Abstract
In this work, we describe the asymmetric synthesis of a series of fluorinated and non-fluorinated quaternary α-amino acid derivatives. This methodology involves the diastereoselective addition of chiral 2-p-tolylsulfinyl benzylcarbanions to either imines containing a 2-furyl moiety or trifluoromethyl α-imino esters. Synthetic practicality of this method is demonstrated by short (two-steps) and convenient preparation of 2-(trifluoromethyl)indoline-2-carboxylates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-Amino acids play a central role in biochemistry and are essential for life. Indeed, they are the building blocks of biologically relevant molecules, such as peptides and proteins, among many others.
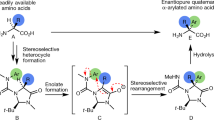
It is well known that conformational changes in the chains of peptides and proteins provoke significant alterations in their secondary and tertiary structures, seriously affecting their properties. In this sense, the synthesis and incorporation of non-natural amino acids into peptidic chains has been extensively studied (Félix 2004). Specifically, α,α-disubstituted quaternary α-amino acids (Fig. 1b) are important since they increase the stability of proteins and restrict their conformational flexibility, giving rise to peptidomimetics with higher resistance against proteases as well as increased levels of lipophilicity and bioavailability. For these reasons, the development of efficient methodologies for the asymmetric synthesis of these type of amino acids has attracted a great deal of interest in organic synthesis (Cativiela and Díaz-de-Villegas 1998, 2000; Park and Kurth 2002; Ohfune and Shinada 2005; Vogt and Bräse 2007; Nájera and Sansano 2007; Soloshonok and Sorochinsky 2010).
However, the synthesis of even more constrained amino acids bearing additional chiral centers and their incorporation into peptides have been less explored (Balaram and Ramaseshan 1991; Hruby et al. 1997; Gibson et al. 1999; Abell 1999; DeGrado 2001), particularlyα,β-dialkyl-α-amino acids (Fig. 1c) which would permit the control of the dihedral angles ϕ, ψ and ω, hence allowing for the organization of amino acid chains by controlling the torsional angles and the position of the side-chains (Soloshonok 2002). Despite their potential, there are only a few reports concerning the synthesis of these compounds in enantiomerically pure form, thus their biological studies are seriously limited (Soloshonok et al. 2001; Qiu et al. 2000).
Soloshonok et al. (2008) described the preparation of α,β-dialkyl-α-amino acids by using chiral Nickel (II) complexes, although with low diastereoisomeric ratio in most cases. In addition, several organocatalytic approaches by addition of azlactones to α,β-unsaturated aldehydes (Cabrera et al. 2008) or nitroalkenes (Alemán et al. 2008) have been described in the last 2 years.
On the other hand, the presence of fluorine atoms in α-amino acids often induces significant changes in the physical properties, biological activities and metabolic profiles of the peptides containing them (Kukhar 2009; Sorochinsky and Soloshonok 2010; Jäckel and Kocksch 2005; Qiu et al. 2004; Purser et al. 2008; Müller et al. 2007). Thus, fluorinated amino acids have recently emerged as valuable building blocks for designing hyperstable protein folds as well as for directing highly specific protein–protein interactions (Jäckelet al. 2004; Hodges and Raines 2005; Golbiket al. 2005). Moreover, some α-trifluoromethyl α-amino acids exhibit anticancer, antibacterial and antihypertensive properties and also the ability of acting as suicide inhibitors of pyridoxalphosphate-dependent enzymes (Sewald et al. 1994; Asensio et al. 2001; Jäckel and Kocksch 2005; Qiu et al. 2004; Purser et al. 2008; Müller et al. 2007).
In this context, we described the asymmetric synthesis of a particular class of α,β-dialkyl-α-amino acids a few years ago. Specifically, we developed a method for the asymmetric synthesis of cyclic β,β-difluorinated-α-amino acid derivatives bearing a quaternary stereocenter. The process relied on the chemo- and diastereoselective addition of allylic organometallic reagents to fluorinated α-imino esters followed by a ring closing metathesis reaction. We were able to achieve complete selectivity in the nucleophilic addition when (R)-phenylglycinol methyl ether was employed as chiral auxiliary (Fustero et al. 2006, 2008a, b, c).
The above-mentioned biological relevance of α,α-disubstituted α-amino acids, in particular those α,β-dialkyl-substituted, spurred our interest in the development of a new strategy for their asymmetric synthesis, as well as in fluorine-containing derivatives. This new approach would be based on the methodology previously developed by our research groups involving the reaction of 2-(p-tolylsulfinyl) benzylcarbanions with aldimines and ketimines, which took place with almost complete control of the configuration at the two adjacent stereogenic centers simultaneously created (García Ruano et al. 2003, 2005a, b, c).
We hypothesized that N-protected imines containing a 2-furyl moiety [one of the most classical ways to mask the acid functionality is the use of the furan ring (Kobayashi et al. 1997; Fustero et al. 2008a, b, c; Hasbullah and Jones 2010)] could react with benzylcarbanions stabilized by a remote p-tolylsulfinyl group in order to obtain α,α-disubstituted-α-amino acid derivatives. We would also be able to obtain α,β-dialkyl-α-amino acids by choosing the appropriate sulfinylbenzylcarbanion. In this manner, the stereochemistry at the quaternary α-amino acid stereocenter and the benzylic one could be controlled in a single step.
In this paper, we report the results obtained in the reaction of 2-p-tolylsulfinyl benzylcarbanions derived from 1 with N-p-tolylsulfinylimines and N-p-methoxyphenyl (PMP) fluorinated imines 2 bearing a 2-furyl substituent. The application of these reactions to the preparation of enantiomerically pure quaternary α-amino acid derivatives through the oxidative elaboration of the furan ring is also described (Fig. 2).
Materials and methods
NMR spectra were obtained on a Bruker 300 spectrometer, running at 300 and 75 MHz for 1H and 13C, respectively. Chemical shifts (δ) are reported in ppm relative to residual solvent signals (CHCl3, 7.26 ppm for 1H NMR, CDCl3, 77.0 ppm for 13C NMR). 13C NMR spectra were acquired on a broad-band decoupled mode. Optical rotations were measured by a Perkin–Elmer 241 polarimeter. All reactions were carried out in anhydrous solvents and under argon atmosphere. THF and Et2O were distilled from sodium-benzophenone under argon, and CH2Cl2 was distilled from P2O5. Flash column chromatography was performed using silica gel Merk-60 (230–400 mesh). n-BuLi (2.5 M solution in hexanes) was purchased from Aldrich.
Commercially available starting materials and solvents were used without further purification. Sulfoxides 1 (García Ruano et al. 2003, 2005a, b, c), imines (S)-2a (Jiang et al. 2005), (S)-2b (Leverett et al. 2006) and (S)-2d (García Ruano et al. 2008), addition product 3g (García Ruano et al. 2008) and trifluoromethyl α-imino ester 8 (Watanabe et al. 1982a, b) had previously been described.
(S)-4-Methyl N-(2,2,2-trifluoro-1-(furan-2-yl) ethylidene) benzenesulfinamide [(S)-2c]
[α] 25D = +146.3 (c 1.0, CHCl3).1H NMR (CDCl3, 300 MHz): δ 7.86 (dd, J = 0.6, 1.7 Hz, 1H), 7.76 (dd, J = 1.7, 6.6 Hz, 2H), 7.33–7.28 (m, 3H), 6.68 (dd, J = 1.9, 3.8 Hz, 1H), 2.40 (s, 3H). 13C NMR (CDCl3, 75 MHz): δ 148.9, 146.1 (q, 2 J CF = 35.7 Hz), 144.0, 142.9, 142.8, 130.0, 125.4, 121.9 (q, 4 J CF = 2.9 Hz), 118.7 (q, 1 J CF = 281.2 Hz), 113.5, 21.5. 19F NMR (CDCl3, 282.4 MHz): δ −66.94 (s, 3F). HRMS (EI+): m/z calcd for C13H10F3NO2S [M]+301.0384, found: 301.0389.
General procedure for the synthesis of addition products 3 and 9
A solution of n-BuLi (0.49 mmol, 2.5 M in hexane) was added dropwise to i-Pr2NH (0.74 mmol) in THF (2 mL) at 0°C. After stirring for 10 min, the mixture was cooled at –78°C and then a solution of sulfoxide 1 (0.41 mmol) in THF (2 mL) was added. After 10 min, the corresponding imine (2 or 8) (0.45 mmol) dissolved in THF (2 mL) was added at –78°C. When the reaction was completed (the reaction was followed by TLC), the mixture was hydrolyzed (2 mL aqueous saturated NH4Cl), extracted (3x10 mL Et2O), washed (2 × 10 mL NaCl sat), dried (MgSO4) and the solvent evaporated. The residue was purified by flash column chromatography.
N-{(1S,2S)-1-(Furan-2-yl)-2-[2-((S)-(p-tolylsulfinyl)phenyl]propyl} (S)-p-tolylsulfinamide(3a)
By means of the general procedure described above, compound 3a was obtained as yellow oil in 82% yield. [α] 20D =−37.0 (c 0.5, CH2Cl2). 1H NMR (CDCl3, 300 MHz): δ 7.98 (dd, J = 1.5, 7.6 Hz, 1H), 7.50–7.47 (m, 2H), 7.39 (dd, J = 1.3, 7.6 Hz, 1H), 7.34 (d, J = 8.1 Hz, 2H), 7.26, (d, J = 1.1 Hz, 1H), 6.93 (d, J = 8.0 Hz, 2H), 6.82 (d, J = 8.0 Hz, 2H), 6.63 (d, J = 8.1 Hz, 2H), 6.15 (dd, J = 1.9, 3.2 Hz, 1H), 5.98 (d, J = 3.2 Hz, 1H), 4.38 (dd, J = 8.9, 10.8 Hz, 1H), 3.79 (d, J = 8.8 Hz, 1H), 3.68 (dq, J = 10.9, 6.8 Hz, 1H), 2.26 (s, 3H), 2.11 (s, 3H), 0.84 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 153.7, 143.1, 142.7, 141.8, 141.7, 141.6, 141.2, 140.5, 131.9, 129.7, 128.9 m 127.6 m 127.4, 126.0, 125.9, 125.3, 110.2, 107.6, 57.3, 38.4, 21.2, 21.1, 19.4. MS (ESI+): [M + Na]+calcd for C27H27NO3S2Na 500.1324; found 500.1306.
N-{(2S,3S)-2-(Furan-2-yl)-3-[2-((S)-(p-tolylsulfinyl)phenyl]butan-2-yl}(S)-p-tolylsulfinamide (3b)
By means of the general procedure described above, compound 3b was obtained as yellow oil in 51% yield. [α] 20D = +44.8 (c 1.0, CH2Cl2). 1H NMR (CDCl3, 300 MHz): δ 7.70 (dd, J = 1.5, 7.6 Hz, 1H), 7.52 (dt, J = 1.4, 7.5 Hz, 1H), 7.45–7.39 (m, 2H), 7.32 (d, J = 7.8 Hz, 1H), 7.25 (d, J = 9.4 Hz, 2H), 7.07, (d, J = 8.5 Hz, 4H), 6.94 (d, J = 8.2 Hz, 2H), 6.30 (dd, J = 1.9, 3.3 Hz, 1H), 6.24 (d, J = 3.3 Hz, 1H), 5.99 (s, 1H), 4.09 (q, J = 7.0 Hz, 1H), 2.33 (s, 3H), 2.27 (s, 3H), 1.73 (s, 3H), 0.72 (d, J = 7.1 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 158.4, 143.9, 143.5, 143.0, 141.7, 140.8, 140.3, 131.8, 130.0, 129.6, 129.1, 128.9, 127.1, 125.7, 125.1, 110.3, 106.7, 60.0, 41.8, 21.3, 18.1, 15.9.MS (ESI+): [M + H]+calcd for C28H30NO3S2 492.1667; found 492.1653.
N-{(2S)-2-(Furan-2-yl)-1-[2-((S)-(p-tolylsulfinyl)phenyl]propan-2-yl}(S)-p-tolylsulfinamide (3c/3c′)
By means of the general procedure described above, compounds 3c/3c′ were obtained as yellow oil in 50% yield and a 60:40 mixture of diastereoisomers. Minor diastereoisomer: 1H NMR (CDCl3, 300 MHz): δ 7.79–6.88 (m, 13H), 6.38 (d, J = 1.9 Hz, 1H), 6.22 (d, J = 3.1 Hz, 1H), 4.75 (s, 1H), 4.05 (d, J = 17.2 Hz, 1H), 3.89 (d, J = 17.2 Hz, 1H), 2.39 (s, 3H), 2.36 (s, 3H), 2.11 (s, 3H). 13C NMR (CDCl3, 75 MHz): δ 155.5, 145.0, 143.0, 142.4, 142.0, 140.9, 134.7, 131.7, 131.5, 130.5, 130.0, 130.0, 128.2, 126.7, 125.8, 125.7, 110.7, 108.7, 59.0, 46.6, 29.7, 5.1, 21.3. Major diastereoisomer: 1H NMR (CDCl3, 300 MHz): δ 7.90 (d, J = 7.9 Hz, 1H), 7.55 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 8.6 Hz, 2H), 7.44 (d, J = 8.3 Hz, 2H), 7.37 (t, J = 6.9 Hz, 1H), 7.27 (d, J = 6.8 Hz, 2H), 7.20 (d, J = 8.0 Hz, 2H), 6.47 (d, J = 7.6 Hz, 1H), 6.35 (dd, J = 1.7, 3.1 Hz, 1H), 6.15 (d, J = 3.1 Hz, 1H), 4.80 (s, 1H), 3.51 (d, J = 13.6 Hz, 1H), 3.32 (d, J = 13.6 Hz, 1H), 2.39 (s, 3H), 2.33 (s, 3H), 1.88 (s, 3H); 13C NMR (CDCl3, 75 MHz): δ 155.0, 144.9, 142.9, 142.2, 141.8, 141.6, 141.3, 134.2, 131.5, 130.5, 130.0, 129.6, 128.2, 125.6, 125.5, 124.8, 110.7, 109.1, 59.5, 43.7, 24.7, 21.5, 21.4. MS (TOF ES+): [M + Na]+calcd for C27H27NO3S2Na500.1324; found 500.1320.
N-{(2R,3S)-2-(Furan-2-yl)-3-[2-((S)-p-tolylsulfinyl)phenyl]butan-2-yl}(R)-p-tolylsulfinamide (Epi-3b)
By means of the general procedure described above, compound epi -3b was obtained as yellow oil in 46% yield. [α] 20D = −13.3 (c 0.8, CH2Cl2). 1H NMR (CDCl3, 300 MHz): δ 7.51–7.47 (m, 2H), 7.44 (d, J = 8.2 Hz, 2H) 7.38 (dd, J = 1.4,7.5 Hz, 1H), 7.32 (dd, J = 1.4, 7.6 Hz, 1H), 7.26 (d, J = 8.2 Hz, 2H), 7.26 (d, J = 8.2 Hz, 2H), 7.19 (d, J = 8.6 Hz, 2H), 7.16 (d, J = 8.2 Hz, 2H), 7.03 (d, J = 7.7 Hz, 1H), 6.40 (dd, J = 1.9, 3.3 Hz, 1H), 6.37 (d, J = 3.2 Hz, 1H), 5.00 (s, 1H), 4.28 (q, J = 7.1 Hz, 1H), 2.36 (s, 3H), 2.35 (s, 3H), 1.68 (s, 3H), 1.04 (d, J = 7.1 Hz, 3H) 13C NMR (CDCl3, 75 MHz): δ 156.2, 144.2, 143.2, 142.9, 142.3, 14.9, 140.8, 140.3, 131.7, 129.7, 129.6, 129.4, 128.4, 127.8, 125.5, 125.4, 110.3, 109.5, 61.6 (2C), 22.2, 21.3 (2C), 17.3.MS (TOF ES+): [M + H]+calcd for C28H30NO3S2 492.1667; found 492.1671.
N-{(2S,3S)-2-(Furan-2-yl)-3-[2-(methylsulfinyl) phenyl]-4-benzylbutan-2-yl}(S)-p-tolylsulfinamide (3d)
By means of the general procedure described above, compound 3d was obtained as yellow oil in 60% yield. [α] 20D = −14.8 (c 1.0, CH2Cl2). 1H NMR (CDCl3, 300 MHz): δ 7.82 (d, J = 7.5 Hz, 1H), 7.58–7.43 (m, 5H), 7.22 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 8.3 Hz, 2H), 7.02, (d, J = 6.7 Hz, 2H), 6.91 (d, J = 8.1 Hz, 2H), 6.82 (d, J = 8.2 Hz, 2H), 6.65 (d, J = 7.6 Hz, 2H), 6.41 (dd, J = 1.9, 2.7 Hz, 1H), 6.34 (d, J = 3.3 Hz, 1H), 4.55 (s, 1H), 4.21 (dd, J = 2.9, 10.8 Hz, 1H), 3.05 (dd, J = 2.9, 13.3 Hz, 1H), 2.88 (dd, J = 10.9, 13.1 Hz, 1H), 2.38 (s, 3H), 2.22 (s, 3H), 1.79 (s, 3H). 13C NMR (CDCl3, 75 MHz): δ 157.9, 144.9, 142.6, 141.9, 141.9, 140.6, 140.3, 140.0, 138.7, 130.7, 129.9, 129.2, 128.8, 128.2, 127.8, 126.2, 126.2, 126.1, 125.7, 110.6, 107.6, 60.5, 50.3, 38.6, 21.5, 21.3, 19.7, 14.2. MS (ESI+): [M + Na]+calcd for C34H33NO3S2Na 590.1794; found 590.1804.
N-{(2S,3S)-2-(Furan-2-yl)-3-[2-(methylsulfinyl)phenyl]-4-allylbutan-2-yl}(S)-p-tolylsulfinamide (3e)
By means of the general procedure described above, compound 3e was obtained as yellow oil in 42% yield. [α] 20D =−23.0 (c 1.0, CH2Cl2). 1H NMR (CDCl3, 300 MHz): δ 7.78 (dd, J = 1.5, 7.6 Hz, 1H), 7.59–7.54 (m, 1H), 7.49 (dd, J = 1.4, 7.5 Hz, 1H), 7.46 (dd, J = 0.8, 1.8 Hz, 1H), 7.37(dd, J = 1.3, 7.5 Hz, 1H), 7.32 (d, J = 8.3 Hz, 2H), 7.10 (d, J = 7.9 Hz, 2H), 7.08 (d, J = 7.9 HZ, 2H), 6.95 (d, J = 8.2 Hz, 2H), 6.36 (dd, J = 1.8, 3.3 Hz, 1H), 6.30 (dd, J = 0.8, 3.3 Hz, 1H), 60.70 (s, 1H), 4.57–4.34 (m, 4H), 4.11–4.06 (m 2H), 2.37 (s, 3H), 2.30 (s, 3H), 1.75 (s, 3H). 13C NMR (CDCl3, 75 MHz): δ 158.3, 144.3, 142.9, 141.7, 141.4, 140.3, 134.7, 131.5, 129.9, 129.7, 129.0, 128.7, 127.1, 125.7, 125.4, 116.2, 110.4, 106.9, 59.8, 47.1, 35.5, 21.3, 21.3, 18.2.MS (TOF ES+): [M + H]+calcd for C30H32NO3S2518.1824; found 518.1830.
4-Methoxy-N-[(2S)-1,1,1-trifluoro-2-(furan-2-yl)-3-(2-(S)-(phenylsulfinyl)phenyl)propan-2-yl) aniline (3f)
By means of the general procedure described above, compound 3f was obtained as yellow oil in 73% yield. [α] 25D = +28.9 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 7.69 (dd, J = 1.3, 7.7 Hz, 1H), 7.30–7.26 (m, 4H), 7.15–7.13 (m, 3H), 6.52–6.45 (m, 4H), 6.32 (dd, J = 1.7, 3.4 Hz, 1H), 6.27–6.24 (m, 2H), 5.90 (br s, 1H), 3.61 (d, J = 14.0 Hz, 1H), 3.58 (s, 3H), 3.00 (d, J = 13.9 Hz, 1H), 2.27 (s, 3H). 13C NMR (CDCl3, 75 MHz): δ 153.0, 148.4, 143.8, 142.2, 140.9, 140.9, 138.4, 134.4, 133.7, 131.2, 129.9, 128.3, 128.1, 128.0 (q, 1 J CF = 292.1 Hz), 124.7, 118.4, 113.8, 111.2, 110.8, 64.6 (q, 2 J CF = 26.0 Hz), 55.4, 36.8, 21.2. 19F NMR (CDCl3, 282.4 MHz): δ −81.96 (s, 3F). HRMS (EI+): m/z calcd for C27H25F3NO3S [M + H]+500.1507, found: 500.1505.
(2S,3S)-Ethyl 2-(4-methoxyphenylamino)-3-[2-(S)-(p-tolylsulfinyl)phenyl]-2-(trifluoromethyl) butanoate (9a)
By means of the general procedure described above, compound 9a was obtained as a light yellow solid in 62% yield. Mp = 39–41°C. [α] 25D = +5.8 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 7.66–7.61 (m, 2H), 7.42–7.33 (m, 4H), 7.21–7.16 (m, 2H), 6.77 (d, J = 9.0 Hz, 2H), 6.65 (d, J = 9.2 Hz, 2H), 4.88 (br s, 1H), 4.37 (q, J = 7.2 Hz, 1H), 4.06–3.89 (m, 2H), 3.67 (s, 3H), 2.30 (s, 3H), 1.14 (d, J = 7.2 Hz, 3H), 0.99 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 167.9, 154.4, 144.2, 141.1, 140.5, 140.2, 137.9, 131.9, 131.2, 129.7, 128.5, 128.4, 125.1, 125.2 (q, 1 J CF = 290.9 Hz), 120.4, 114.0, 71.1 (q, 2 J CF = 24.7 Hz), 61.9, 55.5, 39.9, 21.3, 18.0, 13.7. 19F NMR(CDCl3, 282.4 MHz): δ −73.84 (s, 3F). HRMS (EI+): m/z calcd for C27H29F3NO4S [M + H]+520.1769, found: 520.1766.
(2R,3S)-Ethyl 2-(4-methoxyphenylamino)-3-[2-(S)-(p-tolylsulfinyl)phenyl]-2-(trifluoromethyl) butanoate (9a′)
By means of the general procedure described above, compound 9a′ was obtained as a light yellow solid in 20% yield. Mp = 48–50°C. [α] 25D = +24.4 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 7.71 (d, J = 7.2 Hz, 1H), 7.45–7.36 (m, 5H), 7.22–7.19 (m, 2H), 6.59–6.47 (m, 4H), 4.90 (br s, 1H), 4.33–4.18 (m, 2H), 4.25 (q, J = 7.2 Hz, 1H), 3.63 (s, 3H), 2.32 (s, 3H), 1.21 (t, J = 7.2 Hz, 3H), 1.05 (d, J = 7.1 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 167.3, 153.7, 143.4, 141.3, 141.0, 140.9, 138.1, 132.0, 130.9, 129.9, 129.1, 128.2, 125.6 (q, 1 J CF = 291.6 Hz), 124.8, 119.3, 113.8, 70.8 (q, 2 J CF = 24.7 Hz), 62.0, 55.5, 38.2, 21.3, 17.9, 13.8. 19F NMR (CDCl3, 282.4 MHz): δ −67.47 (s, 3F). HRMS (EI+): m/z calcd for C27H29F3NO4S [M + H]+520.1769, found: 520.1749.
(2S)-Ethyl 3,3,3-trifluoro-2-(4-methoxyphenylamino)-2-[2-(S)-(p-tolylsulfinyl)benzyl]propanoate (9b)
By means of the general procedure described above, compound 9b was obtained as yellow oil in 38% yield. [α] 25D = −5.1 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 7.80 (dd, J = 1.4, 7.8 Hz, 1H), 7.44, 7.37 (m, 3H), 7.36–7.25 (m, 2H), 7.19–7.16 (m, 2H), 6.67–6.59 (m, 4H), 4.67 (br s, 1H), 3.96–4.06 (m, 2H), 3.66 (s, 3H), 3.51 (dd, J = 16.5, 45.5 Hz, 2H), 2.30 (s, 3H), 0.86 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 167.6, 154.5, 143.8, 141.8, 140.7, 136.2, 133.3, 131.2, 130.1, 128.2, 125.9, 125.7, 124.6 (q, 1 J CF = 292.1 Hz), 121.2, 114.3, 68.4 (q, 2 J CF = 25.6 Hz), 63.1, 55.4, 29.9, 21.3, 13.5. 19F NMR (CDCl3, 282.4 MHz): δ −73.25 (s, 3F). HRMS (EI+): m/z calcd for C26H26F3NO4S [M]+505.1535, found: 505.1535.
(2R)-Ethyl 3,3,3-trifluoro-2-(4-methoxyphenylamino)-2-[2-(S)-(p-tolylsulfinyl)benzyl]propanoate (9b′)
By means of the general procedure described above, compound 9b′ was obtained as yellow oil in 13% yield. [α] 25D = −35.4 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 7.89 (dd, J = 1.7, 7.9 Hz, 1H), 7.41–7.30 (m, 5H), 7.19–7.14 (m, 2H), 6.63–6.56 (m, 4H), 5.09 (br s, 1H), 4.12–4.05 (m, 2H), 3.64 (s 3H), 3.40 (dd, J = 15.6, 49.0 Hz, 2H), 2.30 (s, 3H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 168.0, 155.2, 144.0, 141.9, 140.8, 135.7, 133.3, 130.9, 130.0, 129.1, 128.1, 128.2 (q, 1 J CF = 276.2 Hz),126.1, 125.1, 122.5, 114.3, 68.2 (q, 2 J CF = 25.4 Hz), 62.9, 55.5, 29.9, 21.4, 13.4. 19F NMR (CDCl3, 282.4 MHz): δ −72.43 (s, 3F). HRMS (EI+): m/z calcd for C26H26F3NO4S [M]+505.1535, found: 505.1520.
(2S,3S)-Ethyl 2-(4-methoxyphenylamino)-3-[2-(S)-(p-tolylsulfinyl)phenyl)-2-(trifluoromethyl]hex-5-enoate (9c)
By means of the general procedure described above, compound 9c was obtained as yellow oil in 38% yield. [α] 25D = −43.1 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 7.63–7.57 (m, 2H), 7.48–7.31 (m, 4H), 7.19–7.14 (m, 2H), 6.71–6.63 (m, 4H), 4.95–4.87 (m, 1H), 4.75–4.63 (m, 3H), 4.41 (dd, J = 5.2, 9.1 Hz, 1H), 4.15–3.98 (m, 2H), 3.67 (s, 3H), 2.64–2.58 (m, 2H), 2.28 (s, 3H), 1.07 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 167.8, 154.5, 145.9, 141.3, 140.2, 137.4, 137.3, 133.8, 131.5, 130.9, 129.8, 128.9, 129.1, 125.4, 125.1 (q, 1 J CF = 290.5 Hz), 120.6, 114.0, 118.0, 71.8 (q, 2 J CF = 24.7 Hz), 62.2, 55.5, 44.9, 36.7, 21.3, 13.7. 19F NMR (CDCl3, 282.4 MHz): δ −73.96 (s, 3F). HRMS (EI+): m/z calcd for C29H31F3NO4S [M + 1]+546.1926, found: 546.1918.
(2S,3R)-Ethyl 2-(4-methoxyphenylamino)-3-[2-(S)-(p-tolylsulfinyl)phenyl)-2-(trifluoromethyl]hex-5-enoate (9c′)
By means of the general procedure described above, compound 9c′ was obtained as yellow oil in 12% yield. [α] 25D = −0.9 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 774 (dd, J = 1.7, 9.2 Hz, 1H), 7.59 (dd, J = 1.3, 7.9 Hz, 1H), 7.47–7.38 (m, 4H), 7.21–7.18 (m, 2H), 6.56 (d, J = 9.1 Hz, 2H), 6.42 (d, J = 9.0 Hz, 2H), 4.68–4.49 (m, 3H), 4.29 (dq, J = 1.0, 7.2 Hz, 2H), 4.19 (dd, J = 3.3, 11.9 Hz, 1H), 3.62 (s, 3H), 2.78–2.70 (m, 1H), 2.33–2.20 (m, 1H), 2.31 (s, 3H), 1.24 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 167.4, 154.1, 145.4, 141.3, 140.6, 137.7, 137.6, 133.6, 132.3, 131.8, 130.0, 128.7, 128.4, 125.7 (q, 1 J CF = 291.6 Hz), 125.2, 120.0, 117.6, 113.9, 70.7 (q, 2 J CF = 24.7 Hz), 62.3, 55.5, 43.4, 36.7, 21.3, 13.9. 19F NMR (CDCl3, 282.4 MHz): δ −67.84 (s, 3F). HRMS (EI+): m/z calcd for C29H31F3NO4S [M + 1]+546.1926, found: 546.1907.
General procedure for the synthesis of N-acetylα-amino acid derivatives 4
To a solution of the corresponding compound 3 (0.41 mmol) in MeOH (5 ml), TFA (1.23 mmol) was added. The solution was stirred for about 2 h, and thereafter (followed by TLC) the residue was purified by strong cation exchange (SCX) chromatography. Then, to a solution of the yellow amine residue (0.41 mmol) in THF (2 ml) acetic anhydride (0.656 mmol) and Et3N (1.23 mmol) were added and stirred for 12 h. When the reaction was completed (followed by TLC), the reaction mixture was extracted (3 × 10 mL Et2O), washed (2 × 10 mL H2O), dried (MgSO4) and the solvent evaporated. Then, the corresponding residue was solved in a mixture of CH3CN/H2O/CCl4 (4/4/4 ml), NaIO4 (0.656 mmol) and RuCl3 (0.041 mmol) were added at room temperature. The solution was stirred overnight, and followed by TLC. When the reaction was completed the product was extracted with AcOEt (4 ml), dried (MgSO4), and the solvent evaporated. Finally, the obtained yellow oil was solved in MeOH (5 ml) and trimethylsilyldiazomethane was slowly added (1.23 mmol). When the reaction was completed (followed by TLC) the solvent was evaporated and the residue was purified by flash column chromatography.
(2S, 3S)-Methyl-3-[2-(p-tolylsulfonyl)phenyl]-2-acetamidobutanoate (4a)
By means of the general procedure described above, compound 4a was obtained as yellow oil in 55% yield (three steps). [α] 20D = +55.9 (c 0.93, CH2Cl2). 1H NMR (CDCl3, 300 MHz): δ 8.11 (d, J = 8.0 Hz, 1H), 7.54 (dt, J = 1.3, 8.5 Hz, 1H), 7.38 (d, J = 8.5 Hz, 2H), 7.27 (d, J = 8.0 Hz, 2H), 6.57 (d, J = 8.2 Hz, 2), 4.50 (dd, J = 8.3, 11.2 Hz, 1H), 3.82 (qd, J = 6.9, 11.3 Hz, 1H), 3.71 (s, 3H), 2.36 (s, 3H), 1.62 (s, 3H), 0.77 (d, J = 6.9 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 172.0, 169.7, 144.5, 142.3, 139.3, 138.6, 134.4, 130.0, 128.8, 128.7, 127.6, 127.4, 58.4, 52.2, 36.1, 22.6, 21.6, 18.1. MS (ESI+): [M + Na]+calcd for C20H23NO5SNa 412.1189; found 412.1172.
(2S,3S)-Methyl 2-(acetylamino)-2-methyl-3-[2-(p-tolylsulfonyl)phenyl]butanoate (4b)
By means of the general procedure described above, compound 4b was obtained as yellow oil in 42% yield (three steps). [α] 20D = +60.2 (c 0.4, CH2Cl2). 1H NMR (CDCl3, 300 MHz): δ 8.29 (dd, J = 1.4, 7.9 Hz, 1H), 7.72 (d, J = 8.3 Hz, 2H), 7.64 (s, 1H), 7.60 (dd, J = 1.4, 7.6 Hz, 1H), 7.52–7.45 (m, 2H), 7.31 (d, J = 8.5 Hz, 2H), 3.89 (q, J = 7.0 Hz, 1H), 3.77 (s, 3H), 2.41 (s, 3H), 1.89 (s, 3H), 1.58 (s, 3H), 0.67 (d, J = 7.1 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 158.3, 143.8, 143.3, 142.7, 141.7, 140.9, 140.6, 140.4, 131.9, 130.0, 129.6, 129.1, 129.0, 127.1, 125.7, 125.0, 110.2, 106.7, 59.9, 50.6, 41.7, 21.3, 18.0, 15.8. MS (ESI+): [M + Na]+calcd for C21H25NO5SNa 426.1351; found 426.1340.
General procedure for the Nickel–Raney desulfinylation reaction
A solution of the corresponding sulfoxide 3f, g or 9/9′(0.50 mmol) in THF (5 mL) was added to a suspension of Ni–Raney (2.5 g) in THF (5 mL). The reaction was stirred for 2 h, filtered through a Celite pad, and the residue was purified by flash column chromatography.
(S)-4-Methoxy-N-(1,1,1-trifluoro-2-(furan-2-yl)-3-phenylpropan-2-yl)aniline (5a)
By means of the general procedure described above, compound 5a was obtained as colorless oil in 89% yield. [α] 25D = +56.5 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 7.43 (d, J = 0.8 Hz, 1H), 7.42–7.15 (m, 3H), 6.96–6.93 (m, 2H), 6.58 (dd, J = 2.3, 6.7 Hz, 2H), 6.32 (dd, J = 2.5, 6.4 Hz, 4H), 3.82 (br s, 1H), 3.64 (s, 3H), 3.43 (dd, J = 14.3, 18.5 Hz, 2H). 13C NMR (CDCl3, 75 MHz): δ 154.1, 148.9, 142.5, 137.3, 134.1, 130.7, 128.1, 127.2, 125.6 (q, 1 J CF = 289.4 Hz), 119.9, 114.2, 111.0, 110.9, 64.6 (q, 2 J CF = 25.8 Hz), 55.4, 40.3. 19F NMR (CDCl3, 282.4 MHz): δ −101.4 (s, 3F). HRMS (EI+): m/z calcd for C20H19F3NO2[M + H]+362.1368, found: 362.1378.
4-Methoxy-N-[(2S,3S)-1,1,1-trifluoro-2-(furan-2-yl)-3-phenylbutan-2-yl]aniline (5b)
By means of the general procedure described above, compound 5b was obtained as colorless oil in 96% yield. [α] 25D = +57.3 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 7.45 (d, J = 1.8 Hz, 1H), 7.34–7.25 (m, 5H), 6.66–6.60 (m, 2H), 6.54–6.53 (m, 1H), 6.49 (dd, J = 1.8, 3.3 Hz, 1H), 6.39–6.34 (m, 2H), 3.84 (br s, 1H), 3.65 (q, J = 7.2 Hz, 1H), 3.64 (s, 3H), 1.27 (d, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 153.5, 148.8, 142.0, 140.2, 138.0, 129.3, 128.4, 127.6, 126.3 (q, 1 J CF = 292.1 Hz), 118.8, 113.9, 111.1 (q, 4 J CF = 2.2 Hz), 110.7, 67.0 (q, 2 J CF = 25.1 Hz), 55.4, 46.3, 17.4. 19F NMR (CDCl3, 282.4 MHz): δ −67.76 (s, 3F). HRMS (EI+): m/z calcd for C21H20F3NO2[M]+375.1446, found: 375.1453.
(2S,3S)-Ethyl 2-(4-methoxyphenylamino)-3-phenyl-2-(trifluoromethyl)butanoate (10a)
By means of the general procedure described above, compound 10a was obtained as colorless oil in 70% yield. [α] 25D = +28.39 (c 0.8, CHCl3). 1H NMR (CDCl3, 300 MHz): δ7.37–7.32 (m, 5H), 6.79–6.70 (m, 4H), 4.18–4.07 (m, 2H), 3.89 (br s, 1H), 3.74 (s, 3H), 3.68 (q, J = 7.2 Hz, 1H), 1.55 (d, J = 7.4 Hz, 3H), 1.14 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 168.2, 154.5, 139.2, 137.2, 129.1, 128.4, 127.9, 125.0 (q, 1 J CF = 291.0 Hz), 121.0, 114.0, 71.8 (q, 2 J CF = 24.3 Hz), 62.1, 55.5, 45.1, 16.0, 13.7. 19F NMR (CDCl3, 282.4 MHz): δ −74.20 (s, 3F). HRMS (EI+): m/z calcd for C20H22F3NO3[M]+381.1552, found: 381.1540.
(2R,3S)-Ethyl 2-(4-methoxyphenylamino)-3-phenyl-2-(trifluoromethyl)butanoate (10a′)
By means of the general procedure described above, compound 10a′ was obtained as colorless oil in 72% yield. [α] 25D = −9.96 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ7.39–7.32 (m, 5H), 6.75–6.59 (m, 4H), 4.38–4.25 (m, 2H), 3.71 (s, 3H), 3.76–3.71 (m, 2H), 1.49 (d, J = 7.1 Hz, 3H), 1.30 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 167.8, 154.2, 138.7, 137.5, 129.4, 128.6, 128.1, 125.2 (q, 1 J CF = 290.5 Hz), 120.0, 114.0, 71.5 (q, 2 J CF = 24.7 Hz), 62.2, 55.5, 43.2, 17.2, 13.9. 19F NMR (CDCl3, 282.4 MHz): δ −76.19 (s, 3F). HRMS (EI+): m/z calcd for C20H22F3NO3[M]+381.1552, found: 381.1544.
(S)-Ethyl 2-benzyl-3,3,3-trifluoro-2-(4-methoxyphenylamino)propanoate (10b)
By means of the general procedure described above, compound 10b was obtained as a white solid in 77% yield. Mp = 45–47°C. [α] 25D = +18.4 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ7.22–7.20 (m, 3H), 7.09–7.06 (m, 2H), 6.82–6.75 (m, 4H), 4.48 (br s, 1H), 4.28–4.15 (m, 2H), 3.76 (s, 3H), 3.53 (dd, J = 14.5, 83.2 Hz, 2H), 1.22 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 168.0, 154.3, 136.9, 133.7, 130.1, 128.2, 127.3, 124.8 (q, 1 J CF = 289.9 Hz), 120.6, 114.4, 68.8 (q, 2 J CF = 26.2 Hz), 62.9, 55.5, 35.1, 13.7. 19F NMR (CDCl3, 282.4 MHz): δ −81.65 (s, 3F). HRMS (EI+): m/z calcd for C19H20F3NO3[M]+367.1395, found: 367.1385.
(2S,3S)-Ethyl 2-(4-methoxyphenylamino)-3-phenyl-2-(trifluoromethyl)hexanoate (10c)
By means of the general procedure described above, compound 10c was obtained as a white solid in 74% yield. Mp = 71–73°C. [α] 25D = −28.5 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ7.33–7.31 (m, 5H), 6.76–6.69 (m, 4H), 4.11 (q, J = 7.1 Hz, 2H), 3.95 (br s, 1H), 3.73 (s, 3H), 3.45 (dd, J = 2.6, 12.2 Hz, 1H), 2.04–2.00 (m, 1H), 1.85–1.80 (m, 1H), 1.16–0.96 (m, 2H), 1.14 (t, J = 7.2 Hz, 3H), 0.83 (t, J = 7.3 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 168.0, 154.4, 137.2, 136.9, 129.9, 128.3, 127.9, 125.1 (q, 1 J CF = 290.5 Hz), 120.4, 114.1, 72.0 (q, 2 J CF = 24.2 Hz), 62.1, 55.5, 50.9, 31.3, 20.9, 13.7, 13.6. 19F NMR (CDCl3, 282.4 MHz): δ −73.99 (s, 3F). HRMS (EI+): m/z calcd for C22H26F3NO3[M]+409.1865, found: 409.1865.
General procedure for the tert-BuLi desulfinylation reaction
A solution of t-BuLi (0.22 mmol, 1.7 M in hexane) was added dropwise to a solution of the corresponding sulfoxide 9c or 9c′ (0.1 mmol) in THF (2 mL) at –78°C. After 10 min stirring, the mixture was hydrolyzed (2 mL saturated NH4Cl), extracted (3 × 10 mL AcOEt), washed (2 × 10 mL NaCl sat), dried (MgSO4) and the solvent evaporated. The residue was purified by flash column chromatography.
(2S,3S)-Ethyl 2-(4-methoxyphenylamino)-3-phenyl-2-(trifluoromethyl)hex-5-enoate (10d)
By means of the general procedure described above, compound 10d was obtained as colorless oil in 62% yield. [α] 25D = −7.5 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ736–7.32 (m, 5H), 6.79–6.71 (m, 4H), 5.48–5.37 (m, 1H), 4.99–4.86 (m, 2H), 4.15 (q, J = 7.2 Hz, 2H), 3.96 (br s, 1H), 3.74 (s, 3H), 3.52 (dd, J = 3.5, 11.6 Hz, 1H), 2.82–2.71 (m, 2H), 1.17 (t, J = 7.1 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 167.9, 154.6, 137.0, 136.3, 135.3, 130.1, 128.3, 128.0, 125.0 (q, 1 J CF = 291.0 Hz), 121.0, 117.2, 114.1, 71.9 (q, 2 J CF = 24.5 Hz), 62.2, 55.5, 51.0, 33.9, 13.7. 19F NMR (CDCl3, 282.4 MHz): δ −74.24 (s, 3F). HRMS (EI+): m/z calcd for C22H24F3NO3[M]+407.1708, found: 407.1710.
(2R,3S)-Ethyl 2-(4-methoxyphenylamino)-3-phenyl-2-(trifluoromethyl)hex-5-enoate (10d′)
By means of the general procedure described above, compound 10d′ was obtained as colorless oil in 70% yield. [α] 25D = +32.8 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ7.39–7.35 (m, 5H), 6.69–6.58 (m, 4H), 5.48–5.37 (m, 1H), 5.00–4.86 (m, 2H), 4.36–4.24 (m, 2H), 3.71 (s, 3H), 3.59 (dd, J = 4.0, 11.7 Hz, 1H), 2.79–2.59 (m, 2H), 1.28 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 167.7, 154.3, 137.4, 136.0, 135.3, 130.3, 128.6, 128.3, 125.1 (q, 1 J CF = 289.9 Hz), 120.2, 117.2, 114.0, 71.4 (q, 2 J CF = 25.4 Hz), 62.2, 55.5, 49.5, 34.9, 13.9. 19F NMR (CDCl3, 282.4 MHz): δ −76.97 (s, 3F). HRMS (EI+): m/z calcd for C22H24F3NO3[M]+407.1708, found: 407.1706.
General procedure for the synthesis of N-Boc-α-amino acid derivatives 7
N-PMP protected amines 5 (0.15 mmol) were dissolved in CH3CN/CCl4/H2O (3: 1.5: 1.5 mL) under brisk stirring. Then, NaIO4 (0.18 mmol) and RuCl3·3H2O (0.015 mmol) were added. After stirring at room temperature for 2 h, the reaction salts were filtered off and washed with CH2Cl2. The organic layer was washed with saturated aqueous NaHCO3, dried over anhydrous Na2SO4 and the solvents removed under reduced pressure. The crude reaction mixture was then dissolved in MeOH (1.5 mL) and added to a stirred solution of p-toluenesulfonic acid (0.33 mmol) and Girard T (0.33 mmol). The reaction mixture was stirred for 10 min and next, methanol was removed under reduced pressure. The resulting residue was dissolved in AcOEt (10 mL), quenched with 1 M K2HPO4, and extracted with AcOEt (4 × 10 mL). The combined organic layers were washed with brine (3 × 10 mL), dried over anhydrous Na2SO4 and concentrated in vacuum. The resulting crude reaction mixture was diluted with CH2Cl2 (2.5 mL), and (Boc)2O (0.75 mmol) and K2CO3 (0.45 mmol) were added at 0°C. After stirring for 4 h, the reaction was quenched with H2O and the aqueous layer was extracted with EtOAc (3 × 10 mL). The combined organic layers were washed with brine, dried over anhydrous Na2SO4 and the solvents removed under reduced pressure. The crude reaction mixture was purified by means of flash column chromatography on silica gel.
4-[(2S,3S)-1,1,1-Trifluoro-2-(furan-2-yl)-3-phenylbutan-2-ylimino]cyclohexa-2,5-dienone (6b)
By means of the general procedure described above, after the oxidation step compound 6b was obtained by crystallization with Et2O as a brown solid. Mp = 114–116°C. [α] 20D = +13.9 (C 0.27, CH2Cl2). 1H NMR (CDCl3, 300 MHz): δ 7.47 (s, 1H), 7.53–7.23 (m, 5H), 6.60 (dd, J = 3.3, 10.7 Hz, 1H), 6.55–6.51 (m, 3H), 6.04 (dd, J = 2.2, 10.4, 1H), 5.68 (dd, J = 2.4, 10.6 Hz, 1H), 3.84 (q, J = 7.2 Hz, 1H), 1.63 (d, J = 6.6 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 187.1, 160.8, 148.8, 143.8, 142.4, 140.5, 131.9, 131.1, 130.2, 128.8 (q, 1 J CF = 240.0 Hz), 128.3, 127.8, 111.2, 111.0, 73.2 (q, 2 J CF = 96.7 Hz), 26.5, 17.1. MS (ESI+): [M + Na]+calcd for C20H16NO2F3Na 382.1030; found 382.1025.
(S)-tert-Butyl 1,1,1-trifluoro-2-(furan-2-yl)-3-phenylpropan-2-ylcarbamate (7a)
By means of the general procedure described above, compound 7a was obtained as yellow oil in 48% yield (three steps). [α] 25D = +17.6 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ7.40 (d, J = 1.3 Hz, 1H), 7.18–7.16 (m, 3H), 6.93–6.91 (m, 2H), 6.90–6.78 (m, 2H), 4.05 (br s, 1H), 3.43 (dd, J = 14.0, 23.3 Hz, 2H), 1.45 (s, 9H). 13C NMR (CDCl3, 75 MHz): δ 152.2, 144.3, 142.7, 141.7, 130.7, 128.2, 127.4, 125.3 (q, 1 J CF = 288.8 Hz), 111.2, 110.9, 83.2, 64.0 (q, 2 J CF = 26.4 Hz), 40.3, 27.7. 19F NMR (CDCl3, 282.4 MHz): δ −82.38 (s, 3F). HRMS (EI+): m/z calcd for C18H20F3NO3[M]+355.1395, found: 355.1323.
tert-Butyl (2S,3S)-1,1,1-trifluoro-2-(furan-2-yl)-3-phenylbutan-2-ylcarbamate (7b)
By means of the general procedure described above, compound 7b was obtained as yellow oil in 55% yield (three steps). [α] 25D = +35.6 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ7.45 (dd, J = 0.8, 1.8 Hz, 1H), 7.36–7.31 (m, 3H), 7.26–7.23 (m, 2H), 6.78 (dd, J = 2.3, 6.8 Hz, 2H), 4.05 (br s, 1H), 3.64 (q, J = 7.2 Hz, 1H), 1.51 (s, 9H), 1.26 (d, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 152.3, 143.8, 142.2, 140.0, 129.2, 128.6, 127.8, 126.3 (q, 1 J CF = 291.6 Hz), 111.3, 110.7, 83.1, 66.3 (q, 2 J CF = 25.8 Hz), 46.4, 27.7, 17.5. 19F NMR (CDCl3, 282.4 MHz): δ −68.47 (s, 3F). HRMS (EI+): m/z calcd for C19H22F3NO3[M]+369.1552, found: 369.1537.
General procedure for the synthesis of indolines 11
To a solution of the corresponding amine 10′(0.2 mmol) in THF (0.1 M) at 0 C a solution of KHDMS (0.3 mmol, 0.5 M in toluene) was added under argon atmosphere, and the temperature was raised to room temperature. The reaction was followed by TLC and once the reaction was completed, the mixture was treated with a saturated solution of NaHCO3 (10 mL) and extracted with CH2Cl2 (3 × 10 mL). The organic phase was dried with anhydrous Na2SO4, and the solvent was eliminated under reduced pressure. Finally, compounds 11 were purified by means of flash column chromatography.
(2R,3S)-Ethyl 1-(4-methoxyphenyl)-3-methyl-2-(trifluoromethyl)indoline-2-carboxylate (11a)
By means of the general procedure described above, compound 11a was obtained as a white solid in 51% yield. Mp = 66–68°C. [α] 25D = +9.2 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ7.34 (d, J = 8.9 Hz, 2H), 7.05–6.98 (m, 2H), 6.92–6.89 (m, 2H), 6.77–6.72 (m, 1H), 6.18 (d, J = 7.9 Hz, 1H), 4.23 (q, J = 7.2 Hz, 2H), 4.01 (q, J = 7.1 Hz, 1H), 3.83 (s, 3H), 1.45 (d, J = 7.2 Hz, 3H), 1.24 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 165.8, 158.6, 151.0, 132.6, 129.1, 130.8, 128.1, 124.8 (q, 1 J CF = 284.4 Hz), 122.6, 118.6, 114.4, 107.4, 81.1 (q, 2 J CF = 26.4 Hz), 61.7, 55.4, 43.2, 15.6, 14.1. 19F NMR (CDCl3, 282.4 MHz): δ −81.28 (s, 3F). HRMS (EI+): m/z calcd for C20H20F3NO3[M]+379.1395, found: 379.1394.
(2R,3S)-Ethyl 3-allyl-1-(4-methoxyphenyl)-2-(trifluoromethyl)indoline-2-carboxylate (11b)
By means of the general procedure described above, compound 11b was obtained as a white solid in 64% yield. Mp = 50–52°C. [α] 25D = +14.6 (c 1.0, CHCl3). 1H NMR (CDCl3, 300 MHz): δ 7.33 (d, J = 8.9 Hz, 2H), 7.21 (d, J = 7.3 Hz, 1H), 7.04–6.98 (m, 1H), 6.92–6.89 (m, 2H), 6.71 (dt, J = 1.0, 7.4 Hz, 1H), 6.17 (d, J = 7.7 Hz, 1H), 6.04–5.90 (m, 1H), 5.22–5.16 (m, 2H), 4.23–4.15 (m, 2H), 3.97 (dd, J = 5.5, 8.4 Hz, 1H), 3.83 (s, 3H), 2.63–2.56 (m, 2H), 1.23 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 75 MHz): δ 165.7, 158.6, 151.0, 135.5, 132.6, 130.9, 130.9, 128.2, 124.8 (q, 1 J CF = 285.5 Hz), 123.8, 118.5, 117.5, 114.4, 107.7, 80.2 (q, 2 J CF = 26.4 Hz), 61.8, 55.4, 47.3, 36.4, 13.9. 19F NMR (CDCl3, 282.4 MHz): δ −81.89 (s, 3F). HRMS (EI+): m/z calcd for C22H22F3NO3[M]+405.1552, found: 405.1552.
Results and discussion
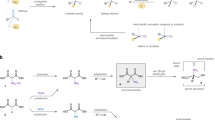
First, we prepared the starting materials, i.e. the ortho-sulfinylbenzyl derivatives 1 and the N-protected 2-furyl imines 2. The former ones were obtained according to literature protocols (García Ruano et al. 2003, 2005a, b, c).
N-Sulfinylimines 2a, b were synthesized through a condensation reaction between the corresponding carbonylic compound and (S)-p-toluenesulfonamide in the presence of Ti(OEt)4 as a water scavenger (Davis et al. 1993; García Ruano et al. 2005a, b, c), thus obtained pure products in good yields after column chromatography purification (Fig. 3a), while trifluoromethylimine 2d was prepared by the Staudinger (aza-Wittig) reaction of furyltrifluoromethylketone (Soloshonok et al. 1986, 1987, 1988; Kerdesky and Basha 1991) with N-PMP iminophosphorane (Stauffer et al. 2000) (Fig. 3b).
With imines 2 in hand, we went on to deal with the nucleophilic addition of stabilized γ-sulfinylcarbanions derived from sulfoxides 1. The reaction of (S)-1a with N-sulfinylated 2-furyl aldimine (S)-2a yielded compound 3a as the only isomer in 82% yield (Table 1, entry 1). The same reaction with N-sulfinylketimine (S)-2b yielded a single diastereoisomer anti-3b although in moderate yield (Table 1, entry 2), probably as a consequence of the enolization of this methyl-substituted ketimine. As we had previously observed with other N-sulfinylketimines, when the carbanion derived from (S)-2-p-tolylsulfinyl toluene (1b) reacted with ketimine (S)-2b, a 60:40 mixture of diastereoisomers 3c and 3c′ was detected in the crude reaction mixture (Table 1, entry 3). Interestingly, the reaction of the sulfinylcarbanion derived from (S)-1a with N-sulfinylketimine (R)-2b gave syn-amine derivative epi-3b (Table 1, entry 4). Comparison of the results in entries 2 and 4 suggests that this methodology allows for the synthesis of all possible diastereoisomers by choosing the appropriate configuration at both sulfinyl groups. Other substitutions at the benzylic center were also tolerated, thus obtaining benzyl derivative 3d and allyl derivative 3e both as unique diastereoisomers (Table 1, entries 5–6).
Regarding the trifluoromethyl-substituted imines, when N-sulfinylketimine (S)-2c was reacted with the sulfinylcarbanion (S)-1a under the same conditions, we observed a complex mixture of products (Table 1, entry 7). Therefore, we changed the nitrogen protecting group from the p-toluenesulfinyl to the p-methoxyphenyl (PMP) groupFootnote 1 (García Ruano et al. 2008). In this way, N-PMP trifluoromethylfurylimine 2d took part in a mono-induction process, in which only the sulfinyl group of 1 would induce asymmetry in the formation of the new chiral centers. To our delight, both sulfinylcarbanions (S)-1a and (S)-1b led to the corresponding addition products 3f and 3g as single diastereoisomers in good yields (Table 1, entries 8, 9).
The next step in our study comprised the transformation of adducts 3 into the corresponding quaternary α-amino acid derivatives. In the case of the non-fluorinated compounds it involved the desulfinylation of the nitrogen and the oxidative elaboration of the furane moiety. To illustrate this process, 3a and 3b were treated with TFA (hydrogenolysis of the N–S bonds) and the resulting amino sulfoxides (purified by SCX column) were then acetylated. Further oxidation (of both, the sulfoxide and the furan ring) with NaIO4/RuCl3 followed by esterification with trimethylsilyldiazomethane afforded α-amino esters 4 in good yields (Fig. 4).
We also tried to exemplify the synthesis of α-trifluoromethyl α-amino acid derivatives by using an analogous sequence. Thus, starting from compounds 3f and 3g, we removed the p-toluenesulfinyl group by treatment with Ni Raney in THF/H2O to give desulfinylated products 5 in very good yields. Then, in order to release the amino group under oxidative conditions (RuCl3/NaIO4), we observed the formation of quinonimine intermediates 6, which proved to be very difficult to hydrolyze Different attempts with H2O, 10% aqueous HCl or H2SO4 either at room or high (100°C) temperature gave only starting material or complex mixtures. Fortunately, we could hydrolyze these intermediates by using a Girard reagent in a polar protic solvent (Girard and Sandulesco 1936). These reagents are quaternary ammonium salts bearing an acetylhydrazine functionality and they have been widely used to extract carbonylic compounds from natural product mixtures (Wheeler et al. 1961, 1962; Watanabe et al. 1982a, b). Thus, the treatment of a solution of intermediates 6 in MeOH with Girard T in the presence of p-toluenesulfonic acid followed by addition of a K2HPO4 solution and an extractive work up (del Pozo et al. 2001) led to the corresponding free amine intermediates which, without further purification, were protected as N-Boc carbamates 7 under standard conditions. Although this protocol allowed us to isolate both N-Boc protected amines 7 in good yields from PMP derivatives 5, we were not able to oxidize the furane ring, and we obtained complex mixtures of products after trying various conditions (Fig. 5).
In this context, we were able to obtain crystals of compound 6b suitable for X-ray analysis,Footnote 2 which allowed us to confirm the relative configuration of this type of derivatives. Interestingly, this X-ray structure showed a π-stacking interaction between the quinonimine ring and the phenyl group at the β-position, with a typical distance connecting the rings (4.0 Ǻ) (Fig. 6). It is likely that due to this hindered conformation and the relatively stable π-π stacking, the hydrolysis of these quinone-type intermediates was difficult to achieve.Footnote 3
However, not being able to oxidize the furane ring in the fluorinated derivatives, we decided to slightly change our strategy for the preparation of quaternary fluorinated α-amino acids. This new approach consisted of the addition of our 2-p-tolylsulfinylbenzylcarbanions of (S)-1 to fluorinated α-imino esters, which are interesting synthetic intermediates for the preparation of β-fluorinated α-amino acids (Fustero et al. 2006, 2008a, b, c). Thus, the N-PMP imino ester derived from commercially available ethyl trifluoropyruvate was easily obtained by means of an aza-Wittig reaction, and it was used as electrophile in the reaction with different stabilized sulfinylcarbanions. These reactions took place in good yields although a 3/1 diastereoisomeric mixture was obtained in all cases independently of the substitution at the benzylic position (Table 2). However, the diastereoisomers could easily be separated by column chromatography.
The absolute configuration of compounds 9 was determined related to the known (S S) stereochemistry of the chiral auxiliary by analysis of the X-ray diffraction pattern of a suitable crystal obtained by slow evaporation of a solution of the minor diastereoisomer 9a′ in hexanes/diethyl ether (Fig. 7).2
Once compounds 9 and 9′ had been obtained as single diastereoisomers, the sulfoxide group was removed to obtain the desired α-trifluoromethyl α-amino acid derivatives. Therefore, we carried out the desulfinylation of either diastereoisomer 9 and 9′ separately by treatment with either Ni Raney or t-BuLi. These reactions proceeded in good yields (Table 3). In the case of the β-allyl substituted compound 9c, the reaction with Ni Raney caused the hydrogenation of the double bond, thus obtaining the β-propyl substituted product 10c in 74% yield (Table 3, entry 5). In order to avoid reductive conditions, compounds 9c and 9c′ were desulfinylated by using t-BuLi at –78°C. In this manner, the allyl substitution at the benzylic position was also obtained in good yields (Table 3, entries 6, 7).
Another strategy for removing the sulfinyl group was developed by our research groups in 2008 (García Ruano et al. 2008), which involved an anionic-anionic asymmetric tandem process including an intramolecular-nucleophilic aromatic substitution of the p-tolylsulfinyl group by the nitrogen atom. This process gave rise to optically pure fluorinated indolines. Likewise, if an appropriate base is added to addition products 9/9′, fluorinated α-amino acid derivatives inserted into an indoline skeleton (Horton et al. 2003; Anas and Kagan 2009; Liu et al. 2010) were obtained. These kind of compounds, e.g. 2-(alkyl)indoline-2-carboxylates, have been explored for the treatment of anxiety and depression (Kondo et al. 2006, 2008), and they have also been studied for the prevention and treatment of hypertension, congestive cardiac failure, and renal diseases (Uemoto et al. 2000). Notwithstanding the importance of these drugs, the synthesis of fluorine-containing analogues has not been reported to date.
With these thoughts in mind, we discovered that only minor diastereoisomer 9′ was able to adopt the appropriate conformation for cyclizing in the presence of potassium hexamethyldisilazane (KHMDS) affording, for the first time, the preparation of 2-trifluoromethylated 2-indoline carboxylates 11 in good yields without epimerization of the chiral centers (Fig. 8). The existing dependence between the configuration of the precursor and the success of the cyclization is in agreement with the results previously reported (García Ruano et al. 2009).
Conclusions
We have described a new strategy for the asymmetric synthesis of different types of quaternary α-amino acid derivatives 4 and 10. The approach involved the reaction of imines 2 bearing a 2-furyl moiety as the masked acid functionality with stabilized ortho-sulfinylbenzylcarbanions 1 and the subsequent nitrogen deprotection and furan oxidation. The nucleophilic addition was highly selective and led to the corresponding amines with two vicinal stereogenic centers, one of them quaternary. For the preparation of fluorinated analogues of α-amino acids, the reaction of the sulfinylcarbanions with a trifluoromethyl α-imino ester (8) allowed for the attainment of the final α-amino acid derivatives 10 just after a desulfinylation step. We were also able to synthesize another interesting type of α-amino acids, namely 2-(trifluoromethyl)indoline-2-carboxylates 11 in only two steps.
Notes
Our research groups’ previous results showed that these fluorinated PMP-derived imines reacted with p-tolyl sulfinyl carbanions in good yields and selectivities (see García-Ruano et al. 2008).
The authors have deposited atomic coordinates for 6b and 9a´ with the Cambridge Crystallographic data Centre (deposition numbers CCDC 805405 & 805406). The coordinates can be obtained, on request, from the Director, Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge Cb2 1EZ, UK.
The stability of products 6 can be attributed to the strong electron withdrawing effect of the CF3 group as well as its strong stereocontrolling effect. In certain cases it can act as a bigger group than the tert-butyl one (see, for example Soloshonok et al. 1996).
References
Abell A (1999) Advances in amino acid mimetics and peptidomimetics. JAI Press Inc, Greenwich, pp 191–220
Alemán J, Milelli A, Cabrera S, Reyes E, Jørgensen KA (2008) Asymmetric 1, 4-addition of oxazolones to nitroalkenes by bifunctional cinchona alkaloid thiourea organocatalysts: synthesis of α, α-disubstituted α-amino acids. Chem Eur J 14:10958–10966
Anas S, Kagan HB (2009) Routes toward enantiopure 2-substituted indolines: an overview. Tetrahedron Asymm 20:2193–2199
Asensio A, Bravo P, Crucianelli M, Farina A, Fustero S, García-Soler J, Meille SV, Panzeri W, Viani F, Volonterio A, Zanda M (2001) Synthesis of non-racemic α-trifluoromethylα-amino acids from sulfinimines of trifluoropyruvate. Eur J Org Chem 1449–1458 (references cited therein)
Balaram P, Ramaseshan S (1991) Molecular conformation and biological interactions. Indian Academy of Science, Bangalore
Cabrera S, Reyes E, Alemán J, Milelli A, Kobbelgaard S, Jørgensen KA (2008) Organocatalytic asymmetric synthesis of α, α-disubstituted α-amino acids and derivatives. J Am Chem Soc 130:12031–12037
Cativiela C, Díaz-de-Villegas MD (1998) Stereoselective synthesis of quaternary α-amino acids. Part 1: acyclic compounds. Tetrahedron Asymm 9:3517–3599
Cativiela C, Díaz-de-Villegas MD (2000) Stereoselective synthesis of quaternary α-amino acids. Part 2: cyclic compounds. Tetrahedron Asymm 11:645–732
Davis FA, Reddy RE, Szweczyk JM, Portonovo PS (1993) Asymmetric synthesis of sulfinimines: chiral ammonia imine synthons. Tetrahedron Lett 34:6229–6232
DeGrado WF (2001) Special issue on protein design. Chem Rev 101:3025–3032
delPozo C, Alonso E, López-Ortiz F, Fernández-Marí F, Bayod M, González J (2001) Synthesis of 1,1-dioxopenicillanoyloxymethyl 6-[D-α (benzylideneaminophenylacetamido)] penicillanate and analogs. New intermediates in the preparation of sultamicillin. Tetrahedron 57:6209–6214
Félix A (2004) Synthesis of peptides and peptidomimetics. In: Goodman M, Félix A, Moroder L, Toniolo C (eds) Methods in organic chemistry (Houben-Weyl). Georg ThiemeVerlag, Sttutgart
Fustero S, Sánchez-Roselló M, Rodrigo V, del Pozo C, Sanz-Cervera JF, Simón-Fuentes A (2006) Asymmetric synthesis of new β, β-difluorinated cyclic quaternary α-amino acid derivatives. Org Lett 8:4129–4132
Fustero S, Sánchez-Roselló M, Rodrigo V, Sanz-Cervera JF, Piera J, Simón-Fuentes A, del Pozo C (2008a) Solution-, solid-phase and fluorous synthesis of β, β-difluorinated cyclic quaternary α-amino acid derivatives. A comparative study. ChemEur J 14:7019–7029
Fustero S, Rodrigo V, Sánchez-Roselló M, Mojarrad F, Vicedo A, Moscardó T, del Pozo C (2008b) Solution and fluorous phase synthesis of β, β-difluorinated 1-amino-1-cyclopentane carboxylic acid derivatives. J Fluorine Chem 129:943–950
Fustero S, Román R, Sanz-Cervera JF, Simón-Fuentes A, Cuñat AC, Villanova S, Murguía M (2008c) Improved regioselectiviy in pyrazole formation through the use of fluorinated alcohols as solvents: synthesis and biological activity of fluorinated tebufenpyrad analogs. J Org Chem 73:3523–3529
García Ruano JL, Alemán (2003) Stereocontrolled reactions mediated by a remote sulfoxide group: synthesis of optically pure anti-β-amino alcohols. Org Lett 5:4513–4516
García Ruano JL, Alemán J, Soriano F (2003) Facile synthesis of optically pure 1, 2-diaryl (and 1-alkyl-2-aryl) ethyl and propylamines. Org Lett 5:677–680
García Ruano JL, Alemán, Parra A (2005a) Highly stereoselectivebenzylation of N-sulfinylketimines. J Am Chem Soc 127:13048–13054 (references cited therein)
García Ruano JL, Alemán J, Cid MB, Parra A (2005b) A general method for the preparation of N-sulfonylaldimines and ketimines. Org Lett 7:179–182
García Ruano JL, Alemán J, Aranda M, Arévalo MJ, Padwa A (2005c) Highly stereoselective vinylogous Pummerer reaction mediated by Me3SiX. Org Lett 7:19–22
García Ruano JL, Alemán J, Catalán S, Marcos V, Monteagudo S, Parra A, del Pozo C, Fustero S (2008) Anionic-anionic asymmetric tandem reactions: one-pot synthesis of optically pure fluorinated indolines from 2-p-tolylsulfinyl alkylbenzenes. Angew Chem Int Ed 47:7941–7944
García Ruano JL, Parra A, Marcos V, del Pozo C, Catalán S, Monteagudo S, Fustero S (2009) Asymmetric synthesis of indolines through intramolecular shifting of aromatic sulfinyl groups. Role of the π, π-stacking interactions in these unusual SNAr processes. J Am Chem Soc 131:9432–9441
Gibson SE, Guillo N, Tozer MJ (1999) Towards Control of χ-Space: conformationally constrained analogues of Phe, Tyr, Trp and His. Tetrahedron 55:585–615
Girard A, Sandulesco G (1936) Sur une nouvelle série de réactifs du groupe carbonyle, leur utilisation à l’extraction des substances cétoniques et à la caractérisation microchimique des aldéhydes et cétones. HelvChimActa 19:1095–1107
Golbik R, Yu C, Weyher-Stingl E, Huber R, Moroder L, Budisa N, Schiene-Fischer C (2005) Peptidylprolyl cis/trans-isomerases: comparative reactivities of cyclophilins, FK506-binding proteins, and parvulins with fluorinated oligopeptide and protein substrates. Biochem 44:16026–16034
Hasbullah SA, Jones S (2010) Evaluating the use of chiral anthracene templates to access pyroglutamic acids. Tetrahedron Asymm 10:2719–2725
Hodges JA, Raines RT (2005) Stereoelectronic and steric effects in the collagen triple helix: toward a code for strand association. J Am Chem Soc 127:15923–15932
Horton DA, Bourne GT, Smythe ML (2003) The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem Rev 103:893–930
Hruby VJ, Lu G, Haskell-Luevano C, Shenderovich MD (1997) Design of peptides, proteins and peptidomimetics in chi space. Biopol (Pept Science) 43:219–266
Jäckel C, Kocksch B (2005) Fluorine in peptide design and protein engineering. Eur J Org Chem 4483–4503
Jäckel C, Seufert W, Thust S, Koksch B (2004) Evaluation of the molecular interactions of fluorinated amino acids with native polypeptides. Chem Bio Chem 5:717–720
Jiang ZY, Chan WH, Lee WM (2005) Synthesis of enantiopure sulfinimines (Thioxime S-oxides) catalyzed by Yb(OTF)3 from p-toluenesulfinamide and aldehydes in mild reaction conditions. J Org Chem 70:1081–1083
Kerdesky FAJ, Basha A (1991) A facile synthesis of aryl trifluoromethylketones. Tetrahedron Lett 32:2003–2004
Kobayashi Y, Nakano M, Okui H (1997) Chiral synthesis of (+)-aspicilin by using a furyl group as the masked γ-oxo-α, β-unsaturated carboxylic acid. Tetrahedron Lett 38:8883–8886
Kondo M, Kaoru M, Kohayakawa H (2006) PCT IntAppl WO 2006075619 A1 20060720
Kondo M, Kaoru M, Kobayakawa H (2008) Jpn. KokaiTokkyoKoho JP 2008019173 A 20080131
Kukhar VP, Sorochinsky AE, Soloshonok VP (2009) Practical synthesis of fluorine-containing α- and β-amino acids: recipes from Kiev, Ukraine. Future Med Chem 1:793–819
Leverett CA, Cassidy MP, Padwa A (2006) Application of the aza-Achmatowicz oxidative rearrangement for the stereoselective synthesis of the Cassia and Prosopis alkaloid family. J Org Chem 71:8591–8601
Liu D, Zhao G, Xiang L (2010) Diverse strategies for the synthesis of indoline scaffold. Eur J Org Chem 3975–3984
Müller K, Faeh C, Diederich F (2007) Fluorine in pharmaceuticals: looking beyond intuition. Science 317:1881–1886
Nájera C, Sansano JM (2007) Catalytic asymmetric synthesis of α-amino acids. Chem Rev 107:4584–4671
Ohfune Y, Shinada, T (2005) Enantio- and diastereoselective construction of α,α-disubstituted α-amino acids for the synthesis of biologically active compounds. Eur J Org Chem 5127–5143
Park KH, Kurth MJ (2002) Cyclic amino acid derivatives. Tetrahedron 58:8629–8659
Purser S, Moore PR, Swallow S, Gouverneur V (2008) Fluorine in medicinal chemistry.Chem Soc Rev 37:320–330
Qiu W, Soloshonok VA, Cai C, Tang X, Hruby VJ (2000) Convenient, Large-scale asymmetric synthesis of enantiomerically pure trans-cinnamylglycine and -α-alanine. Tetrahedron 56:2577–2582
Qiu XL, Meng WD, Qing FL (2004) Synthesis of fluorinated amino acids. Tetrahedron 60:6711–6745
Sewald N, Seymour LC, Burger K, Osipov SN, Kolomiets AF, Fpkin AV (1994) Asymmetric synthesis of α-trifluoromethyl substituted aminoacids via 3-hydroxy-3-trifluoromethyl-2, 5-diketopiperazines. Tetrahedron Asymmetr 5:1051–1060
Soloshonok VA (2002) Highly diastereoselective michael addition reactions between nucleophilic glycine equivalents and β-substituted-α, β-unsaturated carboxylic acid derivatives; a general approach to the stereochemically defined and sterically χ-constrained α-amino acids. Curr Org Chem 6:341–364
Soloshonok VA, Gerus II, Yagupolskii YL (1986) N-(Methoxycarbonyl)imine of trifluoropyruvic acid. Zh Org Khim 22:1335–1337
Soloshonok VA, Gerus II, Yagupolskii YL, Kukhar VP (1987) Fluorine-containing amino acids. III. α-Trifluoromethyl-α-Amino Acids. Zh Org Khim 23:2308–231
Soloshonok VA, Yagupolskii YL, Kukhar VP (1988) Fluorine-containing amino acids. V. Imines of trifluoropyruvic acid in the synthesis of N-substituted trifluoroalanines. Zh Org Khim 24:1638–1644
Soloshonok VA, Avilov DV, Kukhar VP (1996) Asymmetric aldol reactions of trifluoromethyl ketones with a chiral Ni(II) complex of glycine: stereocontrolling effect of the trifluoromethyl group. Tetrahedron 52:12433–12442
Soloshonok VA, Tang X, Hruby VJ, Van Meervelt L (2001a) Asymmetric synthesis of α, β-dialkyl-α-phenylalanines via direct alkylation of a chiral alanine derivative with racemic α-alkylbenzyl bromides. A case of high enantiomer differentiation at room temperature. Org Lett 3:341–343
Soloshonok VA, Tang X, Hruby VJ (2001b) Large scale asymmetric synthesis of novel sterically constrained 2′, 6′-dimethyl- and α, 2′, 6′-trimethyltyrosine and -phenylalanine derivatives via alkylation of chiral equivalents of nucleophilic glycine and alanine. Tetrahedron 57:6375–6382
Soloshonok VA, Boettiger TU, Bolene SB (2008) Asymmetric synthesis of (2S,3S)- and (2R,3R)-α,β-dialkyl-α-amino acids via alkylation of chiral Nickel(II) complexes of aliphatic α-amino acids with racemicα-alkylbenzyl bromides. Synthesis 2594–2602
Soloshonok VA, Sorochinsky, AE (2010) Practical methods for the synthesis of symmetrically α,α-disubstituted α-amino acids. Synthesis 2319–2344
Sorochinsky AE, Soloshonok VA (2010) Asymmetric synthesis of fluorine-containing amines, amino alcohols, α- and β-amino acids mediated by chiral sulfinyl group. J Fluorine Chem 131:127–139
Stauffer SR, Sun J, Katzenellenbogen BS, Katzenellenbogen JA (2000) Acyclic amides as estrogen receptor ligands: synthesis, binding, activity and receptor interaction. Bioorg Med Chem 8:1293–1316
Uemoto K, Koide T, Uchibori T, Watanabe N, Sato H, Yamada S, Kimura T, Kojo K (2000) Jpn. KokaiTokkyoKoho JP 2000256318 A 20000919
Vogt H, Bräse S (2007) Recent approaches towards the asymmetric synthesis of α, α-disubstituted α-amino acids. Org Biomol Chem 5:406–430
Watanabe T, Sugawara S, Miyadera T (1982a) Synthesis of α-amino-cycloheptatriene-1-acetic acids and their 7-acilaminocephalosporin derivatives. Chem Pharm Bull 30:2579–2582
Watanabe H, Hashizume Y, Uneyama K (1982b) Homologation of trifluoroacetimidoyl iodides by palladium-catalyzed carbonylation. An approach to α-amino perfluoroalkanoic acids. Tetrahedron Lett 33:4333–4336
Wheeler OH (1962) The Girard reagents. Chem Rev 62:205–221
Wheeler OH, Gaind VS, Rosado O (1961) Formation of Girard-T derivatives. J Org Chem 26:3537–3538
Acknowledgments
We would like to thank the Spanish Ministerio de Ciencia e Innovación (CTQ2010-19774, CTQ-2009-12168), Generalitat Valenciana (PROMETEO/2010/061) and Comunidad de Madrid (“programa AVANCAT CS2009/PPQ-1634”) for their financial support. M. S.-R. and J. A. would like to express their gratitude for a Juan de la Cierva and Ramón y Cajal contract, respectively, and L.M. and C.B. for their predoctoral fellowships.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fustero, S., Sánchez-Roselló, M., Báez, C. et al. Asymmetric synthesis of quaternary α-amino acid derivatives and their fluorinated analogues. Amino Acids 41, 559–573 (2011). https://doi.org/10.1007/s00726-011-0881-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-0881-7