Abstract
In this paper, we report on a catanionic vesicles-based strategy to reduce the cytotoxicity of the diacyl glycerol arginine-based synthetic surfactants 1,2-dimyristoyl-rac-glycero-3-O-(N α-acetyl-l-arginine) hydrochloride (1414RAc) and 1,2-dilauroyl-rac-glycero-3-O-(N α-acetyl-l-arginine) hydrochloride (1212RAc). The behavior of these surfactants was studied either as pure components or after their formulation as pseudo-tetra-chain catanionic mixtures with phosphatidylglycerol (PG) and as cationic mixtures with 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) used as control. The antimicrobial activity of the negatively charged formulations against Acinetobacter baumannii was maintained with respect to the surfactant alone, while a significant improvement of the antimicrobial activity against Staphylococcus aureus was observed, together with a strong decrease of hemolytic activity. The influence of the net charge of the catanionic vesicles on membrane selectivity was studied using model membranes. The dynamics of surface tension changes induced by the addition of 1414RAc/PG aqueous dispersions into phospholipid monolayers composed of zwitterionic DPPC as model system for mammalian membranes and of negatively charged PG mimicking cytoplasmic membrane of Gram-positive bacteria was followed by tensiometry. Our results constitute a proof of principle that tuning formulation can reduce the cytotoxicity of many surfactants, opening their possible biological applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interfacial and self-assembly properties of surfactants in aqueous environments have been investigated for many decades because of their basic interest in physical chemistry, biophysics and material science, as well as due to their enormous practical relevance. Surfactants are used as additives in pharmaceutical and dermatological formulations. Thus, the concentration used in commercial formulations requires avoidance of adverse side effects such as irritation and/or damage to skin, eyes and mucous membrane. Therefore, developing new non-irritant biocompatible surfactants is of great interest.
Amino acids are not only essential components of living organisms, but are also appealing raw materials for biocompatible surfactants. Cationic acyl amino acids are particularly attractive because of their antibacterial activity (Infante et al. 1985; Xia et al. 1995). To modulate their properties, a large number of N-acyl amino acids have been synthesized and tested for their surfactant properties and interactions with artificial membranes (Miyagishi et al. 1989; Sanson et al. 1987; Epand et al. 1998; Peypoux et al. 2004). According to the information gathered through these experiments, N-fatty acid acylated amino acids deserve their classification into a separate class of lipids worthy of in-depth studies.
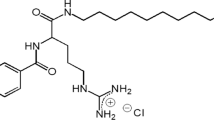
In the last two decades, a number of papers addressing the synthesis and the study of properties of biocompatible cationic amino acid-based surfactants of different structures have been published (Infante et al. 1985; Perez et al. 1996; Pinazo et al. 2009). Acyl-glycerol amino acid conjugates constitute a class of specific lipoamino acid surfactants that share properties with glycerides and phospholipids. They consist of one or two aliphatic chains and one amino acid headgroup, linked together through ester bonds to the glycerol backbone (Moran et al. 2001, 2002). The arginine glyceride conjugates were obtained in the quest to find improved and cheaper soft antimicrobial surfactants (Fig. 1) (Perez et al. 2004a, b). Their use in food and cosmetic applications as well as topical disinfectants has been studied by Benavides et al. (2004) and Vinardell et al. (2008). These compounds consist of a glycerol backbone esterified at positions 1 and 2 with aliphatic acid chains and with the carboxylic group of an arginine residue at position 3 (Fig. 1) (Perez et al. 2004a, b). The modulation of their physicochemical properties, and hence of their associated biological activities (Pinazo et al. 2004; Lozano et al. 2008), is achieved by tuning their charge–hydrophobicity balance, either by acetylation of the α-amino group or by variation of the length of the hydrophobic chains, giving rise to a wide collection of analogs with diverse behavior in biological systems.
In an attempt to explore the potential applications of these arginine-based surfactants into applications close to clinical settings, a set of four monocationic arginine-based surfactants, 1,2-dimyristoyl-rac-glycero-3-O-(N α-acetyl-l-arginine) hydrochloride (1414RAc), 1,2-dilauroyl-rac-glycero-3-O-(N α-acetyl-l-arginine) hydrochloride (1212RAc), as well as the non-N α-acetylated versions (1414R and 1212R, respectively) were synthesized, with a purity higher than 99%. Their antimicrobial and hemolytic activities were examined.
Catanionic vesicles have been extensively used as promoters for chemical and enzymatic reactions and, more recently, as a way to improve the delivery of either DNA or other small size drugs into eukaryotic cells (Bramer et al. 2007). The surfactant ions used in the preparation of catanionic mixtures can be of single or multiple chain. Mixing oppositely charged single chain surfactant ions gives pseudo-double-chain catanionic surfactants (Kamenka et al. 1992; Bonincontro et al. 2006). However, a large amount of literature deals with single-double chain surfactants, forming pseudo-triple-chain catanionic mixtures (Marques et al. 2006, 2008; Burgo et al. 2007; Bonincontro et al. 2008). Recently, Brito et al. (2009) has firstly reported on the relation between the acute toxicity and hemolytic activity/potential ocular irritancy of two types of pseudo-triple-chain catanionic vesicles using lysine- and serine-based surfactants. In that study, the micellization behavior of the pure surfactants was related with the spontaneous vesicle formation of the mixtures that occurs only for excess cationic surfactants.
Biological membranes contain a variety of phospholipid classes and their composition depends on the species and on their functionality (Lohner et al. 2008). Gram-negative bacteria are a complex structure consisting of two bilayers, the outer membrane and the inner or cytoplasmic membrane. The outer membrane has a highly asymmetric composition, formed by a polysaccharide layer located in the outer leaflet and, to a large extent, by the zwitterionic phosphatidylethanolamine (PE) in the inner one. The cytoplasmic membrane consists to a large extent of PE and to negatively charged phosphatidylglycerol (PG). In contrast, Gram-positive bacteria only have a cytoplasmic membrane that consists to a large extent of PG. Accordingly, we selected negatively charged PG as a model membrane system for Gram-positive bacteria. The membrane of erythrocytes, used as archetype of mammalian cytoplasmic membranes, consists mainly of 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC), as well as sphingomyelin and cholesterol. Thus, a model membrane consisting of DPPC has been used mimicking mammalian membranes. Therefore, this model membrane can provide information related to cell toxicity.
The present study reports on the biological activity of the monocationic N α-acetylated arginine-based surfactants, which fall into the category of double-chain surfactants and their mixtures with phosphatidylglycerol (PG) forming pseudo-tetra-chain catanionic vesicles (Caria and Khan 1996; Lozano et al. 2009). Vesicles can be prepared both with cationic or anionic net charge. Increased selectivity of the biological selectivity against Gram-positive bacteria was observed for the PG formulations, mostly due to the strong decrease of hemolytic activity compared to the individual arginine-based surfactant. Therefore, this model membrane can provide information related to antibacterial activity.
Materials and methods
The N α-acetylated (1414RAc, 747.5 g/mol; 1212RAc, 691.4 g/mol) and the non-N α-acetylated (1414R, 741.9 g/mol; 1212R, 685.8 g/mol) diacyl glycerol arginine-based surfactants were synthesized according to Perez et al. (2004a, b). Their purity, higher than 99%, was checked by elemental analysis and high-performance liquid chromatography (HPLC); see Table 1 for the analytical data. PG and DPPC were purchased from Sigma with a purity of 99% and used as received. Sodium chloride (>99.5% by weight) was purchased from Fluka. Water was obtained using a Synergy Ultrapure water system from Millipore (resistivity of 18.2 MΩ cm). HPLC-grade ethanol was supplied by Panreac (water ≤0.2%). The non-ionic surfactant Triton X-100 was purchased from Calbiochem.
Preparation of vesicles for biological activity
Previous to any manipulation, the surfactants and the phospholipids were sterilized by solubilization in ethanol and further evaporation in a cell culture flood hood under sterile conditions. Stock dispersions at a total concentration of 10 mM, of pure surfactant, phospholipids or surfactant/phospholipid formulations at surfactant mole fractions of 0.2 and 0.8 were prepared by weight, with further hydration in water. All dispersions were shaken vigorously at room temperature for 5 min and then sonicated at 50°C for 15 min to promote the formation of uniform vesicles. Lower concentrations were obtained by dilution from stock dispersions.
Preparation of erythrocyte suspensions
To assess cytotoxicity, the hemolytic activity of the surfactants was tested as described below. Sheep erythrocytes from defibrinated blood from Biomedix (Madrid, Spain) were washed twice with Hanks medium (136 mM NaCl; 4.2 mM Na2HPO4; 4.4 mM KH2PO4; 5.4 mM KCl; 4.1 mM NaHCO3, pH 7.2). Erythrocytes were resuspended in the same buffer at 2 × 107 erythrocytes/ml. Different volumes of stock solution of surfactants were mixed with Hanks medium in Eppendorf tubes to a final volume of 1 mL; the final concentration of products ranged from 0 to 150 μM. Aliquots of erythrocyte suspension (25 μL) were added to these solutions, and the mixtures were incubated for 4 h at 37°C. Afterward, tubes were centrifuged at 130,000 rpm over 5 min in an Eppendorf Hettich mikro 200 centrifuge. The supernatant was collected, transferred into polypropylene 96-well microplates and measured by turbidimetry at 540 nm in a Bio-Rad 680 Microplate reader. The percent hemolysis was then determined by comparing the absorbance of the supernatant with that of control samples totally hemolyzed. Full hemolysis was considered as that obtained with 0.1% Triton X-100. HC50 is defined as the concentration of surfactant that induces 50% of hemolysis. Data were expressed as the mean ± SD from triplicate samples.
Antimicrobial activity
The microorganisms used in this study were Acinetobacter baumannii ATCC 19606, Staphylococcus aureus CECT 240, Bacillus Cereus LWLI and Brochothrix thermosphata CECT 847. The inoculums were from bacterial growth in Müeller-Hinton Broth (Oxoid) at 37°C for 18 h (Saugar et al. 2006). Aliquots of cell suspension were added to the wells of a microliter plate containing surfactant solutions of different concentration (0–250 μM), and the final density of bacteria in the wells was 1.25 × 106 CFU/ml. After 18 h of incubation, the absorbance was read at 600 nm (Bio-Rad 680 Microplate reader). We constructed the inhibition–concentration curves. The minimum inhibitory concentration (MIC) is defined as the lowest concentration of surfactant at which there is no change in optical density (OD). MIC50 is defined as the lowest concentration of surfactant at which there is a decrease of 50% in OD. Data were expressed as the mean ± SD from triplicate samples.
Dynamic surface tension measurements
Surface tension (γ) measurements as a function of time were carried out at 25°C using a KSV Sigma 700 tensiometer equipped with a Wilhelmy plate, made of filter paper (Whatman ashless, 10 mm width and negligible thickness) as the surface tension sensor. Samples were placed in a cylindrical Teflon trough (4.95 cm diameter, 1 cm depth) with a lateral orifice to inject the dispersions in the subphase. Samples were prepared in plastic tube containers from Falcon to avoid possible ionic contamination from glass. The solutions were shaken vigorously at room temperature for 5 min and then sonicated at 50°C for 15 min to promote the formation of uniform vesicles. The chemical stability of the surfactant in harsher condition had been checked previously (Lozano et al. 2009). The 1414RAc/PG mixtures were prepared by weighing separately surfactant and PG aqueous dispersions at the desired concentration and mixed, followed by the same sonication treatment. Dynamic surface tension of 1414RAc/PG dispersions into a clean air/water interface and their penetration into PG and DPPC-spread monolayers were studied at a total concentration of 100 μM and at surfactant mole fractions of 0, 0.2, 0.8 and 1. The pure 1414RAc isotherms at 20 and 80 μM were also measured. As much as 10 μL of a hexane/ethanol 9:1 (v/v) solution of either PG or DPPC was spread on the saline subphase to form a phospholipid monolayer at 47 mN/m of surface tension, which was close to the accepted value for packing density of biological membranes (Marsh 1996). To minimize pH changes induced by prolonged exposure to atmospheric carbon dioxide, the solutions were examined within 24 h after preparation. After 15 min, 400 μL of 1414RAc/PG dispersions were injected with a syringe, through a lateral hole, into a saline subphase (20 mL of 0.1 M sodium chloride), which was stirred continuously to assure homogeneous concentration in the subphase. The agitation did no affect significantly the stability of the readings. The standard deviation of the surface tension measurements at the plateau was found to be around 0.02 mN/m with a maximum scatter range of 0.07 mN/m. Surface tension was recorded at a sampling interval of 60 s (not all points are shown on the plots for clarity) and with a penetration measurement time of 40 min. Injection of a dye in the same experimental conditions led to a homogeneous solution in less than 90 s.
Results and discussion
Hemolytic activity of surfactants
A major concern about new antimicrobial agents is whether they fail to fulfill specificity requirements of sparing eukaryotic host cells while killing the microorganism targeted. For surfactants, this is usually reflected in an unspecific lysis. This undesirable lysis of eukaryotic cells can be easily measured by hemolysis assays, based on the release of hemoglobin from erythrocytes. To this aim, the diacyl glycerol arginine-based surfactants were incubated with sheep erythrocytes. As the hemolysis was linearly proportional to the erythrocyte concentration of up to 5 × 107 erythrocytes/ml, the assay was routinely carried out with 2 × 107 erythrocyte/ml. Results are shown in Fig. 2. Hemolysis by the arginine-based surfactants showed a sigmoidal curve as a function of surfactant concentration up to 40 μM for N α-acetylated and 140 μM for non-N α-acetylated arginine-based surfactants, in which HC50 were 16.2 ± 2.0, 5.5 ± 2.0, 65.3 ± 7.0 and >150 μM for 1414RAc, 1212RAc, 1414R and 1212R, respectively.
The hemolytic behavior of the non-N α-acetylated arginine-based surfactants depends on the number of carbon atoms in the hydrophobic chains. Thus, the surfactant with 14 carbon atoms in the hydrophobic chains resulted with the highest hemolysis. When the hydrophilicity of the headgroup was decreased, via acetylation of the amino group of the arginine amino acid, a stronger membrane permeabilization was obtained. The results are difficult to rationalize. While N α-acetylation seems to play a major role, the total surfactant length seems to be only a modulating factor. Results on hemolysis that do not follow the trend of increased hemolysis for increased surfactant hydrophobicity have also been found for other surfactants (Fogt et al. 1995).
Antimicrobial activity of surfactants
Recently published results for diacyl glycerol arginine-based surfactants (Perez et al. 2004a, b) reported a moderate antimicrobial activity against a limited number of Gram-positive and Gram-negative bacteria. To get better knowledge about the antimicrobial properties of these new compounds, extended bactericidal activity was tested on four human pathogenic bacteria: the Gram-negative Acinetobacter baumannii and three Gram-positive Staphylococcus aureus, Bacillus cereus and Brochothrix thermosphacta. MIC50 values for the four of them are compiled in Table 2. Even at the highest concentration tested (250 μM), none of the surfactants reached full growth inhibition. Nevertheless, MIC50 values within the range measured were obtained for the N α-acetylated compounds 1414RAc (101.7 ± 1.05 μM for A. baumannii and more than 250 μM for S. aureus), whereas for 1212RAc, MIC50 values were 72.6 ± 4.8 μM for A. baumannii and 250 μM for S. aureus. MIC50 values were higher than 250 μM for the four bacteria tested for the non-N α-acetylated arginine-based surfactants 1414R and 1212R.
Unexpectedly, for the 1414R and 1212R compounds, the substitution of the N α-amino group for an N α-acetylamide group changes the antimicrobial activity dramatically. To understand the molecular basis of these differences in antimicrobial performance, some relevant insight may be gained based on some theoretical considerations and on our experimental observations. On the one hand, the non-N α-acetylated arginine-based surfactants can dissociate in aqueous media in several species following an acid–base equilibrium (Pinazo et al. 2004). A range of pH exists in which the monocationic species predominate. In our studies, at culture pH media (~7), the α-amino group of the amino acid is largely deprotonated, rendering monocationic molecules. Therefore, the structure of these molecules is similar to that of the N α-acetylated arginine-based surfactants (only with a positive charge on the guanidine group); however their antimicrobial activity is much lower (Table 2). In general, the antimicrobial activity is attributed largely to the net positive charge; our results suggest that the relationship between charge and antimicrobial activity appears to be more complex (Papanastasiou et al. 2009; Valko and DuBois 1945). Our results agree with the complexity of physicochemical factors involved in the final antimicrobial outcome described in literature such as adsorption, hydrophobicity, aqueous solubility and diffusion in the test medium (see for example Denyer 1995; Russell 1995; Vieira and Carmona-Ribeiro 2006).
The non-N α-acetylated compounds 1212R and 1414R were considerably less hemolytic than the N α-acetylated ones, although their poor bactericidal activity precluded their use for further experiments.
Biological activity of catanionic vesicles
In an attempt to explore the potential applications of these arginine-based surfactants into applications close to clinical settings and taking into account the results exposed in the previous section, we developed a strategy of formulation to reduce the cytotoxicity while keeping their antimicrobial activities. It is widely known in literature that for cationic surfactants, the higher the antimicrobial activity the higher is the hemolytic activity. This trend has been attributed to the effective cationic charge on the headgroup. Therefore, what we intended was to change this general trend. The most likely option to improve the therapeutic index of the arginine-based surfactants was to tune its density charge by formulation. Mixed vesicles between the monocationic N α-acetylated arginine-based surfactants and both the anionic phospholipid PG, as catanionic vesicles, and the zwitterionic phospholipid DPPC, as control cationic vesicles, were formulated at two different surfactant/phospholipid molar ratios of 0.2 and 0.8. In PG-based formulations, the diacyl arginine compounds formed positively or negatively charged vesicles, depending on the major component in the mixture. This assumption is based on the results obtained in a previous study (Lozano et al. 2009) using the anionic phospholipid DPPA, which presents a structure and charge similar to the phospholipid PG used in the present study. Electrophoretic mobility (ζ-potential) was the property used to determine the charge of the catanionic vesicles at different ratio.
As expected, once corrected for the real concentration of the surfactant, bactericidal and hemolytic activity of the DPPC-based formulations resulted in the absence of synergism with respect to the surfactant alone (data not shown). However, in the formulations with PG, when catanionic vesicles had negative net charge, activity versus A. baumannii fell slightly, and it was maintained when incorporated into positively charged vesicles. In contrast, there was a significant improvement of the selectivity for the PG formulation with anionic vesicles on S. aureus (Table 3), correlated with a strong decrease of hemolytic activity (Pons et al. 2009).
The use of catanionic vesicles with a net negative charge results in a reduction of both hemolytic and Gram-negative lysis activities while increasing against Gram-positive bacteria. Positively charged vesicles as formulated either with DPPC or PG, however, results in negligible effect on the surfactant activities, that is, high hemolysis and activity against Gram-negative bacteria and moderate activity against Gram-positive ones. From these results, it is inferred that hemolysis and Gram-negative bacteria followed the same trend with the formulation effect. Reduction of hemolysis and activity against Gram-negative bacteria can be understood by considering the shielding effect of the formulation. These negatively charged catanionic vesicles will have a reduced affinity for negatively charged surfaces and conversely a higher affinity for positively or zwitterionic charged surfaces. The same effect could be responsible for the increased activity against Gram-positive bacteria. Although the surface charge of the bacteria is also negative, the release of active surfactant imposed by the formulation will be probably under the form of neutral ionic pairs, through the peptidoglycan layer; while the cationic surfactant may still access the plasma membrane, the insertion of the negatively charged phospholipid will be probably more difficult.
Dynamic surface tension for 1414RAc/PG aqueous dispersions
The first stage in surfactant-induced antimicrobial activity and hemolysis include the adsorption of surfactant molecules to components of the membrane surface and their intercalation into the membrane structure (Rideal and Taylor 1957, 1958). Much research has been done to understand the mechanism underlying these processes; however, despite the significant advances in understanding the lysis process, its mechanism is yet unclear. In an attempt to understand the mechanism of action on cells and to correlate the selectivity on the membrane with the net charge of the catanionic vesicles, adsorption of 1414RAc/PG binary mixtures on PG and DPPC-spread monolayers were studied (results are shown in Fig. 3). First, the adsorption isotherms of the catanionic vesicles into a clean air/water interface were studied. The adsorption of molecules at the air/water interface decreases the surface tension. The lower the surface tension, the higher is the adsorption (Lozano et al. 2010). Figure 3a shows the surface tension as a function of time for 1414RAc/PG mixtures at surfactant mole fractions of 0, 0.2, 0.8 and 1. Both anionic phospholipid PG and negatively charged catanionic vesicles show a moderate and slow adsorption rate at the air/water interface, reaching a minimum surface tension value of ca. 60 mN/m during the experimental time. In the adsorption isotherm of PG, a lag time of 7 min can be observed. When the surfactant is present at mole fraction of 0.2, the isotherm shows a lag time of only 3 min. Higher reduction in surface tension is observed when the cationic surfactant 1414RAc is the major component of the mixture, reaching a minimum surface tension value of ca. 20 mN/m. To elucidate if the origin of this activity is the surfactant itself or the synergism between both components in the mixture, it will be necessary to compare each isotherm of the binary systems with the corresponding pure 1414RAc isotherm at the same surfactant concentration. In Fig. 3a the 1414RAc adsorption isotherms at 20 and 80 μM are plotted. In both cases, the adsorption rate of the catanionic formulations with PG is slower compared with the corresponding pure surfactant adsorption rate. From these results, it can be concluded that the synergism observed in the adsorption rate is only with respect to the anionic phospholipid PG. That is, in 1414RAc/PG mixtures, the presence of surfactant favors the PG adsorption both in terms of adsorbed amount as well as in terms of its kinetics.
Dynamic surface tension of mixed 1414RAc/PG dispersions (C total = 100 μM) at surfactant mole fractions of 0 (squares), 0.2 (up triangles), 0.8 (down triangles) and 1 (diamonds) into a clean air/water interface (a), a PG spread monolayer (b), and a DPPC-spread monolayer (c). Dotted lines show the dynamic surface tension of pure 1414RAc at 20 μM (up triangles) and 80 μM (down triangles). Solutions were injected in a saline subphase (0.1 M sodium chloride) at 25°C
To understand the activity against Gram-positive bacteria of the 1414RAc/PG dispersions, penetration experiments of these catanionic mixtures were carried out into PG spread monolayers at 47 mN/m, which corresponds to the natural tension in biological membranes (Marsh 1996). The anionic phospholipid PG is considered to be the major component of the cytoplasmic bacterial membrane (Lohner et al. 2008). Figure 3b shows the dynamic surface tension of an insertion assay of 1414RAc/PG dispersions into PG spread monolayer at the same surfactant mole fractions studied in a clean air/water interface. As expected, negatively charged dispersions, corresponding to surfactant mole fractions of 0 and 0.2, inserted most readily into anionic films composed of PG due to electrostatic attractions, reaching a minimum surface tension value of ca. 22 mN/m. Moreover, the positively charged ones, at surfactant mole fractions of 0.8 and 1, also reduce the surface tension down to 28 mN/m. This decrease in surface tension could be related to a lack of viability in bacterial membranes, but does not agree with the experimental antimicrobial activity tested in this study. From these results, we can conclude that antimicrobial activity against Gram-positive bacteria by surfactants is not governed only by a membrane permeabilization mechanism. From pure 1414RAc isotherms at 20 and 80 μM, the minimum surface tension reached by a negative catanionic system is determined by the pure anionic phospholipid while a positive catanionic system is determined by the pure cationic surfactant.
Figure 3c shows dynamic surface tension for catanionic vesicles studied in DPPC-spread monolayer, used as mammalian membrane model. The injection of both PG dispersion and negatively charged catanionic vesicles into the saline subphase induces a small decrease on the surface tension down to ca. 40 mN/m. This behavior suggests that these anionic vesicles will not disturb the lipidic bilayer of mammalian cells, in agreement with the low hemolytic activity obtained in this study. The opposite behavior is observed for positively charged catanionic vesicles and pure cationic 1414RAc, reducing strongly the surface tension of DPPC-spread monolayer from ca. 47 to around 10 and 25 mN/m, respectively. Minimum surface tension was reached by the negatively charged catanionic vesicles, being lower than that corresponding to the pure surfactant at the same concentration (80 μM). This suggests that hemolytic activity is caused by a synergism between both components in the mixture. Because surface tension reduction is related to the degree of deformation of the mammalian cell membranes tested, the mechanism of hemolysis by 1414RAc/PG dispersions could be predicted by penetration studies using DPPC monolayers as a model of mammalian membranes.
Conclusions
This study demonstrates that the fine-tuning of the cationic character of diacyl glycerol arginine-based surfactants results in optimized antimicrobial activity and selectivity. High selectivity was the result of both enhanced antimicrobial activity against Gram-positive bacteria and reduced hemolytic activity.
The hemolysis activity by XXRAc/PG dispersions has been correlated with a decrease in the surface tension, using penetration studies in DPPC-spread monolayers as model mammalian membranes. From these results, penetration studies have been helpful in understanding the degree of deformation of mammalian cell membranes. However, antimicrobial activity against Gram-positive bacteria cannot be explained only by penetration studies using PG-spread monolayers as cytoplasmic bacterial membranes. This behavior suggests other mechanisms not related to membrane permeabilization.
Our results might be considered as a proof of principle of a strategy, which may reduce the toxicity of many surfactants, opening possibilities for biological applications. In these catanionic vesicles, the delivery rate was strongly dependent on the overall physical behavior of the system and on the driving forces leading to the formation of the aggregate, not only in vitro but also in the presence of biological fluids.
We have shown that formulating these hemolytic surfactants in the form of negatively charged catanionic vesicles reduced their cytotoxicity drastically. Moreover, the antimicrobial activity in the worst case is maintained and in some cases can be enhanced.
Abbreviations
- 1212R:
-
1,2-Dilauroyl-glycero-3-O-l-arginine dihydrochloride
- 1212RAc:
-
1,2-Dilauroyl-rac-glycero-3-O-(N α-acetyl-l-arginine) hydrochloride
- 1414R:
-
1,2-Dimyristoyl-glycero-3-O-l-arginine dihydrochloride
- 1414RAc:
-
1,2-Dimyristoyl-rac-glycero-3-O-(N α-acetyl-l-arginine) hydrochloride
- DPPC:
-
1,2-Dipalmitoyl-sn-glycero-3-phosphatidylcholine
- HC50 :
-
Surfactant concentration inducing 50% of hemolysis
- HPLC:
-
High-performance liquid chromatography
- MIC:
-
Minimum inhibitory concentration
- PG:
-
Phosphatidylglycerol
- SD:
-
Standard deviation
- OD:
-
Optical density
References
Benavides T, Mitjans M, Martinez V, Clapes P, Infante MR, Clothier RH, Vinardell MP (2004) Assessment of primary eye and skin irritants by in vitro cytotoxicity and phototoxicity models: an in vitro approach of new arginine-based surfactant-induced irritation. Toxicology 197:229–237. doi:10.1016/j.tox.2004.01.011
Bonincontro A, Spigone E, Pena MR, Letizia C, La Mesa C (2006) Lysozyme binding onto cat-anionic vesicles. J Colloid Interface Sci 304:342–347. doi:10.1016/j.jcis.2006.09.046
Bonincontro A, Falivene M, La Mesa C, Risuleo G, Pena MR (2008) Dynamics of DNA adsorption on and release from SDS-DDAB cat-anionic vesicles: a multitechnique study. Langmuir 24:1973–1978. doi:10.1021/la701730h
Bramer T, Dew N, Edsman K (2007) Pharmaceutical applications for catanionic mixtures. J Pharm Pharmacol 59:1319–1334. doi:10.1211/jpp.59.10.0001
Brito RO, Marques EF, Silva SG, Vale ML, Gomes P, Araujo MJ, Rodriguez-Borges JE, Infante MR, Garcia MT, Ribosa I, Vinardell MP, Mitjans M (2009) Physicochemical and toxicological properties of novel amino acid-based amphiphiles and their spontaneously formed catanionic vesicles. Colloids Surf B 72:80–87. doi:10.1016/j.colsurfb.2009.03.017
Burgo P, Aicart E, Junquera E (2007) Mixed vesicles and mixed micelles of the cationic–cationic surfactant system: didecyldimethylammonium bromide/dodecylethyldimethylammonium bromide/water. Colloids Surf A 292:165–172. doi:10.1016/j.colsurfa.2006.06.019
Caria A, Khan A (1996) Phase behavior of catanionic surfactant mixtures: sodium bis(2-ethylhexyl) sulfosuccinate–didocyldimethylammonium bromide–water system. Langmuir 12:6282–6290. doi:10.1021/la960581z
Denyer SP (1995) Mechanisms of action of antibacterial biocides. Int Biodeterior Biodegradation 36:227–245
Epand RF, Infante MR, Flanagan TD, Epand RM (1998) Properties of lipoamino acids incorporated into membrane bilayers. Biochim Biophys Acta 1373:67–75
Fogt A, Hägerstrand H, Isomaa B (1995) Effects of N,N′-bisdimethyl-1,2-ethanediamine dichloride, a double-chain surfactant, on membrane-related functions in human erythrocytes. Chem Biol Interact 94:147–155
Infante MR, Molinero J, Erra P, Julia R, Garcia-Dominguez JJ (1985) A comparative-study on surface-active and antimicrobial properties of some N α-lauroyl-l α, omega dibasic amino-acids derivatives. Fette Seifen Anstrichmittel 87:309–313
Kamenka N, Chorro M, Talmon Y, Zana R (1992) Study of mixed aggregates in aqueous-solutions of sodium dodecyl-sulfate and dodecyltrimethylammonium bromide. Colloids Surf 67:213–222
Lohner K, Sevcsik E, Pabst G (2008) Liposome-based biomembrane mimetic systems: implications for lipid–peptide interactions. Adv Planar Lipid Bilayers Liposomes 6:103–137. doi:10.1016/S1554-4516(07)06005-X
Lozano N, Perez L, Pons R, Luque-Ortega JR, Fernandez-Reyes M, Rivas L, Pinazo A (2008) Interaction studies of diacyl glycerol arginine-based surfactants with DPPC and DMPC monolayers, relation with antimicrobial activity. Colloids Surf A 319:196–203. doi:10.1016/j.colsurfa.2007.07.015
Lozano N, Pinazo A, La Mesa C, Perez L, Andreozzi P, Pons R (2009) Catanionic vesicles formed with arginine-based surfactants and 1,2-dipalmitoyl-sn-glycero-3-phosphate monosodium salt. J Phys Chem B 113:6321–6327. doi:10.1021/jp810671p
Lozano N, Pinazo A, Perez L, Pons R (2010) Dynamic properties of cationic diacyl-glycerol-arginine-based surfactant/phospholipid mixtures at the air/water interface. Langmuir 26:2559–2566. doi:10.1021/la902850j
Marques EF, Brito RO, Wang YJ, Silva BFB (2006) Thermotropic phase behavior of triple-chained catanionic surfactants with varying headgroup chemistry. J Colloid Interface Sci 294:240–247. doi:10.1016/j.jcis.2005.07.021
Marques EF, Brito RO, Silva SG, Rodriguez-Borges JE, Vale ML, Gomes P, Araujo MJ, Soderman O (2008) Spontaneous vesicle formation in catanionic mixtures of amino acid-based surfactants: chain length symmetry effects. Langmuir 24:11009–11017. doi:10.1021/la801518h
Marsh D (1996) Lateral pressure in membranes. Biochim Biophys Acta Biomembr 1286:183–223. doi:10.1016/S0304-4157(96)00009-3
Miyagishi S, Asakawa T, Nishida M (1989) Hydrophobicity and surface-activities of sodium salts of N-dodecanoyl amino-acids. J Colloid Interface Sci 131:68–73
Moran C, Infante MR, Clapes P (2001) Synthesis of glycero amino acid-based surfactants. Part 1. Enzymatic preparation of rac-1-O-(N α-acetyl-l-aminoacyl)glycerol derivatives. J Chem Soc, Perkin Trans I, 2063-2070. doi:10.1039/b103132p
Moran C, Infante MR, Clapes P (2002) Synthesis of glycero amino acid-based surfactants. Part 2. Lipase-catalysed synthesis of 1-O-lauroyl-rac-glycero-3-O-(N α-acetyl-l-amino acid) and 1,2-di-O-lauroyl-rac-glycero-3-O-(N α-acetyl-l-amino acid) derivatives. J Chem Soc, Perkin Trans I 1124–1134. doi:10.1039/hb200577h
Papanastasiou EA, Hua Q, Sandouk A, Son U, Christenson AJ, Van Hoek ML, Bishop BM (2009) Role of acetylation and charge in antimicrobial peptides based on human β-defensin-3. APMIS 117:492–499. doi:10.1111/j.1600-0463.2009.02460.x
Perez L, Torres JL, Manresa A, Solans C, Infante MR (1996) Synthesis, aggregation, and biological properties of a new class of gemini cationic amphiphilic compounds from arginine, bis(Args). Langmuir 12:5296–5301
Perez L, Infante MR, Angelet M, Clapes P, Pinazo A (2004a) Glycerolipid arginine-based surfactants: synthesis and surface active properties. Prog Colloid Polym Sci 123:210–216. doi:10.1007/b11628
Perez L, Infante MR, Pons R, Moran MC, Vinardell P, Mitjans M, Pinazo A (2004b) A synthetic alternative to natural lecithins with antimicrobial properties. Colloids Surf B 35:235–242. doi:10.1016/j.colsurfb.2004.03.014
Peypoux F, Laprevote O, Pagadoy M, Wallach J (2004) N-Acyl derivatives of Asn, new bacterial N-acyl d-amino acids with surfactant activity. Amino Acids 26:209–214. doi:10.1007/s00726-003-0056-2
Pinazo A, Perez L, Infante MR, Pons R (2004) Unconventional vesicle-to-ribbon transition behaviour of diacyl glycerol amino acid-based surfactants in extremely diluted systems induced by pH-concentration effects. Phys Chem Chem Phys 6:1475–1481. doi:10.1039/b313313n
Pinazo A, Angelet M, Pons R, Lozano M, Infante MR, Perez L (2009) Lysine–bisglycidol conjugates as novel cationic surfactants. Langmuir 25:7803–7814. doi:10.1021/la901675p
Pons R, Pinazo A, Lozano N, Rivas L, Fernandez-Reyes M, Luque-Ortega JR, Perez L, Infante MR, Moran C (2009) Formulaciones de lipoaminoácidos catiónicos con menor poder hemolítico basadas en la formación de pares cataniónicos. Spanish Pat. Appl. ES1641.261, November 13
Rideal E, Taylor FH (1957) On haemolysis by anionic detergents. Proc R Soc Lond B 146:225–241
Rideal E, Taylor FH (1958) On haemolysis and haemolytic acceleration. Proc R Soc Lond B 148:450–464
Russell AD (1995) Mechanisms of bacterial resistance to biocides. Int Biodeterior Biodegrad 36:247–265
Sanson A, Egret-Chalier M, Bouloussa O, Maget-Dana R, Ptak M (1987) N-acyl aminoacids: amphiphilic properties and interactions with the lipid bilayers. In: Mittal KL, Bothorel P (eds) Surfactant in solution, vol 5, pp 793–805
Saugar JM, Rodriguez-Hernandez MJ, de la Torre BG, Pachon-Ibanez ME, Fernandez-Reyes M, Andreu D, Pachon J, Rivas L (2006) Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: molecular basis for the differential mechanisms of action. Antimicrob Agents Chemother 50:1251–1256. doi:10.1128/AAC.50.4.1251-1256.2006
Valko EI, DuBois AS (1945) Correlation between antibacterial power and chemical structure of higher alkyl ammonium ions. J Bacteriol 50:481–490
Vieira DB, Carmona-Ribeiro AM (2006) Cationic lipids and surfactants as antifungal agents: mode of action. J Antimicrob Chemother 58:760–767. doi:10.1093/jac/dk1312
Vinardell MP, Benavides T, Mitjans M, Infante MR, Clapes P, Clothier R (2008) Comparative evaluation of cytotoxicity and phototoxicity of mono and diacyl glycerol amino acid-based surfactants. Food Chem Toxicol 46:3837–3841. doi:10.1016/j.fct.2008.10.007
Xia J, Xia Y, Nnanna IA (1995) Structure–function relationship of acyl amino acid surfactants: surface activity and antimicrobial properties. J Agric Food Chem 43:867–871
Acknowledgments
We thank the invaluable advice, suggestions, comments and critical reading of the manuscript by Dr. Luís Rivas and Dra. María Fernández-Reyes from the Centro de Investigaciones Biológicas (CIB-CSIC) of Madrid on biological experiments. Financial support from the Spanish Ministry of Science and Innovation (CTQ2007-604091/BQU and CTQ2009-14151-CO2-01) and Generalitat de Catalunya (2009SGR1331) is gratefully acknowledged. We are also grateful to Ms. Imma Carrera for technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lozano, N., Pérez, L., Pons, R. et al. Diacyl glycerol arginine-based surfactants: biological and physicochemical properties of catanionic formulations. Amino Acids 40, 721–729 (2011). https://doi.org/10.1007/s00726-010-0710-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0710-4