Abstract
Individual mouse strains differ significantly in terms of behavior and cognitive function. Strain-specific variation of metabolic protein levels in the hippocampus among various commonly used mouse strains, however, has not been investigated yet. A proteomic approach based on two-dimensional gel electrophoresis (2-DE) coupled with mass spectrometry [high capacity ion trap (HCT)] has been chosen to address this question by determining strain-dependent levels of metabolic proteins in hippocampal tissue of four inbred and one outbred mouse strain. Statistical analysis of protein spots on 2-DE gels of the individual strains (n = 10) revealed significant strain-dependent differences in densities of 39 spots. Subsequent HCT analysis led to the identification of 22 different metabolic proteins presenting with differential protein levels among the five mouse strains investigated. Among those are proteins concerned with the metabolism of amino acid, nucleic acid, carbohydrate and energy. Moreover, proteins known to play a pivotal role in the processes of learning and memory, such as calcium/calmodulin-dependent protein kinase type II alpha chain, were found to present with significant inter-strain variability, which is also in agreement with our previous reports. Strain-specific protein levels of metabolic proteins in the mouse hippocampus may provide some insight into the molecular underpinnings and genetic determination of strain-dependent neuronal function. Furthermore, data presented herein emphasize the significance of the genetic background for the analysis of metabolic pathways in the hippocampus in wild-type mice as well as in gene-targeting experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mouse as model to study processes related to brain functions is undoubtedly one of the most valuable resources in modern neuroscience research. Wild-type mice are used to address questions in the fields of neuropharmacology and neurotoxicology, as well as for morphological and structural analysis aiming to uncover the neurobiological principles underlying brain function. Transgenic and knockout mouse lines are widely used as tools to learn about the function of particular genes and entire pathways, and their involvement in processes relating to neuronal information processing and storage (Schimanski and Nguyen 2004). In these lines of research, the hippocampus, known for its pivotal role in cognition and behavior, is a region of central interest. In addressing questions on cognition and behavior, however, the use of inbred mouse strains has to be carefully evaluated in the context of the known strain-dependent variability in behavioral domains, including hippocampus-dependent learning and memory (Crawley et al. 1997; Fordyce et al. 1994; Upchurch and Wehner 1989), hippocampal long-term potentiation (LTP) (Gerlai 2002; Nguyen et al. 2000), hippocampal gene (Fernandes et al. 2004) and protein expression (Pollak et al. 2006a, b; Sunyer et al. 2009), as well as response to pharmacological treatments (Sunyer et al. 2007, 2008). Comprehensive information on the “protein chemical background” of the individual strains with regard to molecules related to metabolic activities, importantly influencing neuronal function, however, is limited. We therefore decided to use a proteomic approach, based on two-dimensional gel electrophoresis (2-DE) coupled to mass spectrometry [high capacity ion trap (HCT)], to determine strain dependence of the “metabolome” of proteins expressed in the hippocampus of four inbred and one outbred mouse strains. The aim of this study was to establish a protein reference database of strain variability of metabolic proteins in the mouse hippocampus as a tool to determine the most suitable strains to be used in metabolic, pharmacological and toxicological studies, as well as in gene-targeting experiments.
Materials and methods
Animals
The subjects were FVB/Nhim (FVB), C57BL/6JHim (C57), 129S2/SvHim (129 Sv), Balb/cHim (Balb) and Him:OF1 (OF) (n = 10 for each strain) adult male mice, approximately 20 weeks old. Animals were housed in standard transparent laboratory cages in a temperature-controlled colony room (22 ± 1°C). They were maintained on a 12-h artificial light/dark cycle (with lights on at 6:00 a.m.) and provided with food and water ad libitum. Mice were killed by neck dislocation and hippocampi were dissected. Tissue samples were immediately frozen in liquid nitrogen and stored at −80°C until use for analysis. All experiments on animals were in line with the UK. Animals (Scientific procedures) Act, 1996 and associated guidelines (86/609/EEC) and approved by the local animal committee (confirmation number: LF1-TVG-17/002-2004).
Sample preparation
Hippocampal tissue was powdered and resuspended in 1.0 ml of sample buffer consisting of 7 M urea (Merck, Darmstadt, Germany), 2 M thiourea (Sigma, St. Louis, MO, USA), 4% CHAPS (3-[(3-cholamidopropyl) dimethylammonio]-1-propane-sulfonate) (Sigma, St. Louis, MO, USA), 65 mM 1,4-dithioerythritol (Merck, Germany), 1 mM EDTA (ethylenediaminetetraacetic acid) (Merck, Germany), protease inhibitors Complete® (Roche, Basel, Switzerland) and 1 mM phenylmethylsulfonyl chloride. The suspension was sonicated for approximately 15 s. After homogenization, samples were left at room temperature for 1 h and centrifuged at 14,000×g for 1 h. The supernatant was transferred into Ultrafree-4 centrifugal filter unit (Millipore, Bedford, MA, USA) for desalting and concentrating proteins. Protein content of the supernatant was determined by Bradford protein assay system (Bradford 1976). The standard curve was generated using bovine serum albumin and absorbance was measured at 595 nm.
Two-dimensional gel electrophoresis (2-DE)
Samples prepared from each individual mouse (n = 10 per strain) were subjected to 2-DE as described elsewhere (Weitzdoerfer et al. 2002). On immobilized pH 3–10 nonlinear gradient strips at their basic and acidic ends, 700 μg of protein was applied. Focusing was started at 200 V and voltage was gradually increased to 8,000 V over 31 h and then kept constant for a further 3 h (approximately 150,000 Vh totally). After the first dimension, strips (18 cm) were equilibrated for 15 min in the buffer containing 6 M urea, 20% glycerol, 2% SDS and 2% DTT and then for 15 min in the same buffer containing 2.5% iodo-acetamide instead of DTT. After equilibration, strips were loaded on 9–16% gradient sodium dodecylsulfate polyacrylamide gels for second-dimensional separation. Gels (180 × 200 × 1.5 mm) were run at 40 mA per gel. Immediately after the second dimension run, gels were fixed for 18 h in 50% methanol, containing 10% acetic acid. The gels were then stained with colloidal Coomassie Blue (Novex, San Diego, CA, USA) for 12 h on a rocking shaker. Molecular masses were determined by running standard protein markers (Biorad Laboratories, Hercules, CA, USA) covering the range 10–250 kDa. pI values 3–10 were used as given by the supplier of the immobilized pH gradient strips (Amersham Bioscience, Uppsala, Sweden). Excess dye was washed out from the gels with distilled water and the gels were scanned with ImageScanner (Amersham Bioscience). Electronic images of the gels were recorded using Adobe Photoshop and Microsoft Power Point Software.
Quantification of protein levels
Based on the reference protein map of the mouse hippocampus (Pollak et al. 2006b), protein spots representing metabolic proteins were outlined (first automatically and then manually) from every gel (n = 50 in total) and quantified using the Proteomweaver software (Definiens, Munich, Germany). The percentage of the volume of the spots representing a certain protein was determined in comparison with the total proteins present in the 2-DE gel (Pollak et al. 2006a).
Analysis of peptides by Nano-LC–ESI-(CID/ETD)-MS/MS [high capacity ion trap (HCT)]
A total of 39 spots, which showed different levels among the strains, were selected for verification of protein identity. Spots were manually excised and placed into 0.5 mL LoBind Eppendorf tubes. In-gel digestion and sample preparation for HCT analysis were performed as described before (Chen et al. 2006). Gel plugs were washed with 10 mM ammonium bicarbonate and subsequently with 50% acetonitrile in 10 mM ammonium bicarbonate, repeatedly. Addition of 100% acetonitrile resulted in gel shrinking and the shrunk gel plugs were then Speedvac dried in a Speedvac Concentrator 5301 (Eppendorf, Hamburg, Germany). The dried gel pieces were reswollen, in-gel digested with 40 ng/μL trypsin (Promega, Madison, WI, USA) in digestion buffer [consisting of 5 mM Octyl β-D-glucopyranoside (OGP) and 10 mM ammonium bicarbonate] and incubated overnight at 37°C. Peptide extraction was performed with 20 μL of 1% TFA in 5 mM OGP for 30 min, and subsequently 0.1% TFA in 4% acetonitrile for 30 min. The extracted peptides were pooled for HCT analysis.
Using HCT, 40 μl of extracted peptides were analyzed. The HPLC used was a biocompatible Ultimate 3000 system (Dionex Corporation, Sunnyvale, CA, USA) equipped with a PepMap100 C-18 trap column (300 μm × 5 mm) and PepMap100 C-18 analytic column (75 μm × 150 mm). Peptides were first loaded to the trap column and after washing were run on the separation column. The gradient in the separation column was (A = 0.1% formic acid in water, B = 0.08% formic acid in acetonitrile) 4–30% B from 0 to 105 min, 80% B from 105 to 110 min and 4% B from 110 to 125 min. The flow rate was 300 nL/min from 0 to 12 min, 75 nL/min from 12 to 105 min and 300 nL/min from 105 to 125 min. An HCT ultra ETDII PTM discovery system (Bruker Daltonics, Bremen, Germany) was used to record peptide spectra over the mass range of m/z 350–1,500, and MS/MS spectra in information-dependent data acquisition over the mass range of m/z 100–2,800. Repeatedly, MS spectra were recorded followed by three data-dependent CID MS/MS spectra and three ETD MS/MS spectra generated from three highest intensity precursor ions. An active exclusion of 0.4 min after two spectra was used to detect low abundant peptides. The voltage between ion spray tip and spray shield was set to 1,100 V. Drying nitrogen gas was heated to 170°C and the flow rate was 10 L/min. The collision energy was set automatically according to the mass and charge state of the peptides chosen for fragmentation. Multiple charged peptides were chosen for MS/MS experiments due to their good fragmentation characteristics.
MS/MS spectra were interpreted and peak lists were generated by DataAnalysis 4.0 (Bruker Daltonics, Bremen, Germany). Searches were done using the MASCOT 2.2.04 (Matrix Science, London, UK) against the latest UniProtKB database for protein identification. Searching parameters were set as follows: enzyme selected as trypsin with two maximum missing cleavage sites, species limited to human, a mass tolerance of 0.2 Da for peptide tolerance, 0.2 Da for MS/MS tolerance, fixed modification of carbamidomethyl (C) and variable modification of methionine oxidation and phosphorylation (Tyr, Thr, and Ser). Positive protein identifications were based on a significant MOWSE score. After protein identification, an error-tolerant search was done to detect unspecific cleavage and unassigned modifications. Protein identification and modification information returned from MASCOT were manually inspected and filtered to obtain confirmed protein identification and modifications.
Statistical analysis
Values are expressed as means ± standard deviation (SD) of percentage of the spot volume in each particular gel after subtraction of the background values. Between-group differences were analyzed using the Kruskal–Wallis rank sum test for strain effects. Proteins with significant effects were selected by adjusting the resulting p values for multiple testing. GraphPad InStat version (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses and the level of significance was set at P < 0.05 at all instances.
Results
Protein quantification and statistical analysis
Based on an integrated map of the murine hippocampal proteome using five mouse strains (Pollak et al. 2006b), spots representing metabolic proteins were selected for software-assisted quantification and statistical analysis. 39 spots were found to present with statistically significant different protein levels among the five strains examined (see Fig. 1, Tables 1 and 2).
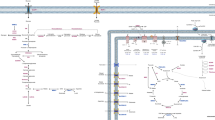
Two-dimensional map of mouse hippocampal metabolic proteins with statistically significant different levels among inbred mouse strains. Mouse hippocampal proteins were extracted and 700 μg were applied on an immobilized. pH 3–10 non-linear gradient strip, followed by 9–16% linear gradient polyacrylamide gel. Gels were stained with Coomassie Blue, spots were analyzed by nano-LC–ESI-(CID/ETD)-MS/MS and proteins were assigned using MASCOT software. SWISS-PROT accession numbers for protein identification are given
Protein identification
Spots with statistically significantly different protein levels were then excised and used for mass spectrometric analysis to confirm their identity and to protein-chemically characterize metabolic proteins with strain-dependent protein levels. HCT analysis led to the unambiguous identification (sequence coverage up to 70% (average 38%) of these 39 spots that were found to represent 22 different metabolic proteins (see Table 1). Proteins identified with strain-dependent protein levels belong to metabolic pathways related to amino acid metabolism (including glutamate dehydrogenase 1 and ornithine aminotransferase, mitochondrial), nucleic acid metabolism (including adenine phosphoribosyltransferase), carbohydrate metabolism (including phosphoglycerate kinase 1 and l-lactate dehydrogenase A chain) and energy metabolism (including ATP synthase subunit alpha, mitochondrial and V-type proton ATPase catalytic subunit A) (see Table 3).
Pair-wise comparisons
Pair-wise comparisons among the individual strains revealed that the most differences were found among the 129Sv and FVB strains (13 significant differences in spot levels) followed by Balb versus OF mouse strains (11 significant differences in spot levels). No statistically significant differences in pair-wise comparisons were found among the FVB/N and OF1 strains (see Table 4).
Discussion
We here show for the first time significant strain dependency of metabolic protein levels in the mouse hippocampus using a proteomic approach. Proteins belonging to several different metabolic systems have been found to present with strain-dependent levels.
Amino acid metabolism
Isocitrate dehydrogenase [NAD] subunit alpha
Mutations in the enzyme cytosolic isocitrate dehydrogenase, resulting in loss of the enzyme’s ability to catalyze conversion of isocitrate to alpha-ketoglutarate, are a common feature of a major subset of primary brain tumors (Dang et al. 2009; Yan et al. 2009). Thus, strain-specific expression of this protein has to be respected particularly in mouse models of neuronal tumors or cell lines derived from a particular strain and used for brain cancer research.
Isovaleryl-CoA dehydrogenase, mitochondrial
Isovaleryl-CoA dehydrogenase is an enzyme of the leucine catabolism pathway. Isovaleryl-CoA dehydrogenase deficiency leads to accumulation of toxic intermediate metabolites causing cell damage and death (Kimmoun et al. 2008). Lowest levels of isovaleryl-CoA dehydrogenase were observed in Balb mice and might affect the bioavailability of leucine thereby impacting on proteins synthesis essential for the processes of learning and memory.
Glutamate dehydrogenase 1 (Glud1)
Glud1 is an important enzyme in the pathway of glutamate synthesis in nerve terminals. Enhanced Glud1 protein levels have been associated with increased glutamate release after neuronal depolarization resulting in excitotoxic effects and consequently neuronal loss (Bao et al. 2009). High levels of Glud1 in the FVB strain may be of relevance when using these mice for electrophysiological studies on hippocampal cells and adaptation of the experimental protocol may have to be considered.
Ornithine aminotransferase (OAT)
OAT is an enzyme involved in the biosynthesis of glutamate by catalyzing the transfer of the delta amino group of Orn to 2-oxoglutarate, resulting in the generation of l-glutamate-gamma-semialdehyde and l-glutamate (Seiler 2000). Moreover, GABA is a feedback regulator of OAT. Strain-dependent regulation of OAT levels might impact the GABAergic and glutaminergic neurotransmitter systems.
Calcium/calmodulin-dependent protein kinase type II alpha chain (CaMKII)
CaMKII is one of the proteins with a pivotal role in the modulation of synaptic transmission, from phosphorylation of neurotransmitter receptors to the induction of cytoskeletal rearrangements leading to activity-dependent modulation of dendritic spines. Using a proteomic approach we confirm significant strain-specific CaMKII protein levels previously described by an immunochemical approach (Pollak et al. 2005). Indeed, as found using Western blotting, quantification of 2-DE gels also revealed highest levels in the 129Sv strain and lowest levels in Balb mice.
Nucleic acid metabolism
Adenine phosphoribosyltransferase (APRT)
APRT is an enzyme importantly involved in the processes of purine salvage. Deficiencies in purine metabolism as a consequence of inborn errors are commonly reflected in neurological defects and behavioral abnormalities (Camici et al. 2010). Although most frequently related to genetically determined deficiencies in hypoxanthine-guanine phosphoribosyltransferase (HRPT), elevated APRT activity is also a common finding in these patients and may also contribute to purine overproduction and symptomatology (Wilson et al. 1982). A defined role for APRT in neuronal function and the relevance of strain-dependent APRT protein levels remain to be clarified.
Ribose-phosphate pyrophosphokinase 1 (phosphoribosyl pyrophosphate synthetase I, PRPS1)
PRPS1 catalyzes the synthesis of phosphoribosylpyrophosphate, essential for nucleotide biosynthesis. Protein levels of PRPS1 were almost double in C57, compared to the FVB strain. Mutations in PRPS1 have been shown to cause hereditary peripheral neuropathy with hearing loss and optic neuropathy (Kim et al. 2007), indicating an important contribution of PRPS1 to physiological neural function.
Carbohydrate metabolism
Fructose-bisphosphate aldolase A
Fructose-bisphosphate aldolase A is required for the breakdown of fructose 1,6-bisphosphate during glycolysis. Of the three iso-enzymes (A, B, and C), the A isoform is primarily found in muscle tissue and also in the brain and other nervous system elements, in comparable levels to the C isoform. The expression of Aldolase A in the hippocampus has been investigated and related to Alzheimer’s disease (Sultana et al. 2007; Mor et al. 2005). We identified strain-specific protein levels of three spots corresponding to Aldolase A distinguished by differences in the pI and probably representing post-translational modifications. Interestingly, strain distribution of protein levels varied among the three spots suggesting the preferential use of particular post-translational modifications in the individual strains.
Fructose-bisphosphate aldolase C
Aldolase C is expressed specifically in the hippocampus and Purkinje cells of the brain. Five spots showing strain-specific protein levels were identified as Aldolase C (ranging from pI 6.6 to 7.9). Interestingly, highest protein levels were observed in the 129Sv strain in spots 1–3 and 5, while 129Sv had lowest protein levels in spot 4 suggesting a strain regulation in the use of the individual Aldolase C spots, possibly representing post-translational modifications.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
GAPDH was initially described as an important enzyme in the sixth step of glycolysis. In addition, it has been proposed that it could be involved in various other cellular functions, from apoptosis induction to receptor-associated kinase properties (Tarze et al. 2007). In the nervous system, GAPDH has been found to be overexpressed in several neurodegenerative diseases, such as Hungtington, Parkinson and Alzheimer diseases (Mazzola and Sirover 2002). Two spots representing GAPDH were identified with strain-dependent protein levels, one corresponding to the theoretical pI of GAPDH at 8.4 and one at around 7.7, possibly due to post-translational modifications, such as acetylation. In both cases, protein levels were lowest in the FVB strain.
l-lactate dehydrogenase A chain (LADH-A)
LADH-A catalyzes the interconversion of lactate and pyruvate with concomitant interconversion of NADH and NAD+, which is the terminal step in the glycolytic sequence. Due to the brain’s high metabolic rate and near complete dependence on glucose for the maintenance of neural activity (Kanatani et al. 1995), alterations in carbohydrate metabolic pathways may importantly influence neuronal function. Strain-dependent regulation of LADH-A protein levels may lead to differences in metabolic rates of the glycolysis pathway, which could contribute to inter-strain variability in cognition and behavior.
Phosphoglycerate kinase 1 (PK1)
PGK1 is the catalyst responsible for transferring a phosphate group from 1,3-biphosphoglycerate to ADP resulting in the formation of ATP and 3-phosphoglycerate. As for LADH-A, inter-strain variability of PGK1 protein levels may relate to variability of metabolic rates during glycolysis, thus potentially impacting on neuronal function.
Pyruvate carboxylase, mitochondrial (PC)
PC mediates the reversible carboxylation of pyruvate to form oxaloacetate as substrate for the citric cycle and the synthesis of phosphoenolpyruvate from pyruvate during gluconeogenesis. PC is of central importance for the intermediary metabolism of neurons, and pyruvate carboxylase deficiency has been associated with malformations of the brain and brain amino acid abnormalities (Perry et al. 1985). PC is represented by two spots showing strain-dependent protein levels and the biological significance of this strain dependency remains to be elucidated.
Pyruvate kinase isozymes M1/M (PK)
PK is involved in the final enzymatic reaction in glycolysis, catalyzing the formation of pyruvate from phosphoenolpyruvate. Protein levels of AH are almost threefold higher in FVB and OF mice than in 129Sv, Balb and C57 strains. Derangement of PK protein levels have been observed in fetal brain tissue in Down syndrome, a cause of mental retardation (Bajo et al. 2002), suggesting an important role of PK in normal brain function.
Triosephosphate isomerase (TPI)
TPI plays an important role in glycolysis and is essential for efficient energy production. TPI deficiency has been associated with progressive neurological dysfunction (Orosz et al. 2006) highlighting the importance of this enzyme for brain function. TPI is represented by two spots with inter-strain differences in protein levels, potentially representing different post-translational modifications of the TPI enzyme. The spot at pI 7.2 shows highest levels in the 129Sv strain, whereas the spot at pI 7.4 has lowest levels in 129Sv mice suggesting strain specificity in the usage of the specific forms of the TPI protein.
Energy metabolism
Aconitate hydratase, mitochondrial (AH)
AH is required for the reversible isomerization of citrate and isocitrate in the citrate cycle, responsible for providing NADH to the respiratory chain. Elevated protein levels of AH have been described in the fetal, but not in the adult, Down syndrome brain (Bajo et al. 2002). Levels of AH proteins are around threefold higher in FVB and OF than in 129Sv, Balb and C57 mouse strains. However, the relevance of these remarkable differences for behavior and cognition in 129Sv mice remains to be further elaborated.
V-type proton ATPase catalytic subunit A
V-ATPase constitutes a multi-subunit enzyme regulating organelle acidification that in turn regulates important processes such as synaptic vesicle proton gradient generation. Blocking V-ATPase in hippocampal neurons significantly affects the electric properties of the cells (Groemer and Klingauf 2007). Thus, homeostasis of V-ATPase protein may importantly regulate brain function. Three spots representing V-ATPase were found to exhibit strain regulation of protein levels with the spot at around 40 kDa, below the calculated molecular weight of the V-ATPase subunit A, possibly constituting a splice variant. In any instance, protein levels were highest in 129Sv mice and this may relate to specifics of brain function described in this strain (Crawley et al. 1997).
V-type proton ATPase subunit B, brain isoform
The brain-specific isoform of the V-ATPase subunit B was displayed in one spot showing inter-strain variability in protein levels. For subunit A, protein levels are highest in 129Sv mice, reaching more than 400% of protein levels observed in the OF and FVB strains.
ATP synthase subunit alpha and beta, mitochondrial
ATP synthase is required for ATP synthesis during oxidative phosphorylation in the mitochondrial respiratory system. Regulation of ATP levels is crucial for neural function and the observed effects of strain genotype on the protein levels of ATP synthase subunit alpha and beta may be a first hind toward inter-strain variability in brain cellular energy production systems.
Creatine kinase U-type, mitochondrial (CK)
CK mediates the catalytic reaction during which creatine and ATP are converted to phosphocreatine and ADP. In neurons, cells with a high rate of consumption of ATP, phosphocreatine as energy reservoir is of major importance, thus designating CK as one of the key enzymes of energy metabolism in brain tissue. Protein levels of CK were highest in OF mice and lowest in the 129Sv strain pointing toward differences in metabolic rates among the individual strains.
Phosphoenolpyruvate carboxykinase [GTP], mitochondrial (PEPCK)
PEPCK catalyzes the conversion of oxaloacetate into phosphoenolpyruvate and carbon dioxide during gluconeogenesis. PEPCK is involved in anaplerosis [de novo formation of intermediates of the tricarboxylic acid (TCA) cycle], which provides an important substitute for losses of TCA cycle intermediates, especially alpha-ketoglutarate, required for the synthesis of glutamate and GABA (Hassel 2000). Thus, strain differences in PEPCK protein levels might affect neurotransmitter synthesis under conditions of high losses of glutamate and GABA in neurons.
The description of strain dependency in the levels of metabolic proteins in the mouse hippocampus is novel and of relevance to the neuroscientific community for several reasons:
First, these data provide evidence for a genetic variation in metabolic brain protein expression. Genetic approaches in future studies mapping these proteins onto the mouse genome may lead to mapping of chromosomal positions not only for genes encoding for metabolic proteins, but also for regulatory loci and modifier genes (Klose et al. 2002).
Second, these data form the basis for further experiments aiming to associate protein expression results with behavioral information that may in turn allow for a more defined assumption about the role of the individual metabolic proteins for specific phenotypes.
Third, strain-dependent levels of metabolic proteins have to be taken into account for pharmacological and toxicological studies using compounds, the metabolization of which involves proteins described herein. Variability in levels of the respective proteins may impact on the pharmacokinetics and bioavailability of the substance studied and should be observed when extending findings obtained in one particular strain to other strains, or relating them to independent sets of experiments carried out in a different strain.
Fourth, when selecting a background strain for gene targeting or transgenic approaches, these strain-specific levels of metabolic proteins should be considered since the interaction of the genetic alteration with the particular genetic background may enhance or mask the effect of the manipulation of the gene(s) studied. Moreover, when genetically engineered mice are bred on a mixed background, careful selection of proper experimental controls is required to ensure that results obtained in genetically modified mice indeed result from the genetic alteration itself and not reflect strain-specific differences.
In summary, although we only quantified and identified a small group of all of the proteins present in the mouse hippocampus, we here describe significant differences in levels of metabolic proteins between five different mouse strains that may provide a reference for future neuroscientific studies based on these mouse strains.
References
Bajo M, Fruehauf J, Kim SH, Fountoulakis M, Lubec G (2002) Proteomic evaluation of intermediary metabolism enzyme proteins in fetal Down’s syndrome cerebral cortex. Proteomics 2:1539–1546
Bao X, Pal R, Hascup KN, Wang Y, Wang WT, Xu W, Hui D, Agbas A, Wang X, Michaelis ML, Choi IY, Belousov AB, Gerhardt GA, Michaelis EK (2009) Transgenic expression of Glud1 (glutamate dehydrogenase 1) in neurons: in vivo model of enhanced glutamate release, altered synaptic plasticity, and selective neuronal vulnerability. J Neurosci 29:13929–13944
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Camici M, Micheli V, Ipata PL, Tozzi MG (2010) Pediatric neurological syndromes and inborn errors of purine metabolism. Neurochem Int 56:367–378
Chen WQ, Kang SU, Lubec G (2006) Protein profiling by the combination of two independent mass spectrometry techniques. Nat Protoc 1:1446–1452
Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R (1997) Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berlin) 132:107–124
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744
Fernandes C, Paya-Cano JL, Sluyter F, D’Souza U, Plomin R, Schalkwyk LC (2004) Hippocampal gene expression profiling across eight mouse inbred strains: towards understanding the molecular basis for behaviour. Eur J Neurosci 19:2576–2582
Fordyce DE, Bhat RV, Baraban JM, Wehner JM (1994) Genetic and activity-dependent regulation of zif268 expression: association with spatial learning. Hippocampus 4:559–568
Gerlai R (2002) Hippocampal LTP and memory in mouse strains: is there evidence for a causal relationship? Hippocampus 12:657–666
Groemer TW, Klingauf J (2007) Synaptic vesicles recycling spontaneously and during activity belong to the same vesicle pool. Nat Neurosci 10:145–147
Hassel B (2000) Carboxylation and anaplerosis in neurons and glia. Mol Neurobiol 22:21–40
Kanatani T, Mizuno K, Okada Y (1995) Effects of glycolytic metabolites on preservation of high energy phosphate level and synaptic transmission in the granule cells of guinea pig hippocampal slices. Experientia 51:213–216
Kim HJ, Sohn KM, Shy ME, Krajewski KM, Hwang M, Park JH, Jang SY, Won HH, Choi BO, Hong SH, Kim BJ, Suh YL, Ki CS, Lee SY, Kim SH, Kim JW (2007) Mutations in PRPS1, which encodes the phosphoribosyl pyrophosphate synthetase enzyme critical for nucleotide biosynthesis, cause hereditary peripheral neuropathy with hearing loss and optic neuropathy (cmtx5). Am J Hum Genet 81:552–558
Kimmoun A, Abboud G, Strazeck J, Merten M, Gueant JL, Feillet F (2008) Acute decompensation of isovaleric acidemia induced by Graves’ disease. Intensive Care Med 34:2315–2316
Klose J, Nock C, Herrmann M, Stuhler K, Marcus K, Bluggel M, Krause E, Schalkwyk LC, Rastan S, Brown SD, Bussow K, Himmelbauer H, Lehrach H (2002) Genetic analysis of the mouse brain proteome. Nat Genet 30:385–393
Mazzola JL, Sirover MA (2002) Alteration of intracellular structure and function of glyceraldehyde-3-phosphate dehydrogenase: a common phenotype of neurodegenerative disorders? Neurotoxicology 23:603–609
Mor F, Izak M, Cohen IR (2005) Identification of aldolase as a target antigen in Alzheimer’s disease. J Immunol 175:3439–3445
Nguyen PV, Abel T, Kandel ER, Bourtchouladze R (2000) Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem 7:170–179
Orosz F, Olah J, Ovadi J (2006) Triosephosphate isomerase deficiency: facts and doubts. IUBMB Life 58:703–715
Perry TL, Haworth JC, Robinson BH (1985) Brain amino acid abnormalities in pyruvate carboxylase deficiency. J Inherit Metab Dis 8:63–66
Pollak DD, Scharl T, Leisch F, Herkner K, Villar SR, Hoeger H, Lubec G (2005) Strain-dependent regulation of plasticity-related proteins in the mouse hippocampus. Behav Brain Res 165:240–246
Pollak DD, John J, Bubna-Littitz H, Schneider A, Hoeger H, Lubec G (2006a) Components of the protein quality control system are expressed in a strain-dependent manner in the mouse hippocampus. Neurochem Int 49:500–507
Pollak DD, John J, Hoeger H, Lubec G (2006b) An integrated map of the murine hippocampal proteome based upon five mouse strains. Electrophoresis 27:2787–2798
Schimanski LA, Nguyen PV (2004) Multidisciplinary approaches for investigating the mechanisms of hippocampus-dependent memory: a focus on inbred mouse strains. Neurosci Biobehav Rev 28:463–483
Seiler N (2000) Ornithine aminotransferase, a potential target for the treatment of hyperammonemias. Curr Drug Targets 1:119–153
Sultana R, Boyd-Kimball D, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA (2007) Proteomics analysis of the Alzheimer’s disease hippocampal proteome. J Alzheimers Dis 11:153–164
Sunyer B, Patil S, Frischer C, Hoger H, Selcher J, Brannath W, Lubec G (2007) Strain-dependent effects of SGS742 in the mouse. Behav Brain Res 181:64–75
Sunyer B, Patil S, Frischer C, Hoeger H, Lubec G (2008) Strain-dependent effects of cognitive enhancers in the mouse. Amino Acids 34:485–495
Sunyer B, An G, Kang SU, Hoger H, Lubec G (2009) Strain-dependent hippocampal protein levels of GABA(B)-receptor subunit 2 and NMDA-receptor subunit 1. Neurochem Int 55:253–256
Tarze A, Deniaud A, Le Bras M, Maillier E, Molle D, Larochette N, Zamzami N, Jan G, Kroemer G, Brenner C (2007) GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene 26:2606–2620
Upchurch M, Wehner JM (1989) Inheritance of spatial learning ability in inbred mice: a classical genetic analysis. Behav Neurosci 103:1251–1258
Weitzdoerfer R, Fountoulakis M, Lubec G (2002) Reduction of actin-related protein complex 2/3 in fetal Down syndrome brain. Biochem Biophys Res Commun 293:836–841
Wilson JM, Daddona PE, Otoadese T, Kelley WN (1982) Adenine phosphoribosyltransferase in patients with disorders of purine and pyrimidine metabolism. J Lab Clin Med 99:163–174
Yan H, Bigner DD, Velculescu V, Parsons DW (2009) Mutant metabolic enzymes are at the origin of gliomas. Cancer Res 69:9157–9159
Conflict of interest statement
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pollak, D.D., Bae, N., Mostafa, G. et al. Strain-dependent expression of metabolic proteins in the mouse hippocampus. Amino Acids 39, 1451–1462 (2010). https://doi.org/10.1007/s00726-010-0609-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0609-0