pathologies in which it seems possible to intervene. Thus, gene therapy for diseases due to mitochondrial dysfunctions and antitumoral chemotherapy may be based on MPT inducers or, conversely, MPT inhibitors, anti-oxidant compounds and modulators of apoptosis regulation proteins. “Mitochondrial medicine” aims at developing drugs targeted directly to mitochondria, i.e., mitochondriotropic agents (Weissig 2005; Torchilin 2006). This point is well-illustrated by the poor effectiveness of antioxidants, such as vitamin E, against diseases with partially oxidative etiogenesis such as Parkinson’s syndrome. The lack of significant effects in these cases is attributed to poor absorption of compounds and their uniform distribution to all organs, tissues and cells (Murphy and Smith 2007). In this regard, several researchers (Murphy and Smith 2007; Sheu et al. 2006; Cochemè et al. 2007) have turned to mitochondrial antioxidant compounds such as decylubiquinone and α-tocopherol, conjugating them with the triphenylphosphonium group (TPP+), lipophilic cations which can cross biological membranes and accumulate in compartments with negative potential such as the mitochondrial matrix. This strategy exploits the ratio between accumulated and external TPP+ concentrations, and is used in bioenergetics to measure the electrical membrane potential, ΔΨ. Several compounds having these characteristics have been synthesized and are used to study redox signaling in cells and to evaluate their effects on oxidative stress models and apoptosis. Until now, they have demonstrated good bioavailability and, vice versa, little or no toxicity, even after prolonged administration (Murphy and Smith 2007). One important implication is that the TPP+ group, in itself, is not particularly toxic. It should be emphasized that cationic conjugates such as those mentioned above have sometimes been observed to exhibit certain selectivity for tumoral tissues. The mitochondria of tumor cells have a higher ΔΨ than that of normal cells (Chen 1988; Fantin et al. 2006; Fantin and Leder 2006) and may sometimes reach 60 mV, corresponding to an accumulation ratio tenfold higher in cancer cells (Fantin and Leder 2006). In view of this peculiar aspect of the mitochondria of tumor cells, some pioneering pharmacological studies have been performed (Fantin and Leder 2004, 2006; Fantin et al. 2002). Among mitochondriotropic agents, the structure and chemical properties of which were described in an exhaustive review (Horobin et al. 2007), polyphenols are considered among the most appropriate to exploit the above effects at mitochondrial level. The polyphenols constitute a group of about 5000 natural molecules, some belonging to classes with different structures, but they all have non-phenolic functions. They are found in nature and in foods, mainly as glycosylated derivatives or bound to polymer structures such as lignins, and include the flavonoids in fruits and vegetables. They were first described by Albert Szent-Gyorgyi, who discovered vitamin C and observed that polyphenols have a synergic effect on it. The best-known flavonoids are polyphenols with low molecular weight, abundant in nature in ester forms as glycosides and acetyls. Quercetin is one of the best-known flavonoids, occurring not only in onions, apples and citrus fruits, but also in the seeds and peel of fruits and vegetables, in the cortex of some plants, and in wine, tea and chocolate. At therapeutic level, administration of quercetin is suggested against allergies, respiratory deficiency, skin inflammation, uric acid intestinal accumulation, inflammation, and diabetes. Several biological effects of polyphenols, still considered putative, have been ascribed to their antioxidant nature (Jovanovic et al. 1994; Cao et al. 1997a, b; Frei and Higdon 2003; Firuzi et al. 2005), that is, to their capacity to act as reducers and radical scavengers. However, they are also easily oxidized catalytically by transition metals such as Cu2+ and Fe3+, leading to the production of hydrogen peroxide and other ROS. In these cases, polyphenols act as oxidative stress inducers, as has been observed in mitochondria treated with some of these compounds. In addition, mitochondria possess a large number of iron–sulfur groups embedded in the respiratory complexes NADH-ubiquinone reductase, succinate ubiquinone reductase and ubiquinol–cytochrome c reductase, whereas copper ions are present in cytochrome c oxidase. Due to the presence of iron and copper ions in the respiratory chain, mitochondria can behave as ROS producers when they interact with polyphenols and their derivatives.
Genistein, a natural isoflavone occurring in plants, is a major component of soybean. It has been shown to have anti-tumoral, anti-oxidant and anti-inflammatory effects, can also prevent cancer in many organs (Peterson 1995) and has beneficial effects in osteoporosis and cardiovascular diseases (Polkowski and Mazurek 2000; Goldwyn et al. 2000). Genistein is a potent inhibitor of α-glucosidase (Dong Sun and Sang Han 2001), an enzyme involved in diseases such as diabetes (Jenkins et al. 1981), cancer (Dennis et al. 1987) and viral attachment (Gruters et al. 1987). The widespread consumption of soy in Asian countries very probably accounts for the low incidence of these chronic disorders in those countries, and suggests that it could play an important role in promoting human health (Goldwyn et al. 2000).
One study linking the therapeutic effects of genistein with its activity as a potential pro-apoptotic agent showed that it can induce the MPT in liver mitochondria (Salvi et al. 2002a, b). The event responsible for transition pore opening is oxidative stress triggered by generation of H2O2, as a result of the interaction of genistein with the mitochondrial respiratory chain. In particular, genistein reacts with the Fe3+ of the bH heme of bc1 complex (complex III), following a previously proposed reaction sequence (Cao et al. 1997a, b), with the final production of H2O2. MPT induction is well-known to be closely associated with the release of several proteins such as cytochrome c, AIF, Smac–Diablo, HtrA2, etc., which prompt the cell toward apoptotic cell death. A clear-cut demonstration of the close correlation between the action of this type of natural compound at mitochondrial level, with triggering of the pro-apoptotic pathway, is that of glycyrrhetinic acid. This compound is the active aglycone of a pentacyclic triterpene glycoside, one of the main components of licorice root. Licorice has long been known as a medicinal plant and its history as a pharmacological drug dates far back into the past (Armanini et al. 2002). Licorice extracts were primarily used as demulcents, expectorants and mild laxatives. Their constituents have several beneficial properties such as anti-inflammatory, anti-hepatotoxic, anti-bacterial, anti-viral and anti-cancer effects (Armanini et al. 2002; WHO 1999). They are also endowed with endocrine properties and, in particular, behave as mineralocorticoids, with estrogenic and antiestrogenic effects (Armanini et al. 2002). However, it should be noted that there is another side in considering flavonoids and polyphenols as antioxidants, since it has been reported that most of these compounds do not act as ROS scavengers but as reducers of reactive nucleophiles or regulators of the signaling pathway (such as Nrf2). In this view, the real antioxidants are α-tocopherol and mitoQ and related compounds (Forman et al. 2014).
It has recently been reported that the compound plastoquinonyl-decyltriphenylphosphonium (SkQ1), at nanomolar concentrations, shows promising biological activity in vivo. SkQ1 can protect against acute pyelonephritis induced by oxidative stress as the result of severe bacterial infections (Plotnikov et al. 2013). Another recent paper has shown that SkQ1 can prevent or slow down some cerebral dysfunctions in accelerated senescence in rats (Stefanova et al. 2014) and suggest that it could be used as a prophylactic to maintain brain health and to treat Alzheimer’s disease.
In view of above effects, one particular aspect is that of the natural polyamines, spermine, spermidine and putrescine, known as essential molecules for a large number of physiological processes (for a review, see Tabor and Tabor 1964; Agostinelli et al. 2004; Toninello et al. 2004). Polyamines interact with mitochondrial membranes at specific sites involved in their electrophoretic transport in mitochondria (Grancara et al. 2014). In particular, spermine can also flow from organelles by promoting the cross-membrane cycling which regulates the induction of MPT (Agostinelli et al. 2010). Polyamines in cells are oxidized by cytosolic (Toninello et al. 2004; Agostinelli et al. 2010) and mitochondrial amine oxidases (Stevanato et al. 2011; Bonaiuto et al. 2015) with the production of hydrogen peroxide and aminoaldehydes, both of which are involved in the induction and/or amplification of MPT. However, this phenomenon, which causes loss of the bioenergetic capacity of mitochondria, together with oxidative stress, is strongly inhibited by polyamines in isolated mitochondria (Toninello et al. 2004). Instead, monoamines as well as the diamine agmatine, have an inhibitory effect at higher concentrations, but at low concentrations they behave as inducers. In particular, this dual effect has been observed with agmatine in liver mitochondria (Battaglia et al. 2007). It should also be noted that, in brain mitochondria, unlike liver mitochondria, the only agent able “to protect” against MPT is spermine (Grancara et al. 2012). According to their cytosolic and matrix concentrations, metabolic conditions, presence of effectors, and cell type, polyamines act as inducing, modulating or preventive agents in intrinsic apoptosis. Although their protective effect is due to inhibition by various mechanisms of MPT induction and retention of pro-apoptotic factors, the inducing effect is explained by the production of ROS, as described above, which, in the absence of sufficient protection by the mitochondrial safety systems, trigger MPT induction and release pro-apoptotic proteins. Polyamines, in particular spermine, can also take part in regulating intrinsic cell death pathways by interacting with the mitochondrial tyrosine phosphorylation/dephosphorylation system (see “Tyrosine phosphorylation, ROS, polyamines and mitochondria”).
As noted above, spermine and the other polyamines can protect against induction of MPT in isolated mitochondria. Several mechanisms have been suggested to explain this effect, including protein phosphorylation/dephosphorylation (Lapidus and Sokolove 1992) and interaction with a specific permeability transition inhibitor site in the matrix or with anionic head groups of inner membrane phospholipids, in particular, the cardiolipin anular domain of AdNT (Lapidus and Sokolove 1993). It has also been proposed that spermine increases the affinity of ADP for its inhibitory binding site (Lapidus and Sokolove 1994). Another proposal is that the binding of spermine to site S1, responsible for polyamine transport, is also competent in inhibiting MPT (Dalla Via et al. 1996). A report on the effect of spermine on MPT induced by the above-mentioned genistein, glycyrrhetinic acid and salicylate provides important information on the protective effect of this polyamine at molecular level (Sava et al. 2006). It demonstrates that these compounds induce oxidative stress, detected by the oxidation of thiol groups, glutathione and pyridine nucleotides, which predispose the membrane to subsequent opening of the transition pore when Ca2+ is also added. In these conditions, spermine can prevent oxidation, even when these species are strongly oxidized, i.e., it behaves like a typical free-radical scavenger. This statement is also confirmed by the protective effect exhibited by spermine on lipid peroxidation and protein oxidation. Again, Casero’s research group demonstrated in vitro DNA protection by spermine against radical scavenging (Ha et al. 1998). The proposed mechanism for this is that spermine reacts with the hydroxyl radical generated by H2O2, producing spermine dialdehyde through a series of intermediates such as bis-N-hydroxyspermine and spermine-bis-oxime (Ha et al. 1998). A very recent paper (Giorgio et al. 2013) proposes another mechanism of spermine protection against MPT: the opening of the transition pore by carbenoxolone, a derivative of glycyrrhetinic acid (Salvi et al. 2005a, b), may be induced via the translocator protein TSPO and connexin 43. The authors propose that MPT is triggered by a series of signal translations from cAMP to AdNT through the acyl CoA binding domain containing protein 3 and TSPO. In turn, AdNT can translate the phosphorylation signal to subunit C of F0-ATP synthase, modulating MPT induction (Azarashvili et al. 2014). It is proposed that carbenoxolone binds to TSPO or the acyl CoA domain, thus increasing the local concentration of cAMP and then inducing TSPO phosphorylation. This event would induce MPT. If spermine can interact with the process of protein phosphorylation, one intriguing proposal is that the polyamine acts at this level to prevent MPT.
Tyrosine phosphorylation, ROS, polyamines and mitochondria
It has become increasingly clear that protein phosphorylation has a profound influence on mitochondria, with protein kinases and phosphatases which regulate the action of critical players involved in the disparate functions of these organelles (Hüttemann et al. 2007; Pagliarini and Dixon 2006; Hebert-Chatelain 2013). More specifically, tyrosine phosphorylation in mitochondria has recently emerged as central to cell biology, several targets being involved in the modulation of virtually all mitochondrial functions, with considerable implications for human disease. Of the substrates found to be tyrosine-phosphorylated, we only mention here: (1) proteins believed to be essential components of the permeability transition pore complex, the multiprotein assembly located at the interface between the outer and inner mitochondrial membranes, including the voltage-dependent anion channel (Lewandrowski et al. 2008), hexokinase type I (Lewandrowski et al. 2008; Pantic et al. 2013), AdNT 1 (Lewandrowski et al. 2008; Feng et al. 2008) and subunit γ of ATP synthase (Di Pancrazio et al. 2006); (2) several subunits of each complex of the respiratory chain (Salvi et al. 2007a, b; Ogura et al. 2012) as well as the electron carrier cytochrome c (Sanderson et al. 2013); (3) metabolic enzymes such as pyruvate dehydrogenase kinase and phosphatase (Hitosugi et al. 2011; Shan et al. 2014); (4) the protein chaperone tumor necrosis factor receptor-associated protein 1 (Yoshida et al. 2013), to name just a few. The actors involved in mitochondrial tyrosine phosphorylation are classified as receptor and non-receptor tyrosine kinases (abbreviated as RTK and NRTK, respectively), which may either temporarily migrate from the plasma membrane to mitochondria in response to diverse cues, thereby integrating the mitochondrial “potential” into the overall cell signaling network, as in the case of hepatocytes (Gringeri et al. 2009), or constitutively reside within the different mitochondrial compartments, as in neurons (Tibaldi et al. 2008). In the former case, epidermal growth factor receptor and Met should be recalled among the RTKs (Cao et al. 2011; Lefebvre et al. 2013) and Src family kinases (SFKs) among the NRTKs, although in the latter SFKs are those thought to be abundantly and stably represented within mitochondria. More importantly, both families of tyrosine kinases have been shown to translocate to mitochondria in response to proliferative stimuli (Tibaldi et al. 2008; Gringeri et al. 2009). In this regard, data accumulated over the past few years have aroused considerable interest in SFKs, which have been shown to be crucial players not only in pathological conditions, such as cancer, but also in regenerative processes, such as liver regeneration (Gringeri et al. 2009). It should be borne in mind that their activity is significantly affected by changes in the redox status of the environment, oxidation enhancing their activity, in sharp contrast with tyrosine phosphatases (PTPs), which are inhibited by oxidative stress (Giannoni and Chiarugi 2014). Thus, high levels of ROS lead to elevated tyrosine phosphorylation, with potentially dramatic changes in mitochondrial function (Zheng et al. 2013). Interestingly, SFKs are also keys to reducing ROS levels by phosphorylating a component of complex II of the respiratory chain, i.e., flotillin-1 (Ogura et al. 2014), which again emphasizes the importance of this class of enzymes as both regulators and effectors in mitochondrial physiology. Because spermine, depending on its concentration and also on the metabolic state of mitochondria, has been found to be both scavenger and producer of ROS (Agostinelli et al. 2007), the existence of a ROS–SFK axis has been hypothesized, which may affect spermine transport: this possibility has been experimentally confirmed, albeit in isolated mitochondria. In fact, the data demonstrate that Src, a member of SFKs, is constitutively present in the brain and is a reasonable candidate for phosphorylation of the spermine channel, as assessed by the use of peroxides, irreversible PTP or SFK inhibitors in rat brain mitochondria (Battaglia et al. 2012). However, the protein channel serving as the spermine transporter has not yet been identified and further research is needed to achieve this structural and functional information, which would pave the way to deeper insights into the connections between ROS, SFKs and spermine transport.
Conclusions
The above observations allow us to postulate that a complex network of signals leading to oxidative and phosphorylative events controls pore opening and intrinsic apoptosis by modulating the activity of cytoplasmic and mitochondrial amine oxidases coupled to kinase signaling pathways. Dysregulation of these events is a sign of several diseases, in particular cancer and neurodegeneration, and the mitochondrial transition pore may represent an intriguing target for pharmacological therapies.
Abbreviations
- MAO:
-
Monoamine oxidase
- AdNT:
-
Adenine nucleotide translocase
- RCI:
-
Respiratory control index
- P/O ratio:
-
Ratio ATP and consumed oxygen
- ROS:
-
Reactive oxygen species
- MPT:
-
Mitochondrial permeability transition
- O ·−2 :
-
Superoxide anion
- RNS:
-
Reactive nitrogen species
- FMN:
-
Flavin mono nucleotide
- CoQH2/CoQ:
-
Cofactor, ubiquinol/ubiquinone ratio
- MnSOD:
-
Mitochondrial superoxide dismutase
- ONOO− :
-
Peroxynitrite
- MAOs:
-
Monoamine oxidases
- FAD:
-
Flavin adenine dinucleotide
- ·NO:
-
Nitric oxide
- mtNOS:
-
Mitochondrial nitric oxide synthase
- MDA:
-
Malondialdehyde
- HNE:
-
Hydroxynonenal
- ALE:
-
Lipoperoxidation
- TPP+ :
-
Triphenylphosphonium
- ΔΨ :
-
Electrical membrane potential
- PTPC:
-
Permeability transition pore complex
- VDAC:
-
Voltage-dependent anion channel
- ANT:
-
Adenine nucleotide translocase
- RTK:
-
Receptor tyrosine kinases
- NRTK:
-
Non-receptor tyrosine kinases
- EGFR:
-
Epidermal growth factor receptor
- SFKs:
-
Src family kinases
- PTPs:
-
Tyrosine phosphatases
References
Adam-Vizi V, Chinopoulos C (2006) Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci 27:639–645
Agostinelli E, Arancia G, Vedova LD, Belli F, Marra M, Salvi M, Toninello A (2004) The biological functions of polyamines oxidation products by amine oxidases: perspectives of clinical applications. Amino Acids 27:347–358
Agostinelli E, Tempera G, Molinari A, Salvi M, Battaglia V, Toninello A, Arancia G (2007) The physiological role of biogenic amines redox reactions in mitochondria. New perspectives in cancer therapy. Amino Acids 33:175–187
Agostinelli E, Tempera G, Viceconte N, Saccoccio S, Battaglia V, Grancara S, Toninello A, Stevanato R (2010) Potential anticancer application of polyamine oxidation products formed by amine oxidase: a new therapeutic approach. Amino Acids 38:353–368
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) The molecular biology of the cell, 4th edn. Garland Science, New York
Aliev G, Smith MA, Seyidov D, Neal ML, Lamb BT, Nunomura A, Gasimov EK, Vinters HV, Perry G, LaManna JC, Friedland RP (2002) The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer’s disease. Brain Pathol 12:21–35
Alvarez S, Valdez LB, Zaobornyj T, Boveris A (2003) Oxygen dependence of mitochondrial nitric oxide synthase activity. Biochem Biophys Res Commun 305:771–775
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Moscow) 70:200–214
Armanini D, Fiore C, Mattarello MJ, Bielenberg J, Palermo M (2002) History of the endocrine effects of licorice. Exp Clin Endocrinol Diabetes 110:257–261
Azarashvili T, Odinokova I, Bakunts A, Ternovsky V, Krestinina O, Tyynelä J, Saris NE (2014) Potential role of subunit c of F0F1-ATPase and subunit c of storage body in the mitochondrial permeability transition. Effect of the phosphorylation status of subunit c on pore opening. Cell Calcium 55:69–77
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483–495
Barja G (1999) Kinetic measurement of mitochondrial oxygen radical production. In: Methods in aging research. CRC press, Boca Raton, pp 533–548
Battaglia V, Rossi CA, Colombatto S, Grillo MA, Toninello A (2007) Different behaviour of agmatine in liver mitochondria: inducer of oxidative stress or scavenger of reactive oxygen species? Biochim Biophys Acta 1768:1147–1153
Battaglia V, Tibaldi E, Grancara S, Zonta F, Brunati AM, Martinis P, Bragadin M, Grillo MA, Tempera G, Agostinelli E, Toninello A (2012) Effect of peroxides on spermine transport in rat brain and liver mitochondria. Amino Acids 42:741–749
Bonaiuto E, Grancara S, Martinis P, Stringaro A, Colone M, Agostinelli E, Macone A, Stevanato R, Vianello F, Toninello A, Di Paolo ML (2015) A novel enzyme with spermine oxidase properties in bovine liver mitochondria: identification and kinetic characterization. Free Radic Biol Med. doi:10.1016/j.freeradbiomed.2015.01.001
Boveris A (1984) Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol 105:429–435
Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134:707–716
Boveris A, Costa LE, Cadenas E (1999) The mitochondrial production of oxygen radicals and cellular aging. In: Cadenas E, Packer L (eds) Understanding the process of aging: the roles of mitochondria, free radicals, and antioxidants. Marcel Dekker, Inc., New York, pp 1–16
Brown GC, Borutaite V (2004) Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta 1658:44–49
Butow RA, Avadhani NG (2004) Mitochondrial signaling: the retrograde response. Mol Cell 14:1–15
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230
Cao G, Sofic E, Prior RL (1997a) Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med 22:749–760
Cao G, Sofic E, Prior RL (1997b) Antioxidant and prooxidant behaviour of flavonoids: structure–activity relationships. Free Radic Biol Med 22:749–760
Cao X, Zhu H, Ali-Osman F, Lo HW (2011) EGFR and EGFRvIII undergo stress- and EGFR kinase inhibitor-induced mitochondrial translocalization: a potential mechanism of EGFR-driven antagonism of apoptosis. Mol Cancer 10:26
Carafoli E (1982) The regulation of intracellular calcium. Adv Exp Med Biol 151:461–472
Carafoli E (2003) Historical review: mitochondria and calcium: ups and downs of an unusual relationship. Trends Biochem Sci 28:175–181
Carafoli E, Crompton M (1978) The regulation of intracellular calcium. Curr Top Membr Trans 10:151–216
Carelli V, Chan DC (2014) Mitochondrial DNA: impacting central and peripheral nervous systems. Neuron 84:1126–1142
Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217:383–393
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Chen LB (1988) Mitochondrial membrane potential in living cells. Annu Rev Cell Biol 4:155–181
Cochemè HM, Kelso GF, James AM, Ross MF, Trnka J, Mahendiran T, Asin-Cayuela J, Blaikie FH, Manas AR, Porteous CM, Adlam VJ, Smith RA, Murphy MP (2007) Mitochondrial targeting of quinones: therapeutic implications. Mitochondrion 7(Suppl. 1):S94–S102
Crompton M, Capano M, Carafoli E (1976) Respiration-dependent efflux of magnesium ions from heart mitochondria. Biochem J 154:735–742
Cuozzo JW, Kaiser CA (1999) Competition between glutathione and protein thiols for disulphide-bond formation. Nat Cell Biol 1:130–135
Dalla Via L, Di Noto V, Siliprandi D, Toninello A (1996) Spermine binding to liver mitochondria. Biochem Biophys Acta 1284:247–253
Dalla Via L, Di Noto V, Toninello A (1999) Binding of spermidine and putrescine to energized liver mitochondria. Arch Biochem Biophys 365:231–238
Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS (1987) Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 236:582–585
Di Mauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348:2656–2668
Di Pancrazio F, Bisetto E, Alverdi V, Mavelli I, Esposito G, Lippe G (2006) Differential steady-state tyrosine phosphorylation of two oligomeric forms of mitochondrial F0F1 ATPsynthase: a structural proteomic analysis. Proteomics 6:921–926
Dong Sun L, Sang Han L (2001) Genistein, a soy isoflavone, is a potent a-glucosidase inhibitor. FEBS Lett 501:84–86
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Du Y, Bales KR, Dodel RC, Hamilton-Byrd E, Horn JW, Czilli DL, Simmons LK, Ni B, Paul SM (1997) Activation of a caspase 3-related cysteine protease is required for glutamate-mediated apoptosis of cultured cerebellar granule neurons. Proc Natl Acad Sci USA 94:11657–11662
Duke RC, Ojcius DM, Young JD (1996) Cell suicide in health and disease. Sci Am 275:80–87
Ernster L, Kuylenstierna B (1969) Structure, composition and function of mitochondrial membranes. In: Ernster L (ed) Mitochondria structure and function. Academic Press, London, pp 5–31
Fantin VR, Leder P (2004) F16, a mitochondriotoxic compound, triggers apoptosis or necrosis depending on the genetic background of the target carcinoma cell. Cancer Res 64:329–336
Fantin VR, Leder P (2006) Mitochondriotoxic compounds for cancer therapy. Oncogene 25:4787–4797
Fantin VR, Berardi MJ, Scorrano L, Korsmeyer SJ, Leder P (2002) A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell 2:29–42
Fantin VR, St-Pierre J, Leder P (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9:425–434
Feng J, Zhu M, Schaub MC, Gehrig P, Roschitzki B, Lucchinetti E, Zaugg M (2008) Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc Res 80:20–29
Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L (2005) Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta 1721:174–184
Fonyo A, Ligeti E, Palmieri F, Quagliariello E (1975) Carrier-mediated tran sport of phosphate in mitochondria. In: Gardos G, Szasz I (eds) Biomembranes, structure and function. Elsevier, North Holland, pp 287–306
Forman HJ, Kennedy JA (1974) Role of superoxide radical in mitochondrial dehydrogenase reactions. Biochem Biophys Res Commun 60:1044–1050
Forman HJ, Davies KJ, Ursini F (2014) How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med 66:24–35
Frei B, Higdon JV (2003) Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr 133:3275S–3284S
Fukuhara N, Tokiguchi S, Shirakawa K, Tsubaki T (1980) Myoclonus epilepsy associated with ragged-red fibres (mitochondrial abnormalities): disease entity or a syndrome? Light-and electron-microscopic studies of two cases and review of literature. J Neurol Sci 47:117–133
Giannoni E, Chiarugi P (2014) Redox circuitries driving Src regulation. Antioxid Redox Signal 20:2011–2025
Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, Lippe G, Bernardi P (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA 110:5887–5892
Goldenthal MJ, Marín-García J (2004) Mitochondrial signaling pathways: a receiver/integrator organelle. Mol Cell Biochem 262:1–16
Goldwyn S, Lazinsky A, Wie H (2000) Promotion of health by soy isoflavones: efficacy, benefit and concerns. Drug Metab Drug Interac 17:261–289
Golstein P (1998) Cell death in us and others. Science 281:1283
Görlach A, Klappa P, Kietzmann T (2006) The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal 8:1391–1418
Grancara S, Battaglia V, Martinis P, Viceconte N, Agostinelli E, Toninello A, Deana R (2012) Mitochondrial oxidative stress induced by Ca2+ and monoamines: different behaviour of liver and brain mitochondria in undergoing permeability transition. Amino Acids 42:751–759
Grancara S, Martinis P, Manente S, García-Argáez AN, Tempera G, Bragadin M, Dalla Via L, Agostinelli E, Toninello A (2014) Bidirectional fluxes of spermine across the mitochondrial membrane. Amino Acids 46:671–679
Green DR, Reed JC (1988) Mitochondria and apoptosis. Science 281:1309–1311
Gringeri E, Carraro A, Tibaldi E, D’Amico FE, Mancon M, Toninello A, Pagano MA, Vio C, Cillo U, Brunati AM (2009) Lyn-mediated mitochondrial tyrosine phosphorylation is required to preserve mitochondrial integrity in early liver regeneration. Biochem J 425:401–412
Gruters RA, Neefjes JJ, Tersmette M, de Goede REY, Tulp A, Huisman HG, Miedema F, Ploegh HL (1987) Interference with HIV induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature 330:74–77
Gunter TE, Gunter KK (2001) Uptake of calcium by mitochondria: transport and possible function. IUBMB Life 52:197–204
Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K (2000) Mitochondrial calcium transport: mechanisms and functions. Cell Calcium 28:285–296
Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC (2003) Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423:456–461
Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA Jr (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 95:11140–11145
Hackenbrock CR (1968) Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc Natl Acad Sci USA 61:598–605
Han D, Antunes F, Canali R, Rettori D, Cadenas E (2003) Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278:5557–5563
Hare ML (1928) Tyramine oxidase: a new enzyme system in liver. Biochem J 22:968–979
Harrington JL, Murphy E (2015) The mitochondrial calcium uniporter: mice can live and die without it. J Mol Cell Cardiol 78:46–53
Hauptmann N, Grimsby J, Shih JC, Cadenas E (1996) The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Arch Biochem Biophys 335:295–304
Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131:596–610
Hayashi T, Rizzuto R, Hajnoczky G, Su TP (2009) MAM: more than just a housekeeper. Trends Cell Biol 19:81–88
Haynes V, Elfering S, Traaseth N, Giulivi C (2004) Mitochondrial nitric-oxide synthase: enzyme expression, characterization, and regulation. J Bioenerg Biomembr 36:341–346
Hebert-Chatelain E (2013) Src kinases are important regulators of mitochondrial functions. Int J Biochem Cell Biol 45:90–98
Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, Ge Q, Gu TL, Polakiewicz RD, Roesel JL, Chen GZ, Boggon TJ, Lonial S, Fu H, Khuri FR, Kang S, Chen J (2011) Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1is important for cancer metabolism. Mol Cell 44:864–877
Horbinski C, Chu CT (2005) Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med 38:2–11
Horobin RW, Trapp S, Weissig VJ (2007) Mitochondriotropics: a review of their mode of action, and their applications for drug and DNA delivery to mammalian mitochondria. J Control Release 121:125–136
Hüttemann M, Lee I, Samavati L, Yu H, Doan JW (2007) Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta 1773:1701–1720
Imai H, Nakagawa Y (2003) Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34:145–169
Jenkins DJ, Taylor RH, Goff DV, Fielden H, Misiewicz JJ, Sarson DL, Bloom SR, Alberti KG (1981) Scope and specificity of acarbose in slowing carbohydrate absorption in man. Diabetes 30:951–954
Jovanovic SV, Steenken S, Tosic M, Marjanovic B, Simic MG (1994) Flavonoids as antioxidants. J Am Chem Soc 116:4846–4851
Kabunga P, Lau AK, Phan K, Puranik R, Liang C, Davis RL, Sue CM, Sy RW (2014) Systematic review of cardiac electrical disease in Kearns–Sayre syndrome and mitochondrial cytopathy. Int J Cardiol 181C:303–310
Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiology 7:153–163
Kearns TP, Sayre GP (1958) Retinitis pigmentosa, external ophthalmophegia, and complete heart block: unusual syndrome with histologic study in one of two cases. AMA Arch Ophthalmol 60:280–289
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kerr JF, Winterford CM, Harmon BV (1994) Apoptosis. Its significance in cancer and cancer therapy. Cancer 73:2013–2026
Kim HR, Won SJ, Fabian C, Kang MG, Szardenings M, Shin MG (2015) Mitochondrial DNA aberrations and pathophysiological implications in hematopoietic diseases, chronic inflammatory diseases, and cancers. Ann Lab Med 35:1–14
Klingenberg M (1979) The ADP, ATP shuttle of the mitochondrion. Trends Biochem Sci 4:249–252
Klingenberg M, Winkler E, Huang S (1995) ADP/ATP carrier and uncoupling protein. Methods Enzymol 260:369–389
Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS (2004) Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279:127–135
Kuroki T, Isshiki K, King GL (2003) Oxidative stress: the lead or supporting actor in the pathogenesis of diabetic complications. J Am Soc Nephrol 14:S216–S220
La Regina G, Silvestri R, Artico M, Lavecchia A, Novellino E, Befani O, Turini P, Agostinelli E (2007) New pyrrole inhibitors of monoamine oxidase: synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. J Med Chem 50:922–931
Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4:181–189
LaNoue KF, Schoolwerth AC (1979) Metabolite transport in mitochondria. Annu Rev Biochem 48:871–922
Lapidus RG, Sokolove PM (1992) Inhibition by spermine of the inner membrane permeability transition of isolated rat heart mitochondria. FEBS Lett 313:314–318
Lapidus RG, Sokolove PM (1993) Spermine inhibition of the permeability transition of isolated rat liver mitochondria: an investigation of mechanism. Arch Biochem Biophys 306:246–253
Lapidus RG, Sokolove PM (1994) The mitochondrial permeability transition: interactions of spermine, ADP, and inorganic phosphate. J Biol Chem 269:18931–18936
Leber T (1871) Ueber hereditaere und congenital-angelegte Sehnervenleiden. Arch Ophthalmol 17:249–291
Lefebvre J, Muharram G, Leroy C, Kherrouche Z, Montagne R, Ichim G, Tauszig-Delamasure S, Chotteau-Lelievre A, Brenner C, Mehlen P, Tulasne D (2013) Caspase-generated fragment of the Met receptor favors apoptosis via the intrinsic pathway independently of its tyrosine kinase activity. Cell Death Dis 4:e871
Lewandrowski U, Sickmann A, Cesaro L, Brunati AM, Toninello A, Salvi M (2008) Identification of new tyrosine phosphorylated proteins in rat brain mitochondria. FEBS Lett 582:1104–1110
Liu Y, Fiskum G, Schubert D (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80:780–787
Loschen G, Flohé L, Chance B (1971) Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Lett 18:261–264
Loschen G, Azzi A, Richter C, Flohé L (1974) Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett 42:68–72
MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA (2005) Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab 288:E1–E15
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1:120–129
Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by chemi-osmotic type of mechanism. Nature 191:144–148
Muller F (2000) The nature and mechanism of superoxide production by the electron transport chain. J Am Aging Assoc 23:227–253
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Murphy MP, Smith RA (2007) Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47:629–656
Naqui A, Chance B, Cadenas E (1986) Reactive oxygen intermediates in biochemistry. Annu Rev Biochem 55:137–166
Navarro A, Boveris A (2008) Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants. Adv Drug Deliv Rev 60:1534–1544
Newmeyer DD, Ferguson-Miller S (2003) Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112:481–490
Nicholls DG, Crompton M (1980) Mitochondrial calcium transport. FEBS Lett 111:261–268
Ogura M, Yamaki J, Homma MK, Homma Y (2012) Mitochondrial c-Src regulates cell survival through phosphorylation of respiratory chain components. Biochem J 447:281–289
Ogura M, Yamaki J, Homma MK, Homma Y (2014) Phosphorylation of flotillin-1 by mitochondrial c-Src is required to prevent the production of reactive oxygen species. FEBS Lett 588:2837–2843
Packer MA, Porteous CM, Murphy MP (1996) Superoxide production by mitochondria in the presence of nitric oxide forms peroxynitrite. Biochem Mol Biol Int 40:527–534
Pagliarini DJ, Dixon JE (2006) Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem Sci 31:26–34
Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE (2012) The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol 682:12–20
Palacios-Callender M, Quintero M, Hollis VS, Springett RJ, Moncada S (2004) Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc Natl Acad Sci USA 101:7630–7635
Palmieri F, Klingenberg M (1979) Direct methods for measuring metabolite transport and distribution in mitochondria. Methods Enzymol 56:279–301
Pantic B, Trevisan E, Citta A, Rigobello MP, Marin O, Bernardi P, Salvatori S, Rasola A (2013) Myotonic dystrophy protein kinase (DMPK) prevents ROS-induced cell death by assembling a hexokinase II-Src complex on the mitochondrial surface. Cell Death Dis 4:e858
Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP (1984) Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol 16:481–488
Peterson G (1995) Evaluation of the biochemical targets of genistein in tumor cells. J Nutr 125:S784–S789
Pfeiffer DR, Gunter TE, Eliseev R, Broekemeier KM, Gunter KK (2001) Release of Ca2+ from mitochondria via the saturable mechanisms and the permeability transition. IUBMB Life 52:205–212
Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Manskikh VN, Pulkova NV, Galkina SI, Skulachev VP, Zorov DB (2013) Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc Natl Acad Sci USA 110:E3100–E3108
Polkowski K, Mazurek AP (2000) Biological properties of genistein. A review of in vitro and in vivo effects. Acta Pol Pharm 57:135–155
Puskin JS, Gunter TE, Gunter KK, Russell PR (1976) Evidence for more than one Ca2+ transport mechanism in mitochondria. Biochemistry 15:3834–3842
Radi R, Turrens JF, Chang LY, Bush KM, Crapo JD, Freeman BA (1991) Detection of catalase in rat heart mitochondria. J Biol Chem 266:22028–22034
Raha S, Robinson BH (2000) Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci 25:502–508
Rhee SG, Kang SW, Chang TS, Jeong W, Kim K (2001) Peroxiredoxin, a novel family of peroxidases. IUBMB Life 52:35–41
Rich P (2003) Chemiosmotic coupling: the cost of living. Nature 421:583
Rizzuto R, Bernardi P, Pozzan T (2000) Mitochondria as all-round players of the calcium game. J Physiol 529(Pt 1):37–47
Rottenberg H, Scarpa A (1974) Calcium uptake and membrane potential in mitochondria. Biochemistry 13:4811–4817
Rubattu S, Pagliaro B, Pierelli G, Santolamazza C, Castro SD, Mennuni S, Volpe M (2014) Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. Int J Mol Sci 16:823–839
Salvesen GS, Dixit VM (1999) Caspase activation: the induced proximity model. Proc Natl Acad Sci USA 96:10964–10967
Salvi M, Toninello A (2004) Effects of polyamines on mitochondrial Ca2+ transport. Biochim Biophys Acta 1661:113–124
Salvi M, Brunati AM, Bordin L, La Rocca N, Clari G, Toninello A (2002a) Characterization and location of Src-dependent tyrosine phosphorylation in rat brain mitochondria. Biochim Biophys Acta 1589:181–195
Salvi M, Brunati AM, Clari G, Toninello A (2002b) Interaction of genistein with the mitochondrial electron transport chain results in opening of the membrane transition pore. Biochim Biophys Acta 1556:187–196
Salvi M, Stringaro A, Brunati AM, Agostinelli E, Arancia G, Clari G, Toninello A (2004) Tyrosine phosphatase activity in mitochondria: presence of Shp-2 phosphatase in mitochondria. Cell Mol Life Sci 61:2393–2404
Salvi M, Brunati AM, Toninello A (2005a) Tyrosine phosphorylation in mitochondria: a new frontier in mitochondrial signaling. Free Radic Biol Med 38:1267–1277
Salvi M, Fiore C, Battaglia V, Palermo M, Armanini D, Toninello A (2005b) Carbenoxolone induces oxidative stress in liver mitochondria, which is responsible for transition pore opening. Endocrinology 146:2306–2312
Salvi M, Battaglia V, Brunati AM, La Rocca N, Tibaldi E, Pietrangeli P, Marcocci L, Mondovì B, Rossi CA, Toninello A (2007a) Catalase takes part in rat liver mitochondria oxidative stress defense. J Biol Chem 282:24407–24415
Salvi M, Morrice NA, Brunati AM, Toninello A (2007b) Identification of the flavoprotein of succinate dehydrogenase and aconitase as in vitro mitochondrial substrates of Fgr tyrosine kinase. FEBS Lett 581:5579–5585
Sanderson TH, Mahapatra G, Pecina P, Ji Q, Yu K, Sinkler C, Varughese A, KumarR Bukowski MJ, Tousignant RN, Salomon AR, Lee I, Hüttemann M (2013) Cytochrome c is tyrosine 97 phosphorylated by neuroprotective insulin treatment. PLoS One 8:e78627
Sava IG, Battaglia V, Rossi CA, Salvi M, Toninello A (2006) Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic Biol Med 41:1272–1281
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30:1191–1212
Scheffler IE (2008) Mitochondrial mutations and disease. In: Scheffler IE (ed) Mitochondria, 2nd edn. Wiley, Hoboken, pp 345–416
Schon EA, DiMauro S, Hirano M (2012) Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet 13:878–890
Schwartzman RA, Cidlowski LA (1993) Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev 14:133–151
Shan C, Kang HB, Elf S, Xie J, Gu TL, Aguiar M, Lonning S, Hitosugi T, ChungTW Arellano M, Khoury HJ, Shin DM, Khuri FR, Boggon TJ, Fan J (2014) Tyr-94 phosphorylation inhibits pyruvate dehydrogenase phosphatase 1 and promotes tumor growth. J Biol Chem 289:21413–21422
Sheu SS, Nauduri D, Anders MW (2006) Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta 1762:256–265
Slater EC (1953) Mechanism of phosphorylation in the respiratory chain. Nature 172:975–978
Sparagna GC, Gunter KK, Sheu SS, Gunter TE (1995) Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem 270:27510–27515
Stefanova NA, Muraleva NA, Skulachev VP, Kolosova NG (2014) Alzheimer’s disease-like pathology in senescence-accelerated OXYS rats can be partially retarded with mitochondria-targeted antioxidant SkQ1. J Alzheimers Dis 38:681–694
Stevanato R, Cardillo S, Braga M, De Iuliis A, Battaglia V, Toninello A, Agostinelli E, Vianello F (2011) Preliminary kinetic characterization of a copper amine oxidase from rat liver mitochondria matrix. Amino Acids 40:713–720
Susin SA, Zamzami N, Kroemer G (1998) Mitochondria as regulators of apoptosis, doubt no more. Biochim Biophys Acta 1366:151–165
Szabó C, Ischiropoulos H, Radi R (2007) Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6:662–680
Tabor H, Tabor CW (1964) Spermidine, spermine, and related amines. Pharmacol Rev 16:245–300
Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462
Tibaldi E, Brunati AM, Massimino ML, Stringaro A, Colone M, Agostinelli E, Arancia G, Toninello A (2008) Src-Tyrosine kinases are major agents in mitochondrial tyrosine phosphorylation. J Cell Biochem 104:840–849
Toninello A, Miotto G, Siliprandi D, Siliprandi N, Garlid KD (1988) On the mechanism of spermine transport in liver mitochondria. J Biol Chem 263:19407–19411
Toninello A, Dalla Via L, Siliprandi D, Garlid KD (1992) Evidence that spermine, spermidine, and putrescine are transported electrophoretically in mitochondria by a specific polyamine uniporter. J Biol Chem 267:18393–18397
Toninello A, Dalla Via L, Stevanato R, Yagisawa S (2000) Kinetics and free energy profiles of spermine transport in liver mitochondria. Biochemistry 39:324–331
Toninello A, Salvi M, Mondovì B (2004) Interaction of biologically active amines with mitochondria and their role in the mitochondrial-mediated pathway of apoptosis. Curr Med Chem 11:2349–2374
Torchilin VP (2006) Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng 8:343–375
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Valente S, Tomassi S, Tempera G, Saccoccio S, Agostinelli E, Mai A (2011) Novel reversible monoamine oxidase a inhibitors: highly potent and selective 3-(1h-pyrrol-3-yl)-2-oxazolidinones. J Med Chem 54:8228–8232
Voet D, Voet JG (2004) Electron transport and oxidative phosphorylation. Biochemistry, 3rd edn. Wiley, USA, pp 797–842
Vogt S, Riehl A, Koch V, Kadenbach B (2007) Regulation of oxidative phosphorylation by inhibition of its enzyme complexes via reversible phosphorylation. Curr Enzym Inhib 3:189–206
Votyakova TV, Reynolds IJ (2001) DeltaPsi(m)-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem 79:266–277
Wallace DC (1997) Mitochondrial DNA in aging and diseases. Sci Am 227:40–47
Weissig V (2005) Targeted drug delivery to mammalian mitochondria in living cells. Expert Opin Drug Deliv 2:89–102
WHO, World Health Organization (1999) Radix glycyrrhizae. In: Monographs on selected medicinal plants, vol 1. WHO, Geneva, pp 183–194
Winterbourn CC (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4:278–286
Winterbourn CC, French JK, Claridge RF (1978) Superoxide dismutase as an inhibitor of reactions of semiquinone radicals. FEBS Lett 94:269–272
Wohlrab H (2005) The human mitochondrial transport protein family: identification and protein regions significant for transport function and substrate specificity. Biochim Biophys Acta 1709:157–168
Yen K, Lee C, Mehta H, Cohen P (2013) The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol 50:R11–R19
Yoshida S, Tsutsumi S, Muhlebach G, Sourbier C, Lee MJ, Lee S, Vartholomaiou E, Tatokoro M, Beebe K, Miyajima N, Mohney RP, Chen Y, Hasumi H, Xu W, Fukushima H, Nakamura K, Koga F, Kihara K, Trepel J, Picard D, Neckers L (2013) Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc Natl Acad Sci USA 110:E1604–E1612
Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182:367–377
Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez Monterrey I, Castedo M, Kroemer G (1996) Mitochondrial control of nuclear apoptosis. J Exp Med 183:1533–1544
Zhang K, Kaufman RJ (2008) From endoplasmic-reticulum stress to the inflammatory response. Nature 454:455–462
Zheng H, Li S, Hsu P, Qu CK (2013) Induction of a tumor-associated activating mutation in protein tyrosine phosphatase Ptpn11 (Shp2) enhances mitochondrial metabolism, leading to oxidative stress and senescence. J Biol Chem 288:25727–25738
Acknowledgments
The authors would like to thank Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) and the Istituto Pasteur-Fondazione Cenci Bolognetti (EA). This work is dedicated to our dear friend and esteemed colleague, Dr. Giampiero Tempera, Ph.D., who died on 19 in December 2014.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: F. Galli.
Rights and permissions
About this article
Cite this article
Grancara, S., Zonta, F., Ohkubo, S. et al. Pathophysiological implications of mitochondrial oxidative stress mediated by mitochondriotropic agents and polyamines: the role of tyrosine phosphorylation. Amino Acids 47, 869–883 (2015). https://doi.org/10.1007/s00726-015-1964-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-1964-7