Abstract
Cysteine sulfinic acid decarboxylase (Csad) is the rate-limiting enzyme in the de novo biosynthesis of taurine. There are a number of physiological roles of taurine, such as bile salt synthesis, osmoregulation, lipid metabolism, and oxidative stress inhibition. To investigate the role of de novo synthesis of taurine during embryonic development, zebrafish csad was cloned and functionally analyzed. Semi-quantitative RT-PCR showed that csad transcripts are maternally deposited, while whole-mount in situ hybridization demonstrated that csad is expressed in yolk syncytial layer and various embryonic tissues such as notochord, brain, retina, pronephric duct, liver, and pancreas. Knockdown of csad significantly reduced the embryonic taurine level, and the affected embryos had increased early mortality and cardiac anomalies. mRNA coinjection and taurine supplementation rescued the cardiac phenotypes suggesting that taurine originating from the de novo synthesis pathway plays a role in cardiac development. Our findings indicated that the de novo synthesis pathway via Csad plays a critical role in taurine homeostasis and cardiac development in zebrafish early embryos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taurine (2-aminoethane sulfonic acid) is one of the most abundant free amino acids in vertebrates, and it plays diverse, yet vital biological roles (Huxtable 1992). The best-known physiological role of taurine is as conjugates of bile acids that play key roles in dietary fat absorption and cholesterol metabolism (Jacobsen and Smith 1968). Cells of both mammals and fish respond to an anisosmotic environment by in or out trafficking of taurine through taurine transporter (TauT) and a putative volume-sensitive anion exchanger (Lambert 2004; Haskew-Layton et al. 2008; Shennan 2008; Shennan et al. 1993, 2006; Chow et al. 2009). In addition, accumulating data indicate that taurine modulates membrane potential by interacting with γ-aminobutyric acid type A (GABAA), GABAB, and glycine receptors and hence functions as a neural transmitter (Albrecht and Schousboe 2005). There is convincing evidence for a protective effect of taurine against apoptosis mediated by oxidative stress and endoplasmic reticulum stress in various cellular and animal models (Schaffer et al. 2009; Suzuki et al. 2002; Nonaka et al. 2001; Chang et al. 2004; Chen et al. 2008; Ghosh et al. 2009; Men et al. 2010; Pan et al. 2010; Rosemberg et al. 2010a; Das et al. 2011). Taurine deficiency has been suggested to be involved in pathological conditions in retina, brain, heart, the reproductive system, and the immune system (Sturman 1993; Yamori et al. 2010; Schaffer et al. 2003; Jammoul et al. 2010; Ito et al. 2008) indicating that taurine homeostasis is important in general animal health.
The sources of taurine are de novo synthesis and dietary intake. Most mammals are able to synthesize taurine endogenously, although the rate of taurine synthesis varies across species (Worden and Stipanuk 1985). Since the activities of key enzymes in taurine biosynthetic pathways are extremely low in the liver, some species such as the cat and human are more dependent on dietary intake (Jacobsen and Smith 1963; Knopf et al. 1978; Park et al. 1991), which depends on the expression of TauT in the intestinal epithelia (Barnard et al. 1988; Miyamoto et al. 1989). Since taurine is present ubiquitously, it’s not surprising that taurine biosynthesis is also found in extra-hepatic tissues such as central nervous system, ocular tissues, epididymis, perirenal white adipose tissues, and brown adipose tissue (Tappaz et al. 1998; Heinamaki 1988; Ide et al. 2002). While a taurine synthesis pathway that does not require Csad recently is suggested (Dominy et al. 2007), Csad has been confirmed as the rate-limiting enzyme for taurine biosynthesis in the liver (de la Rosa and Stipanuk 1985). Interestingly, csad is also expressed in the female mammary gland. Furthermore, taurine concentration is increased in plasma, mammary gland, and milk of rats during pregnancy and early stages of lactation, and accordingly taurine has been suggested to play important roles in developing embryos and neonatal mammals (Ueki and Stipanuk 2007; Aerts and Van Assche 2002; Pasantes-Morales and Hernandez-Benitez 2010; Sturman 1993; Ito et al. 2008; Verner et al. 2007).
Taurine is present in both vertebrate oocytes and embryos where it may function as an osmolyte (Schultz et al. 1981; Dumoulin et al. 1992; Dumoulin et al. 1997; Devreker et al. 1999). Consistent with maternally provided taurine, preimplantation mouse embryos express high levels of TauT to facilitate the uptake of extracellular taurine (Van Winkle et al. 1994). Although homozygous TauT null (taut −/−) mice showed no specific embryonic lethality, a greatly decreased taurine level was observed in a variety of tissues, accompanied by primary defects including skeletal muscle deficiency and retinal degeneration, while other defects such as decreased fertility and dysfunctions of heart and kidney were also observed later in life (Heller-Stilb et al. 2002; Warskulat et al. 2004; Huang et al. 2006; Warskulat et al. 2006b; Ito et al. 2008). In short, these studies showed that TauT is essential for mouse embryos to maintain taurine homeostasis, with various organ defects emerging with long-term deficiency of taurine.
In teleost fish, TauT has also been speculated to be important in maintaining stable taurine levels, because the yolk of pelagic fish embryos contains high levels of taurine that decreases with embryonic development (Rønnestad and Fyhn 1993; Rønnestad et al. 1993). Recently, TauT has been cloned and characterized in zebrafish, and knockdown of TauT during zebrafish development only produced slightly increased apoptosis in the central nervous system at 24 h post-fertilization (hpf) with no obvious phenotypes (Kozlowski et al. 2008). These data imply that TauT may not be a key player in maintaining taurine homeostasis during zebrafish embryogenesis. In this study, we aimed at evaluating the role of Csad, the key enzyme for taurine de novo synthesis, in taurine homeostasis during zebrafish early embryogenesis initially by characterization of its evolutionarily conserved protein property and correlative expression profiles. In addition, we provide several lines of evidence indicating that Csad is responsible for the maintenance of taurine homeostasis via de novo biosynthesis pathway, and the loss of Csad function resulted in the increased embryo mortality and cardiac anomalies in early zebrafish embryos.
Materials and methods
Zebrafish strain and maintenance
The AB strain wild-type zebrafish was housed at the density of 2 fish per 3-L tank in the aquatic facility with an automatic recirculation system. The water in this facility was maintained at 28.5 °C with a light cycle of 14/10 h light/dark, and the fish were fed with live adult brine shrimp twice a day everyday to keep the zebrafish in good breeding condition (Westerfield 2000). Embryos were collected after spontaneous spawning after the removal of the divider between male and female zebrafish in the mating chamber immediately after the entering of the light cycle. After collection is completed, the embryos were allowed to develop and staged by hours or days after fertilization at 28.5 °C (Kimmel et al. 1995).
Molecular cloning and 5′-RACE
To evaluate the complete sequences of csad at 5′-end, rapid amplification of 5′ complementary DNA ends (5′-RACE) was performed using GeneRacer kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. In brief, total RNA was extracted from 50 of 24 hpf zebrafish embryos using TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol, dephosphorylated and de-capped. After the GeneRacer RNA oligo was ligated to the 5′-end of every mRNA previously protected by a 5′-cap, reverse transcription and nested polymerase chain reaction (PCR) were used to amplify the 5′-end of csad transcripts. Two PCR products were visualized in agarose gel, purified using the QIAquick Gel Extraction Kit (Qiagen, Valencia, California), subcloned using the blunt-end CloneJET™ PCR Cloning Kit (Fermentas, Vilnius, Lithuania) and sequenced (Center for Biotechnology, National Taiwan Univesrity). The sequences of gene specific primers for nested PCR are listed in Table 1.
To clone the full-length coding sequence of csad, total RNA was extracted as previously described. Reverse-transcription was done with 2 μg of total RNA with random primers and a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). To perform PCR amplification, 2 μL of cDNA, 1 μL of specific primers (10 mM) and Phusion® High-Fidelity DNA Polymerase (Finnzymes Oy, Espoo, Finland) were mixed in a total volume of 50 μL and the reactions were performed in a thermo cycler (PCT-200, MJ Research) with initial denaturation at 94 °C for 2 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 1 s, extension at 72 °C for 1 min and 45 s, and final extension at 72 °C for 7 min. After the PCR products were confirmed as a single band in the agarose gel, they were purified, subcloned, and sequenced as previously described.
To synthesize mRNA by T7 RNA polymerase with cistronic expression of green fluorescence reporter for microinjection, a complete sequence of the T7 promoter was introduced into pIRES2-eGFP (Clontech Laboratories, Palo Alto, CA, USA) by ligating the annealed oligos containing T7 promoter sequence into NheI and BglII cutting sites to generate pT7-IRES2-eGFP, and the csad coding sequence was subcloned into the pT7-IRES2-eGFP at EcoRI and SalI cutting sites to generate pT7-CSAD-IRES2-EGFP. For transfection experiments, pT7-CSAD1Δstop-FLAG-IRES2-EGFP and pT7-CSAD2Δstop-FLAG-IRES2-EGFP vectors were constructed by ligating the PCR product of the csad without stop codon into pT7-IRES2-eGFP and introducing the sequence of FLAG tag immediately at the end of csad sequence. The sequences of primers that were used to generate plasmids mentioned above are listed in Table 1.
To analyze the temporal expression profile of csad, total RNA was extracted from zebrafish embryos at 0, 3, 5, 8, 12, 16, 24, 48, and 72 hpf. A total of 50 embryos at each stage were collected and homogenized with 0.5 mL TRIzol® Reagent. After separation, precipitation, washing, and resuspension, the RNA samples were digested with DNase I (Ambion, Austin, TX, USA) to remove the contamination of genomic DNA. The extracted RNA was evaluated for quality and quantity by gel electrophoresis and absorbance at 260 and 280 nm. Reverse transcription and PCR amplification were performed as previously described with the duration of extension set to 30 s. The sequences of primer pairs are designed using Primer3 software (Rozen and Skaletsky 2000) and listed in Table 1.
Western blotting
Human embryonic kidney cell-line 293T (HEK293T) cells were seeded at 2 × 105 cells/well in 6-well plates and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY) containing 10 % fetal bovine serum (FBS; Gibco) for 24 h. HEK293T cells were then transiently transfected with pT7-CSAD1Δstop-FLAG-IRES2-EGFP, pT7-CSAD2Δstop-FLAG-IRES2-EGFP, or pT7-IRES2-EGFP plasmids using the TransIT®-LT1 Transfection Reagent (Mirus Bio Corporation, Madison, WI, USA) and incubated in FBS-free DMEM for 24 h. The secreted fraction was obtained as the culture medium in each well and concentrated using the Amicon Ultra Centrifugal 30 K filter device (Millipore Co., Billerica, MA, USA). To obtain the cytosolic fraction, cells were washed briefly with PBS, and the total protein was extracted using the RIPA buffer (Sigma Chemical Co., St. Louis, MO, USA). Bradford Reagent (Sigma Chemical Co., St. Louis, MO, USA) was used to quantify the total protein harvested from medium and cell lysates. Aliquots of 5 μg of total protein from cell lysates and 50 μg of total protein from medium samples were subjected to sodium dodecyl sulfate-12 % polyacryamide gel electrophoresis and then were transferred onto a polyvinylidene fluoride (PVDF) membrane (Perkin Elmer-Cetus, Norwalk, CT, USA). After blocking, the membranes were hybridized with a 1:2000 dilution of DDDDK tag antibody (ab21536, Abcam, Cambridge, MA, USA), and the signals were detected with a 1:5000 dilution of goat anti-rabbit antibody (Abcam, Cambridge, MA, USA) and Chemiluminescence Reagent (Immobilon Western, Millipore Co., Billerica, MA, USA).
Whole-mount in situ hybridization
To evaluate the spatial expression profiles of csad, whole -mount in situ hybridization was performed as previously described (Thisse and Thisse 2008; Liu et al. 2008). In brief, both sense and antisense digoxigenin-labeled riboprobes were synthesized by in vitro transcription from cDNAs encoding csad. Embryos were dechorionated, fixed, digested with 10 μg/mL proteinase K (Amresco, Solon, OH, USA) and re-fixed. Embryos were then pre-hybridized for 5 h without probe and then hybridized with 35 ng/200 μL probe at 70 °C overnight. After washing, hybridized embryos were blocked for 3 h at room temperature and then incubated at 4 °C overnight with Anti-Digoxigenin-AP Fab fragments (Roche Applied Science, Mannheim, Germany) diluted at 1:5000. After washing, hybridizations were detected by NBT/BCIP Stock Solution (Roche Applied Science, Mannheim, Germany) and observed under microscope (Leica Z16-APO).
Microinjection
To knockdown the expression of functional Csad, two antisense morpholino oligonucleotides (MOs) were designed against two different exon–intron splicing sites and custom made by Gene Tool LLC (Philomath, OR, USA). All MOs were resuspended in distilled water to make 2 mM stock and diluted to 1, 0.5, and 0.125 mM for the final dosage of 8, 4, and 1 ng with 0.5 % phenol red (Sigma Chemical Co., St. Louis, MO, USA) before use. To overexpress Csad, mRNAs were synthesized with the T7 mMESSAGE mMACHINE® Kit (Ambion, Austin, TX, USA) according to the manufacturer’s manual. The synthesized mRNAs were aliquoted, stored at −80 °C, and mixed with 0.5 % phenol red before use. Microinjection was performed at 1-cell stage embryos as previously described with standard control morpholino and IRES2-eGFP mRNA without the csad coding sequence as negative controls (Liu et al. 2008). In brief, glass capillaries with 1.0 mm O.D. and 0.75 mm I.D. (Sutter Instrument, Novato, CA, USA) were pulled using the P-97 Flaming/Brown type micropipette puller (Sutter Instrument, Novato, CA, USA). Embryos were injected with 0.5–1.5 nL of solutions using a IM-31 Electric Microinjector (Narishige Co., Tokyo, Japan).
After injection, embryos were cultured at 28.5 °C in egg water. For the mRNA rescue experiment, CSAD MO e8i8 and mRNA were pre-mixed and coinjected. For the taurine rescue experiment, embryos were cultured in egg water with or without the supplementation of 83 mg/L (13 mM) taurine (Sigma Chemical Co., St. Louis, MO, USA). While the previously reported supraphysiological concentrations used for taurine supplementation in zebrafish embryos ranged from 0.33 to 100 mM (Rosemberg et al. 2010b; Shao et al. 2012), the previous study showed that the taurine content in 0 hpf zebrafish embryo is about 192 pmol/embryo (~1.3 mM) (Kozlowski et al. 2008). We chose this concentration that is tenfold of physiological concentration of 0 hpf zebrafish embryos to ensure the excessive environmental taurine for 24 hpf zebrafish embryos while it is not exceeding reported ranges.
Taurine assay in zebrafish embryo by capillary electrophoresis
To evaluate the physiological role of Csad during zebrafish early embryogenesis, the concentrations of taurine in zebrafish embryos were assayed by capillary electrophoresis as previously described (Kao et al. 2010). In brief, 50 embryos from each treatment were homogenized in 200 μL of methanol at 24 hpf. 100 μL reaction mixtures (pH 9.3) containing 10 μL of embryo homogenate, NaCN (0.3 mM), naphthalene-2,3-dicarboxaldehyde (0.3 mM), and sodium tetraborate (1.0 mM) were prepared and allowed to react for 40 min at room temperature in the dark immediately before injecting into the capillary. Capillaries were rinsed with 0.5 M sodium hydroxide for 15 min, deionized water for 10 min, and finally 0.5 M Tris–borate (TB) buffer (pH 10.2) containing SDS (50 mM) for 15 min before use. Hydrodynamic injection was applied at 31 cm height for 10 s with 24 nL of injection volume. After injection, the two ends of the capillary were immersed in the 0.1 % poly(ethylene oxide) (PEO) solution prepared in 100 mM TB solution (pH 9.0) containing 30 mM SDS, and the separation was conducted at 15 kV. After each run, the capillary was equilibrated with 1.0 M TB (pH 10.2) containing 40 mM SDS for 1 min. The time for the baseline shift due to detection of PEO (neutral) was used to estimate the electroosmotic flow (EOF) values (Chang et al. 2006). The concentrations of taurine in the samples were calculated according to the differences of the peak heights before and after the spiking with 2 μM standard taurine solution.
Statistical analysis
Nonparametric statistical analyses were performed using Prism 5 software (Graphpad software, San Diego, CA, USA), and a p value less than 0.05 was considered statistically significant. Phenotypic penetrances of MOs were subjected to Kruskal–Wallis test followed by Dunn’s multiple comparisons test, and the embryonic mortality was analyzed by the log-rank test. For phenotypic rescue assay, Mann–Whitney test was performed.
Results
Csad is conserved throughout evolution
To study Csad in zebrafish, we first in silico identified zebrafish csad gene and PCR cloned a 1,449 base pair fragment containing the full open reading frame (ORF) of csad, which was confirmed by DNA sequencing. All the Csad orthologs from various species including human (NP_057073.4), rat (NP_068518.1), mouse (NP_659191.1), chicken (XP_423847.3), Xenopus (XP_002936687.1), and zebrafish (NP_001007349.1) contain a pyridoxal phosphate (PLP)-binding sequence (Asn-Pro-His-Lys, NPHK) (Tappaz et al. 1999), and a conserved enzymatic domain of DOPA decarboxylase family (Fig. 1). At the genome level, the exon structures of csad genes in various species were highly conserved (Fig. 2a). Furthermore, similarity analysis and phylogenetic tree construction using a neighbor joining method with percentage identity distances showed that zebrafish Csad is highly conservative throughout evolution (Fig. 2b, c) (Waterhouse et al. 2009).
Sequence and structural similarity of Csad among various animal species Zebrafish Csad amino acid sequences are aligned with the Csad orthologs from human, rat, mice, chicken, and Xenopus. The enzymatic DOPA decarboxylase domain is predicted, and the PLP-binding sequence is present in all Csad homologs as indicated. The identical amino acids are shown in the black box, while the less conservative regions are enclosed by white box
Zebrafish csad is highly conserved throughout evolution. a Genomic organization of csad in zebrafish, human, mouse, rat, and fugu showed almost identical pattern of exonal distribution in the regions coding for enzymatic domains (bracketed by asterisk). b Zebrafish Csad (NP_001007349.1) shares 81–82 % similarity with Xenopus (ENSXETT00000027732), chicken (XP_423847.3), mice (NP_659191.1), rat (NP_068518.1), and human (NP_057073.4). c Phylogenetic analysis based on Csad protein sequence among Marinobacter (YP_005431365.1), sea urchin (XP_780979.2), zebrafish, Xenopus, chicken, mice, rat, and human using neighbor joining method with percentage identity distances with the software Jalview2
Zebrafish Csad is not a secretory protein
Although the csad genes contain a highly conservative enzymatic domain, the 5′ ends of the genes are more variable among species (Fig. 1). Interestingly, the signal peptides for the secretory pathway are predicted (data not shown) in both human and Xenopus Csad with SignalP 4.0 Server (Bendtsen et al. 2004). To obtain the full sequence of the csad transcript, 5′-RACE was performed with total RNA extracted from 24 hpf embryos, and two transcripts were detected (Fig. 3a). While several in frame start codons were found in both transcripts (Fig. 3b), most of the open reading frames (ORF) were predicted without a secretory signal except one with an ambiguous result (data not shown).
Zebrafish Csad is not a secreted protein. a 5′-RACE analysis resulted in two csad transcription initiation sites and an alternative first exon. BLAT search was performed for both sequences, and both are aligned to chromosome 23 (Ensembl release 66). b The nucleotide and predicted amino acid sequences of zebrafish Csad. Two transcription initiation sites are indicated (hat symbol), and multiple in-frame start codons (asterisk) can be found in the initial nucleotide sequence. c Both csad transcripts were fused to FLAG tag sequence immediately before the stop codon and were cloned in pIRES-eGFP plasmid. The signal of FLAG-tag can only be detected in the cellular fraction of both csad transcript result #1 (C1) and csad transcript result #2 (C2), while neither the cellular fraction of empty plasmid (G) nor the medium fraction of all three treatments were positive in FLAG-tag signal
To resolve this ambiguous result, both transcripts were cloned with the 3′-end fused to a FLAG-tag sequence and transiently expressed in HEK293T cells. Protein samples were harvested from cell lysates and medium and analyzed by western blot using anti-FLAG tag antibody. The CSAD-FLAG fusion proteins generated from both transcripts were detected only in the cell lysate (Fig. 3c) clearly indicating that zebrafish Csad is not a secretory protein.
Csad is expressed maternally and shows specific zygotic expression pattern during zebrafish embryogenesis
To identify the developmental stages in which the csad mRNA is expressed, semi-quantitative RT-PCR was performed with β-actin as the loading control. The csad expression was detected as early as 0 hpf (Fig. 4a), indicating that maternally deposited csad mRNA is present. Detected csad PCR product indicated that its expression levels fluctuated between 5 and 24 hpf and then was maintained until 72 hpf (Fig. 4a).
Temporal and spatial expression patterns of the csad through zebrafish early embryonic stages a RT-PCR analysis using cDNAs generated from embryos at 0, 3, 5, 8, 12, 16, 24, 48, and 72 hpf showing that csad mRNA is present as maternal message and persists through embryogenesis. The β-actin was served as an internal control. Whole-mount in situ hybridization of csad antisense riboprobes was applied to various stages of embryos. csad was ubiquitously expressed in blastomeres at 1-cell stage (b) and 3 hpf (c) and confined to a subpopulation of involuting cells in gastrulating embryos (6 hpf) (d). At 12 hpf, csad transcripts were located in the yolk syncytial layer and notochord (e). At 24 hpf, csad mRNA can be observed in the lens, retina, brain, pronephric duct, yolk syncytial layer, and intersomitic regions (f, g). By 48 hpf, csad mRNA can also be detected in the head mesenchyme, tectum wall, liver (arrows in i and k), pectoral fin bud (arrowheads), and otic vesicles (concaved arrows) (h, i). By 72 hpf, csad transcripts were also observed in the proximal straight tubule of the kidney (arrowheads), and pancreas (concaved arrow) (j, k). ov otic vesicle, fb pectoral fin bud, li liver, pa pancreas, ki kidney
Next, we performed whole-mount in situ hybridization to detect the spatial expression patterns of zebrafish csad mRNA during embryogenesis. In accordance with the RT-PCR, csad was detected ubiquitously in the blastomeres at 1-cell stage (Fig. 4b), 3 hpf (Fig. 4c) until 5 hpf (data not shown), whereas the expression pattern was confined to a sub-population of gastrulating cells with a noticeably decreased level at 6 hpf (Fig. 4d). At the segmentation stage (12 hpf), csad was detected in the yolk syncytial layer and notochord (Fig. 4e). At 24 hpf, csad mRNA was detected in the yolk syncytial layer at the yolk extension, lens, retina, brain, pronephric duct, trunk, and tail (Fig. 4f, g). By 48 hpf, csad transcripts were also observed in the head mesenchyme, midbrain-hindbrain boundary, tectum wall, otic vesicles, liver, and pectoral fin bud (Fig. 4h, i). At 72 hpf, csad expression was detected in the proximal straight tubule of the kidney, liver, and pancreas (Fig. 4j, k). Collectively, these expression pattern profiles indicated that csad mRNA is expressed throughout zebrafish embryogenesis. In accordance with the physiological roles of taurine in adult tissues as a neurotransmitter, osmolyte amino acid, and bile acid conjugate, csad mRNA is present in neural tissues, kidney, and accessory digestive glands such as liver and pancreas during their early development.
Csad mRNA is perturbed by two morpholino oligos
To investigate the physiological roles of Csad in zebrafish embryogenesis, two intron–exon boundary targeted antisense morpholino oligos (MOs), MO i2e3 and MO e8i8, were designed and tested to knockdown the protein levels of Csad (Fig. 5a). To confirm the perturbation of mRNA splicing, RT-PCR using primer sets that flank exon 2–5 and exon 6–9 showed that both MOs could effectively disrupt the splicing of csad mRNA (Fig. 5b). Cloning and sequencing of the mis-spliced RT-PCR products showed that MO i2e3 injection led to the retention of intron 2 in the mature mRNA which contains an in-frame stop codon and therefore gave rise to a nonsense protein product (Fig. 5c), whereas MO e8i8 injection led to the skipping of the entire exon8 and gave rise to a missense protein product because of frameshift (Fig. 5d).
Csad MOs perturb gene specific mRNA maturation. a Two morpholino oligos, MO i2e3 and MO e8i8, were designed against the boundary of intron2-exon3 and exon8-intron8 of csad, respectively. Primer sets for RT-PCR were performed to examine the effects of MO i2e3 (concaved arrows) and MO e8i8 (arrows). b Gel electrophoresis showed that MO i2e3 resulted in a larger PCR product (concaved arrow) compared to wild-type embryos (MO), while MO e8i8 resulted in a smaller PCR product (arrow). c, d Cloning and sequencing of the PCR products indicated that MO i2e3 perturbs the mRNA maturation by retaining intron 2 which introducing an early stop codon and resulted in a truncated protein (c), while MO e8i8 disrupts the mRNA maturation by skipping exon 8 which resulted in a frameship mutation of the protein product (d)
Taurine homeostasis requires de novo synthesis via Csad in zebrafish embryos
Since Csad is the rate-limiting enzyme in taurine de novo biosynthesis, we decided to evaluate the taurine levels of Csad manipulated embryos by capillary electrophoresis. The taurine content in 24 hpf wild-type embryos was 235.2 pmol/embryo, which is similar to 244.3 pmol/embryo in the control-MO morphants (Fig. 6a). In accordance with the mRNA perturbation, the levels of taurine in both MO i2e3 (4 ng) and MO e8i8 (4 ng) injected embryos were decreased to 72.4 and 76.9 pmol/embryo, respectively (Fig. 6a), which comprised a reduction of total taurine levels by over 67 %. To further demonstrate that csad is the key gene for taurine de novo biosynthesis, in vitro synthesized csad mRNA was injected into zebrafish embryos and the taurine level in such embryos at 24 hpf increased to 642.4 pmol/embryo (Fig. 6a). Moreover, the decrease of the embryonic taurine level responded to CSAD MO i2e3 in a dose-dependent manner (Fig. 6b).
Csad controls taurine level in the zebrafish embryos. a The micellar electrokinetic chromatography showed that taurine levels in both Csad morphants (MO i2e3, MO e8i8) were discernibly diminished compared to wild-type embryos and control morpholino oligos treated embryos (Ctrl MO), while csad mRNA can apparently increase taurine level in 24 hpf zebrafish embryos. b The embryonic taurine level decreased upon the increase of the dosage of CSAD MO i2e3 from 1 to 4 ng and 8 ng
Taurine deficiency results in premature embryo death and cardiac defects
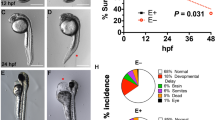
Both MO i2e3 and MO e8i8-injected embryos showed cardiac phenotypes including pericardial edema and cardiac malformation after 48 hpf (Fig. 7a–c). The mortality of 4 ng of the CSAD MO i2e3 morphants were significantly increased to 16.0 ± 1.97, 20.9 ± 2.20, and 21.2 ± 2.21 % at 24, 48, and 72 hpf (n = 344), respectively, compared to the control-MO morphants (3.8 ± 1.17, 5.6 ± 1.47, and 5.6 ± 1.47 %, n = 266) (Fig. 7d). While the mortality of 4 ng of CSAD MO e8i8 morphants (5.0 ± 1.22, 7.5 ± 1.47, and 7.5 ± 1.47 %, n = 321) were comparable to that of the control-MO morphants (Fig. 7d), increasing the dosage of the MO e8i8 to 8 ng discernibly increased the mortality to 50.2 ± 3.50, 94.6 ± 1.58, and 95.1 ± 1.51 % at 24, 48, and 72 hpf, respectively (n = 205) (Fig. 7g). The penetrances of cardiac phenotypes were significantly increased in CSAD MO i2e3 morphants (20.3 ± 2.17 % at 48 hpf and 15.1 ± 1.94 % at 72 hpf, n = 344) and MO e8i8 morphants (14.0 ± 1.94 % at 48 hpf and 13.7 ± 1.92 % at 72 hpf, n = 321) compared to the wild type embryos (0.2 ± 0.22 % at 48 hpf and 1.5 ± 0.57 % at 72 hpf, n = 461) and control-MO morphants (1.1 ± 0.65 % at 48 hpf and 1.5 ± 0.75 % at 72 hpf, n = 266) (Fig. 7e). Furthermore, the overall penetrance of gross phenotypes was significantly increased with the increase of the dosage from 1 and 4 ng to 8 ng in CSAD MO i2e3 (Fig. 7f) and CSAD MO e8i8 (Fig. 7g) morphants (Supplementary material).
Loss of Csad resulted in cardiac anomalies and increased embryonic mortality. a–c Cardiac anomalies including pericardial edema (concaved arrows in b and c) and cardiac tube malformation (arrow heads in b and c) were observed in 48 hpf embryos injected with 4 ng of MO i2e3 (b) or MO e8i8 (c), compared to the normal heart developed in the control morpholino (Ctrl MO) injected embryos (a). Gross phenotypes of Csad knockdown include elevated embryonic mortality (d) and cardiac anomalies (e). Note that the mortality was significantly increased with the injection of 4 ng of MO i2e3 when compared to control morpholino (Ctrl MO), while the penetrances of cardiac phenotypes were significantly increased in both 4 ng of MO i2e3 and MO e8i8 morphants. Both MO i2e3 (f) and e8i8 (g) caused the gross phenotypes in dose-dependent manner. The statistical significance was tested by log-rank test in d and by Kruskal–Wallis test followed by Dunn’s multiple comparisons test in e–g. ***p < 0.001; **p < 0.01; *p < 0.05
To further confirm that the pericardial edema observed in Csad morphants was due to the loss of Csad activity, eGFP or csad mRNA (75 pg) was coinjected with CSAD MO e8i8 into 1-cell stage zebrafish embryos. The penetrances of cardiac phenotypes were significantly decreased with csad mRNA coinjection (8.5 ± 2.60 %, n = 117) compared to eGFP mRNA coinjection (28.1 ± 4.10 %, n = 121, p < 0.001, Mann–Whitney test) (Fig. 8a).
The rescue of the cardiac phenotype in Csad morphants. a The penetrances of cardiac phenotypes in MO e8i8 morphants were significantly reduced by coinjecting 75 pg of csad mRNA compared to GFP mRNA coinjection as control. b The penetrances of cardiac phenotypes in Csad morphants were reduced by 13 mM of taurine supplementation (+taurine) in embryo buffer. The statistical significance was tested by Mann–Whitney test. ***p < 0.001
Since Csad is the key enzyme for taurine de novo biosynthesis and embryonic taurine levels discernibly decreased in Csad morphants, it is reasonable to hypothesize that the cardiac phenotypes in Csad morphants were due to the deficiency of taurine. Therefore, we tested if taurine supplementation to the embryo buffer can rescue the cardiac phenotypes. In accordance with this hypothesis, both pericardial edema and the looping perturbation of the cardiac tube at 48 hpf Csad morphants could be partially alleviated by incubating embryos in 13 mM taurine supplemented buffer (MO i2e3: 5.9 ± 2.18 %, n = 118 and MO e8i8: 6.9 ± 2.52 %, n = 102) compared to non-supplemented Csad morphants (MO i2e3: 20.4 ± 2.17 %, n = 344 and MO e8i8: 14.0 ± 1.94 %, n = 321) (Fig. 8b) and this trend remained in the 72 hpf morphants (data not shown). The pericardial edema in wild type embryos incubated with (0 and 0 %, respectively at 48 and 72 hpf, n = 110) or without (0.3 ± 0.29 and 1.5 ± 0.57 %, respectively at 48 and 72 hpf, n = 461) taurine supplementation remained at extremely low levels (Fig. 8b). We concluded that taurine supplementation can partially rescue the phenotypes caused by Csad knockdown, and these results indicated that the cardiac phenotypes in Csad morphants were largely resulted from taurine deficiency.
Discussion
In this study, we characterized csad molecularly and functionally in zebrafish embryos. The protein sequence of Csad contains a highly conserved domain of the DOPA decarboxylase family that is critical for its enzymatic activity, while the N-terminus of the protein contains a more variable region compared to Csad sequences of other vertebrate animals. 5′-RACE experiments showed two potential transcription initiation sites and several putative in-frame start codons. While the biological meaning of two alternative csad transcripts remains to be elucidated, our data indicated that both csad transcripts generate only cytosolic proteins (Fig. 3), and therefore de novo synthesis of hypotaurine via the Csad pathway is achieved intracellularly in zebrafish embryos. Although how hypotaurine is converted into taurine remains elusive and controversial, it is consistent to the general observation that taurine concentration is higher intracellularly than extracellularly (Huxtable 1992).
It has been suggested that embryonic taurine homeostasis mainly depends on the transfer from the free amino acid pool in the yolk with de novo fetal synthesis being inadequate (Pinto et al. 2012; Brown et al. 2011; Sturman 1993; Hibbard et al. 1990). Accordingly, a previous study showed that TauT are maternally present in zebrafish embryos at 1–4 cell stages with maternal taurine at 192 pmol/embryo, suggesting that yolk-stored taurine plays a role in taurine homeostasis during zebrafish embryogenesis. However, the TauT knockdown experiment has no distinct phenotype (Kozlowski et al. 2008). Our study here showed that csad is maternally and zygotically expressed throughout developmental stages, suggesting that de novo synthesis of taurine also participates in taurine homeostasis during zebrafish embryogenesis (Fig. 4). Furthermore, our results clearly showed decreased taurine levels (by over 67 %) after Csad, the rate-limiting enzyme for taurine biosynthesis, was knocked down, while the taurine level was discernibly increased in embryos injected with csad mRNA. These results indicate that the csad gene cloned in this study plays a key role in taurine de novo biosynthesis and is critical for taurine homeostasis during zebrafish embryogenesis (Fig. 6). An early study indicated that 65 % of sulfur sulfate administered into 24 h chick embryos was recovered as taurine (Machlin et al. 1955). Since zebrafish early embryos contain two csad transcripts with multiple in-frame start codons as our results showed, we were not able to perform the knockdown experiment in the translational blocking manner, and hence the initial synthesis of taurine via maternal csad potentially remained functional in our experiments. Therefore, it is reasonable to speculate that the biosynthesis via Csad contributes more to taurine homeostasis than what we detected.
It is generally believed that the taurine is metabolically inert and is excreted from the body predominantly via kidney or as conjugates of bile acids in adult mammals. Our results showed a noticeable decline of taurine level in 24 hpf Csad morphants before the organogenesis of kidney or digestive organs, suggesting the possibility that taurine could be further metabolized in zebrafish embryos. Although not commonly seen in vertebrates, one possible fate of taurine is to be converted into isethionic acid (Huxtable 1992; Peck and Awapara 1967). An early study showed that only small amounts of taurocholic acid formed in chick embryos (Machlin et al. 1955). Another possible fate of taurine is the formation of various taurine conjugates, such as glutamyltaurine (Huxtable 1992). Although the exact fate of taurine remains elusive, our data indicated that taurine is depleted and needs to be replenished during early zebrafish embryogenesis. According to this and other studies on zebrafish and chickens (Machlin et al. 1955), it is reasonable to speculate that the taurine homeostasis during oviparous embryogenesis requires de novo biosynthesis.
The results from our laboratory and those of others showed that both csad and TauT were maternally expressed suggesting that taurine is important during early zebrafish embryogenesis (Kozlowski et al. 2008). Our results showed increased embryonic mortality when taurine level decreased because of Csad knockdown, and this result is in accordance with the general understanding that sufficient taurine level is important for embryo survival. Although the exact molecular mechanisms are yet to be elucidated, previous studies in diet-restricted cats and TauT-deficient mice showed that fetal taurine deficiency causes abnormalities in renal, retinal, cerebral, and cerebellar development, and that chronic taurine deficiency is related to age-dependent visual and auditory disorders and an increased susceptibility to hepatitis as well as liver fibrosis (Sturman 1993; Han et al. 2000; Heller-Stilb et al. 2002; Warskulat et al. 2006b, 2007). In accordance, our results showed that csad mRNA was detected in the developing pronephros, lens, retina, and brain at 24 hpf and also in liver and otic vesicle at 48 hpf. The punctate expression pattern in the brain of 24 hpf zebrafish embryos suggested only a sub-population of cells in the brain synthesize taurine via the Csad pathway, and this result is in consistent with a recent study that human astrocytes are capable of synthesizing taurine via the Csad pathway, while neuronal cells tend to synthesize taurine via the 2-aminoethanethiol dioxygenase pathway (Vitvitsky et al. 2011). Our results showed that csad mRNA was also detected in pancreas in 72 hpf zebrafish embryos, and interestingly, several lines of evidence showed that fetal taurine plays a role in the development of the endocrine pancreas (Lee et al. 2011; Franconi et al. 2004; Sturman 1993). Collectively, csad may become an ideal target to study the biological activities of taurine with zebrafish as the model animal.
Although the penetrance was not high, the cardiac phenotype was consistently observed in 48 and 72 hpf zebrafish Csad morphants. Both csad mRNA coinjection and taurine supplementation in embryo water successfully rescued this phenotype, indicating that cardiac malformation is caused by the lack of taurine due to Csad knockdown by the microinjection of morpholino oligos. It has been reported that chronic taurine deficiency due to the lack of TauT in mice increases the susceptibility of heart failure and cardiomyopathy, which might involve in the role of taurine in osmoregulation (Ito et al. 2008; Warskulat et al. 2006a). Accordingly, it has been reported that perturbation of osmoregulation contributes to the phenotype of cardiac edema (Hill et al. 2004), which is one of the major cardiac phenotypes we observed. Alternatively, taurine might also play a role in cardiac development via its role in oxidative stress, intracellular calcium homeostasis, and endoplasmic reticulum stress (ER stress), as these are also important factors for cardiomyopathy (Schaffer et al. 2010; Papp et al. 2008; Ceylan-Isik et al. 2011; Das et al. 2011; Huxtable 1993). There might be some existing redundant systems that could compensate for the loss of taurine. For example, organic osmolytes such as glycine, glutamate, and aspartate might compensate for the loss of taurine to control osmoregulation, and antioxidant reagents such as glutathione, ascorbic acid, polyphenols, and super oxide dismutase may alleviate the effects caused by the deficiency of taurine. This may explain the low phenotypic penetrances of Csad morphants in our results.
In conclusion, we have cloned a zebrafish csad gene and demonstrated that there are two transcripts of this gene during zebrafish early embryogenesis. Zebrafish Csad protein is predominantly a cytosolic protein. Csad mRNA transcript is expressed maternally and later in various embryonic tissues, including yolk syncytial layer, notochord, pronephric duct, brain, otic vesicle, pectoral fin bud, liver, and pancreas. Knockdown of zebrafish Csad discernibly reduced taurine levels in embryos and increased early mortality and induced pericardial edema, suggesting that normal taurine level is required for embryo survival and cardiac development. Furthermore, a rescue experiments done by taurine supplementation indicated that the phenotypes in Csad morphants are likely due to taurine deficiency. Together these findings provide robust evidence that taurine de novo synthesis via the Csad pathway plays indispensable roles during early embryonic development in zebrafish.
References
Aerts L, Van Assche FA (2002) Taurine and taurine-deficiency in the perinatal period. J Perinat Med 30(4):281–286
Albrecht J, Schousboe A (2005) Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res 30(12):1615–1621
Barnard JA, Thaxter S, Kikuchi K, Ghishan FK (1988) Taurine transport by rat intestine. Am J Physiol 254(3 Pt 1):G334–G338
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340(4):783–795
Brown LD, Green AS, Limesand SW, Rozance PJ (2011) Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed) 3:428–444
Ceylan-Isik AF, Sreejayan N, Ren J (2011) Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol 50(1):107–116
Chang L, Xu J, Yu F, Zhao J, Tang X, Tang C (2004) Taurine protected myocardial mitochondria injury induced by hyperhomocysteinemia in rats. Amino Acids 27(1):37–48
Chang PL, Chiu TC, Chang HT (2006) Stacking, derivatization, and separation by capillary electrophoresis of amino acids from cerebrospinal fluids. Electrophoresis 27(10):1922–1931
Chen Y, Liu CP, Xu KF, Mao XD, Lu YB, Fang L, Yang JW, Liu C (2008) Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am J Nephrol 28(6):1014–1022
Chow SC, Ching LY, Wong AM, Wong CK (2009) Cloning and regulation of expression of the Na + -Cl–taurine transporter in gill cells of freshwater Japanese eels. J Exp Biol 212(Pt 20):3205–3210
Das J, Ghosh J, Manna P, Sil PC (2011) Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem Pharmacol 81(7):891–909
de la Rosa J, Stipanuk MH (1985) Evidence for a rate-limiting role of cysteinesulfinate decarboxylase activity in taurine biosynthesis in vivo. Comp Biochem Physiol B 81(3):565–571
Devreker F, Van den Bergh M, Biramane J, Winston RL, Englert Y, Hardy K (1999) Effects of taurine on human embryo development in vitro. Hum Reprod 14(9):2350–2356
Dominy JE Jr, Simmons CR, Hirschberger LL, Hwang J, Coloso RM, Stipanuk MH (2007) Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J Biol Chem 282(35):25189–25198
Dumoulin JC, Evers JL, Bakker JA, Bras M, Pieters MH, Geraedts JP (1992) Temporal effects of taurine on mouse preimplantation development in vitro. Hum Reprod 7(3):403–407
Dumoulin JC, van Wissen LC, Menheere PP, Michiels AH, Geraedts JP, Evers JL (1997) Taurine acts as an osmolyte in human and mouse oocytes and embryos. Biol Reprod 56(3):739–744
Franconi F, Di Leo MA, Bennardini F, Ghirlanda G (2004) Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem Res 29(1):143–150
Ghosh J, Das J, Manna P, Sil PC (2009) Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol Appl Pharmacol 240(1):73–87
Han X, Budreau AM, Chesney RW (2000) The taurine transporter gene and its role in renal development. Amino Acids 19(3–4):499–507
Haskew-Layton RE, Rudkouskaya A, Jin Y, Feustel PJ, Kimelberg HK, Mongin AA (2008) Two distinct modes of hypoosmotic medium-induced release of excitatory amino acids and taurine in the rat brain in vivo. PLoS ONE 3(10):e3543
Heinamaki AA (1988) Endogenous synthesis of taurine and GABA in rat ocular tissues. Acta Chem Scand B 42(1):39–42
Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Warskulat U, Haussinger D (2002) Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J 16(2):231–233
Hibbard JU, Pridjian G, Whitington PF, Moawad AH (1990) Taurine transport in the in vitro perfused human placenta. Pediatr Res 27(1):80–84
Hill AJ, Bello SM, Prasch AL, Peterson RE, Heideman W (2004) Water permeability and TCDD-induced edema in zebrafish early-life stages. Toxicol Sci 78(1):78–87
Huang DY, Boini KM, Lang PA, Grahammer F, Duszenko M, Heller-Stilb B, Warskulat U, Haussinger D, Lang F, Vallon V (2006) Impaired ability to increase water excretion in mice lacking the taurine transporter gene TAUT. Pflugers Arch 451(5):668–677
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72(1):101–163
Huxtable RJ (1993) Taurine and the heart. Cardiovasc Res 27(6):1136–1137
Ide T, Kushiro M, Takahashi Y, Shinohara K, Cha S (2002) mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism 51(9):1191–1197
Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, Ueki K, Yoshiyama M, Ikawa M, Okabe M, Schaffer SW, Fujio Y, Azuma J (2008) Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol 44(5):927–937
Jacobsen JG, Smith LH Jr (1963) Comparison of decarboxylation of cysteine sulphinic acid-1-14c and cysteic acid-1-14c by human, dog, and rat liver and brain. Nature 200:575–577
Jacobsen JG, Smith LH (1968) Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 48(2):424–511
Jammoul F, Degardin J, Pain D, Gondouin P, Simonutti M, Dubus E, Caplette R, Fouquet S, Craft CM, Sahel JA, Picaud S (2010) Taurine deficiency damages photoreceptors and retinal ganglion cells in vigabatrin-treated neonatal rats. Mol Cell Neurosci 43(4):414–421
Kao YY, Liu KT, Huang MF, Chiu TC, Chang HT (2010) Analysis of amino acids and biogenic amines in breast cancer cells by capillary electrophoresis using polymer solutions containing sodium dodecyl sulfate. J Chromatogr A 1217(4):582–587
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203(3):253–310
Knopf K, Sturman JA, Armstrong M, Hayes KC (1978) Taurine: an essential nutrient for the cat. J Nutr 108(5):773–778
Kozlowski DJ, Chen Z, Zhuang L, Fei YJ, Navarre S, Ganapathy V (2008) Molecular characterization and expression pattern of taurine transporter in zebrafish during embryogenesis. Life Sci 82(19–20):1004–1011
Lambert IH (2004) Regulation of the cellular content of the organic osmolyte taurine in mammalian cells. Neurochem Res 29(1):27–63
Lee YY, Lee HJ, Lee SS, Koh JS, Jin CJ, Park SH, Yi KH, Park KS, Lee HK (2011) Taurine supplementation restored the changes in pancreatic islet mitochondria in the fetal protein-malnourished rat. Br J Nutr 106(8):1198–1206
Liu IH, Zhang C, Kim MJ, Cole GJ (2008) Retina development in zebrafish requires the heparan sulfate proteoglycan agrin. Dev Neurobiol 68(7):877–898
Machlin LJ, Pearson PB, Denton CA (1955) The utilization of sulfate sulfur for the synthesis of taurine in the developing chick embryo. J Biol Chem 212(1):469–475
Men X, Han S, Gao J, Cao G, Zhang L, Yu H, Lu H, Pu J (2010) Taurine protects against lung damage following limb ischemia reperfusion in the rat by attenuating endoplasmic reticulum stress-induced apoptosis. Acta Orthop 81(2):263–267
Miyamoto Y, Tiruppathi C, Ganapathy V, Leibach FH (1989) Active transport of taurine in rabbit jejunal brush-border membrane vesicles. Am J Physiol 257(1 Pt 1):G65–G72
Nonaka H, Tsujino T, Watari Y, Emoto N, Yokoyama M (2001) Taurine prevents the decrease in expression and secretion of extracellular superoxide dismutase induced by homocysteine: amelioration of homocysteine-induced endoplasmic reticulum stress by taurine. Circulation 104(10):1165–1170
Pan C, Giraldo GS, Prentice H, Wu JY (2010) Taurine protection of PC12 cells against endoplasmic reticulum stress induced by oxidative stress. J Biomed Sci 17(Suppl 1):S17
Papp S, Zhang X, Szabo E, Michalak M, Opas M (2008) Expression of endoplasmic reticulum chaperones in cardiac development. Open Cardiovasc Med J 2:31–35
Park T, Jerkins AA, Steele RD, Rogers QR, Morris JG (1991) Effect of dietary protein and taurine on enzyme activities involved in cysteine metabolism in cat tissues. J Nutr 121(11 Suppl):S181–S182
Pasantes-Morales H, Hernandez-Benitez R (2010) Taurine and brain development: trophic or cytoprotective actions? Neurochem Res 35(12):1939–1943
Peck EJ Jr, Awapara J (1967) Formation of taurine and isethionic acid in rat brain. Biochim Biophys Acta 141(3):499–506
Pinto W, Ronnestad I, Jordal AE, Gomes AS, Dinis MT, Aragao C (2012) Cloning, tissue and ontogenetic expression of the taurine transporter in the flatfish Senegalese sole (Solea senegalensis). Amino Acids 42(4):1317–1327
Rønnestad I, Fyhn HJ (1993) Metabolic aspects of free amino acids in developing marine fish eggs and larvae. Rev Fish Sci 1(3):239–259
Rønnestad I, Groot EP, Fyhn HJ (1993) Compartmental distribution of free amino acids and protein in developing yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus). Mar Biol 116(3):349–354
Rosemberg DB, da Rocha RF, Rico EP, Zanotto-Filho A, Dias RD, Bogo MR, Bonan CD, Moreira JC, Klamt F, Souza DO (2010a) Taurine prevents enhancement of acetylcholinesterase activity induced by acute ethanol exposure and decreases the level of markers of oxidative stress in zebrafish brain. Neuroscience 171(3):683–692
Rosemberg DB, Kist LW, Etchart RJ, Rico EP, Langoni AS, Dias RD, Bogo MR, Bonan CD, Souza DO (2010b) Evidence that acute taurine treatment alters extracellular AMP hydrolysis and adenosine deaminase activity in zebrafish brain membranes. Neurosci Lett 481(2):105–109
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Schaffer S, Solodushko V, Pastukh V, Ricci C, Azuma J (2003) Possible cause of taurine-deficient cardiomyopathy: potentiation of angiotensin II action. J Cardiovasc Pharmacol 41(5):751–759
Schaffer SW, Azuma J, Mozaffari M (2009) Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 87(2):91–99
Schaffer SW, Jong CJ, Ramila KC, Azuma J (2010) Physiological roles of taurine in heart and muscle. J Biomed Sci 17(Suppl 1):S2
Schultz GA, Kaye PL, McKay DJ, Johnson MH (1981) Endogenous amino acid pool sizes in mouse eggs and preimplantation embryos. J Reprod Fertil 61(2):387–393
Shao X, Hu Z, Hu C, Bu Q, Yan G, Deng P, Lv L, Wu D, Deng Y, Zhao J, Zhu R, Li Y, Li H, Xu Y, Yang H, Zhao Y, Cen X (2012) Taurine protects methamphetamine-induced developmental angiogenesis defect through antioxidant mechanism. Toxicol Appl Pharmacol 260(3):260–270
Shennan DB (2008) Swelling-induced taurine transport: relationship with chloride channels, anion-exchangers and other swelling-activated transport pathways. Cell Physiol Biochem 21(1–3):15–28
Shennan DB, McNeillie SA, Curran DE (1993) Stimulation of taurine efflux from human placental tissue by a hypoosmotic challenge. Exp Physiol 78(6):843–846
Shennan DB, Thomson J, Gow IF (2006) Osmoregulation of taurine efflux from cultured human breast cancer cells: comparison with volume activated Cl-efflux and regulation by extracellular ATP. Cell Physiol Biochem 18(1–3):113–122
Sturman JA (1993) Taurine in development. Physiol Rev 73(1):119–147
Suzuki T, Wada T, Saigo K, Watanabe K (2002) Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J 21(23):6581–6589
Tappaz M, Reymond I, Bitoun M, Sergeant A (1998) Cysteine sulfinate decarboxylase (CSD): molecular cloning, sequence and genomic expression in brain. Adv Exp Med Biol 442:25–32
Tappaz M, Bitoun M, Reymond I, Sergeant A (1999) Characterization of the cDNA coding for rat brain cysteine sulfinate decarboxylase: brain and liver enzymes are identical proteins encoded by two distinct mRNAs. J Neurochem 73(3):903–912
Thisse C, Thisse B (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3(1):59–69
Ueki I, Stipanuk MH (2007) Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J Nutr 137(8):1887–1894
Van Winkle LJ, Patel M, Wasserlauf HG, Dickinson HR, Campione AL (1994) Osmotic regulation of taurine transport via system beta and novel processes in mouse preimplantation conceptuses. Biochim Biophys Acta 1191(2):244–255
Verner A, Craig S, McGuire W (2007) Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst Rev 4:CD006072
Vitvitsky V, Garg SK, Banerjee R (2011) Taurine biosynthesis by neurons and astrocytes. J Biol Chem 286(37):32002–32010
Warskulat U, Flogel U, Jacoby C, Hartwig HG, Thewissen M, Merx MW, Molojavyi A, Heller-Stilb B, Schrader J, Haussinger D (2004) Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J 18(3):577–579
Warskulat U, Andree B, Lusebrink J, Kohrer K, Haussinger D (2006a) Switch from actin alpha1 to alpha2 expression and upregulation of biomarkers for pressure overload and cardiac hypertrophy in taurine-deficient mouse heart. Biol Chem 387(10–11):1449–1454
Warskulat U, Borsch E, Reinehr R, Heller-Stilb B, Monnighoff I, Buchczyk D, Donner M, Flogel U, Kappert G, Soboll S, Beer S, Pfeffer K, Marschall HU, Gabrielsen M, Amiry-Moghaddam M, Ottersen OP, Dienes HP, Haussinger D (2006b) Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J 20(3):574–576
Warskulat U, Heller-Stilb B, Oermann E, Zilles K, Haas H, Lang F, Haussinger D (2007) Phenotype of the taurine transporter knockout mouse. Methods Enzymol 428:439–458
Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25(9):1189–1191
Westerfield M (2000) The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio). M. Westerfield, Eugene
Worden JA, Stipanuk MH (1985) A comparison by species, age and sex of cysteinesulfinate decarboxylase activity and taurine concentration in liver and brain of animals. Comp Biochem Physiol B 82(2):233–239
Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M (2010) Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci 17(Suppl 1):S6
Yasuda S, Liu CC, Takahashi S, Suiko M, Chen L, Snow R, Liu MC (2005) Identification of a novel estrogen-sulfating cytosolic SULT from zebrafish: molecular cloning, expression, characterization, and ontogeny study. Biochem Biophys Res Commun 330(1):219–225
Acknowledgments
The authors would like to thank Dr. Gregory J. Cole, Dr. Shu-Yu Wu, and Dr. Harry J. Mersmann for the reviewing and revising of this article. We would also like to acknowledge Dr. Huan-Tsung Chang for his technical support in capillary electrophoresis. This study was supported by the National Science Council in Taiwan (Grant No. NSC99-2313-B-002-030-MY3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Y.-C. Chang and S.-T. Ding contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, YC., Ding, ST., Lee, YH. et al. Taurine homeostasis requires de novo synthesis via cysteine sulfinic acid decarboxylase during zebrafish early embryogenesis. Amino Acids 44, 615–629 (2013). https://doi.org/10.1007/s00726-012-1386-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1386-8