Abstract
Gamma-aminobutyric acid is a non-protein amino acid that is widely present in organisms. Several important physiological functions of gamma-aminobutyric acid have been characterized, such as neurotransmission, induction of hypotension, diuretic effects, and tranquilizer effects. Many microorganisms can produce gamma-aminobutyric acid including bacteria, fungi and yeasts. Among them, gamma-aminobutyric acid-producing lactic acid bacteria have been a focus of research in recent years, because lactic acid bacteria possess special physiological activities and are generally regarded as safe. They have been extensively used in food industry. The production of lactic acid bacterial gamma-aminobutyric acid is safe and eco-friendly, and this provides the possibility of production of new naturally fermented health-oriented products enriched in gamma-aminobutyric acid. The gamma-aminobutyric acid-producing species of lactic acid bacteria and their isolation sources, the methods for screening of the strains and increasing their production, the enzymatic properties of glutamate decarboxylases and the relative fundamental research are reviewed in this article. And the potential applications of gamma-aminobutyric acid-producing lactic acid bacteria were also referred to.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gamma-aminobutyric acid (GABA) is a non-protein amino acid that is widely distributed in nature from microorganisms to plants and animals (Ueno 2000), even in hydrothermal systems (Svensson et al. 2004). It is well known that GABA acts in animals as a major inhibitory neurotransmitter. Besides, GABA has several well-characterized physiological functions, such as induction of hypotension, diuretic effects, and tranquilizer effects (Jakobs et al. 1993; Wong et al. 2003). GABA is also a strong secretagogue of insulin from the pancreas (Adeghate and Ponery 2002) and effectively prevents diabetic conditions (Hagiwara et al. 2004). Quite recently, researches indicated that GABA may improve the concentrations of plasma growth hormone and the rate of protein synthesis in the brain (Tujioka et al. 2009) and inhibit small airway-derived lung adenocarcinoma (Schuller et al. 2008). The GABA content is very low in the temporal cortex, occipital cortex and cerebellum of patients with Alzheimer’s disease (Seidl et al. 2001). In addition to the beneficial bioactivities to humans, GABA production in microbes is also a contribution to pH tolerance and ATP production for themselves (Higuchi et al. 1997; Small and Waterman 1998).
Due to the fact that GABA has the potential as a bioactive component in foods and pharmaceuticals, the development of functional foods containing GABA has been actively pursued. Some GABA-containing foods, such as tea (Abe et al. 1995; Tsushida and Murai 1987), red mold rice (Kono and Himeno 2000; Rhyu et al. 2000), germinated wheat (Nagaoka 2005), soy product (Aoki et al. 2003; Shizuka et al. 2004; Tsai et al. 2006) and rice germ (Oh 2003; Zhang et al. 2006) have been developed. The consumption of GABA-enriched foods has been reported to depress the elevation of systolic blood pressure in spontaneously hypertensive rats (SHRs) (Hayakawa et al. 2004) and mildly hypertensive humans (Inoue et al. 2003).
Glutamic acid decarboxylases (GAD, EC 4.1.1.15) catalyzes the irreversible α-decarboxylation of glutamic acid to produce GABA. GAD can be produced by many microorganisms including bacteria (Capitani et al. 2003; Li et al. 2008; Yang et al. 2008), fungi (Kono and Himeno 2000; Rhyu et al. 2000; Su et al. 2003) and yeasts (Masuda et al. 2008). Lactic acid bacteria (LAB) are an important group of gram-positive bacteria and widely distributed in the environment and frequently exist in fermented food, vegetables and in the intestines of human and animals (Ben Omar et al. 2000; Gardner et al. 2001; Satokari et al. 2003). Many kinds of important products including lactic acid, conjugated linoleic acid, vitamin, aroma compounds, bacteriocins, exopolysaccharides and enzymes can be produced by LAB. LAB can prolong the shelf life of food, enhance the safety, improve food texture, and contribute to the pleasant sensory profile of the end product. LAB possess special physiological activities and are generally regarded as safe (GRAS), and have been extensively utilized in food industries such as dairy products, bread, fermented vegetables, meats and fish, etc. (Karahan et al. 2010; Lee et al. 2006; Leroy and Vuyst 2004; Yan et al. 2008). Also, LAB have been used as probiotics due to their properties such as immunomodulation, inhibition of pathogenic bacteria, control of intestinal homeostasis, resistance to gastric acidity, bile acid resistance, and anti-allergic activity (Hwanhlem et al. 2010; Nishida et al. 2008; Tannock 2004; Tuohy et al. 2003). In recent years, many studies have therefore focused on the GABA production by using LAB as bacterial cell factories (Cho et al. 2007; Kim et al. 2009; Komatsuzaki et al. 2005; Yokoyama et al. 2002).
This review is focused on the GABA-producing LAB species, isolation methods and isolation sources for GABA-producing LAB, the ways to enhance GABA production, the enzymatic properties of GADs and their relative molecular studies, and the potential applications of GABA-producing LAB.
GABA-producing LAB species
Currently, several LAB species/subspecies have been reported to show GABA-producing ability with a vast difference in production, including Lactobacillus brevis (Kim et al. 2007, 2009; Li et al. 2008; Siragusa et al. 2007; Yokoyama et al. 2002), Lactococcus lactis (Lu et al. 2009; Nomura et al. 1999a, b; Siragusa et al. 2007), Lb. paracasei (Komatsuzaki et al. 2005; Siragusa et al. 2007), Lb. delbrueckii subsp. bulgaricus (Siragusa et al. 2007), Lb. buchneri (Cho et al. 2007; Park and Oh 2006c), Lb. plantarum (Siragusa et al. 2007), Lb. helveticus (Sun et al. 2009) and Streptococcus salivarius subsp. thermophilus (Yang et al. 2008). Among them the Lb. brevis produced the highest amount of GABA (345.83 mM) (Li et al. 2009b). Among them, most of the GABA-producing LAB strains belong to lactobacilli. The data are summarized in Table 1.
Isolation sources
Interestingly, almost all of the strains were isolated from traditional fermented foods such as kimchi (Lu et al. 2008; Park and Oh 2007b; Seok et al. 2008), cheese (Nomura et al. 1998; Park and Oh 2006b; Siragusa et al. 2007), sourdough (Rizzello et al. 2008), paocai (Li et al. 2008), etc. which have a common trait with an acidic pH, only with the exception of Lb. brevis CGMCC 1306 from fresh milk without pasteurization (Huang et al. 2007a). In addition, all the reported isolation sources contain a high content of glutamate. It is clear that traditional fermented foodstuffs enriched in glutamate are important isolation sources for screening GABA-producing LAB.
Seventeen GABA-producing LAB strains of 31 colonies from cheese (Nomura et al. 1998), 61 of 440 from cheese (Siragusa et al. 2007), and 23 of 1,000 from paocai (Li et al. 2008), indicate that GABA-producing LAB form a dominant group in some fermented foods. Meanwhile, more than one species in cheese (Siragusa et al. 2007) implies a possible species diversity in some fermented foods. It is noteworthy that GABA-producing strains from the samples with high GABA content may exhibit a relatively higher GABA-producing ability than those from the samples with low GABA content. For example, Siragusa et al. (2007) reported the best GABA-producing strains, L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, and L. brevis PM17, were isolated from Pecorino di Filiano, Pecorino del Reatino, Pecorino Umbro, and Pecorino Marchigiano cheeses, respectively, which had the highest concentrations of GABA. Nomura et al. (1998) screened L. lactis ssp. lactis 01-4, 01-7, 53-1, and 53-7 with the highest GABA production from the cheese starters with the highest levels of GABA.
Although many GABA-producing LAB strains have been isolated and identified, a further isolation and characterization research is needed because screening various types of GABA-producing LAB is important for the food industry (Komatsuzaki et al. 2005). In a further screening, the isolation sources should be expanded to as many as possible fermented foods to obtain GABA-producing LAB strains. This will lead to a wider application area and higher flexibility of starter cultures.
Methods for screening GABA-producing LAB
Several methods are suitable for the detection of GABA in biological fluids, such as amino acid analyzer (Komatsuzaki et al. 2005; Kono and Himeno 2000), gas chromatography (GC) (Kagan et al. 2008), high performance liquid chromatography (HPLC) (Kim et al. 2009; Rossetti and Lombard 1996), capillary liquid chromatographic/tandem mass spectrometric method (Song et al. 2005), and the flow-injection analysis (FIA) method based on GABase (Horie and Rechnitz 1995). However, these methods require tedious sample preparation steps and are time consuming and can only analyze one sample each time. It is clear that they are not ideal methods in the screening work. Planar chromatography (Cho et al. 2007; Li et al. 2008, 2009a; Sethi 1999; Yokoyama et al. 2002), pH indicator method (PIM) (Yang et al. 2006) and enzyme-based microtiter plate assay (EBMPA) (Tsukatani et al. 2005) do not need expensive equipments, and are suitable for a parallel analysis of large numbers of samples, and therefore can be applied in high-throughput screening of GABA-producing strains.
For the PIM method, cells must be washed clean through several centrifugation and washing steps before they react with l-glutamic acid for a very long time (8–24 h). This method seems to be somewhat tedious and time-consuming. The EBMPA method needs the expensive GABase. In addition, components in culture medium may affect on the enzymatic reaction of GABase. There exists some difficulty to eliminate the interference factors. For planar chromatography, no any sample pretreatment and expensive chemical reagent are needed. Compared to the PIM and EBMPA methods, planar chromatography is a simple, convenient and inexpensive method for analysis of GABA. Many GABA-producing LAB strains have been isolated from some food samples by this method (Cho et al. 2007; Li et al. 2008; Park and Oh 2005; Seok et al. 2008). The recently developed pre-staining planar chromatography has almost the same R f values of the acids to those of the traditional method. On other hand, the pre-staining method is more clean, simple, convenient, inexpensive and reproducible (Li et al. 2009a).
To reduce the workload and research cost, it is necessary to detect the content of GABA in samples to preliminarily determine whether GABA-producing LAB occur in the samples before screening (Li et al. 2008; Siragusa et al. 2007). The suspicious GABA-producing samples are then inoculated in the special medium (containing glutamate) for LAB isolation. After cultivation, the suspicious GABA-producing cultures are selected from single colonies. The suspicious GABA-producing strains are further screened by HPLC. Finally, HPLC–MS should be used to confirm the results (Li et al. 2008).
Improvement of GABA production by optimizing fermentation conditions
The GABA-producing ability varies widely among the strains of LAB (Table 1), and is affected significantly by culture conditions and medium composition. Therefore, it is important to optimize these conditions for enhancing the GABA production. The optimal conditions for GABA fermentation are various among the different LAB strains, and the major factors affecting the GABA production have been characterized, including carbon sources, glutamate concentration, culture temperature, pyridoxal 5′-phosphate (PLP, coenzyme), and pH (Cho et al. 2007; Komatsuzaki et al. 2005; Li et al. 2009b; Lu et al. 2008; Yang et al. 2008). Among them, pH, temperature and glutamate concentration were considered as the common important factors for all the strains. The content of intracellular GABA is extremely low and difficult to be extracted from cells (Komatsuzaki et al. 2005), hence only extracellular GABA needs to be determined during the optimization.
Optimization based on GAD properties
The GABA-synthesis is catalyzed by GAD. Therefore, the fermentation conditions can be optimized based on biochemical characteristics of GAD. Komatsuzaki et al. (2005) optimized the fermentation conditions of Lb. paracasei NFRI 7415 according to the GAD properties and successfully increased the GABA production from 60 to 302 mM. Yang et al. (2008) also applied this strategy to enhance the GABA production of S. salivarius subsp. thermophilus Y2. The results suggest that the elucidation of biochemical properties of LAB GAD facilitates the optimization of fermentation processes.
Grading-controlling fermentation
High cell density is required for effective synthesis of GABA. For some strains, the optimal cell growth conditions do not fit the optimal GABA-synthesis conditions. In this circumstance, grading-controlling fermentation can be used to enhance GABA yielding. First, high density cells should be cultivated under the optimal growth conditions, and then the fermentation should be carried out in the optimal conditions for the GABA-synthesis. Yang et al. (2008) designed a two-stage pH and temperature control strategy, based on the differences on the optimal culture conditions and the optimal GAD reaction conditions of S. salivarius subsp. thermophilus Y2, to achieve a high concentration of GABA in the fermentation.
Immobilized cells
Immobilized cell technologies have developed rapidly over the last 30 years and have been widely used in fermentation processes (Junter and Jouenne 2004). Choi et al. (2006) applied recycled immobilized Lb. brevis GABA 057 to produce GABA. The converted glutamate increased from 2% (w/v) to 12% (w/v). The constructed immobilized cells could be reused at least for four times. Huang et al. (2007b) reported a bioprocess of production of GABA by using immobilized LAB cells. GABA yield was significantly improved, especially when continuous fermentation is combined with cell immobilization techniques to increase the GABA concentration in the fermentor. Hence the technique holds a great promise for the efficient production of GABA.
Enzymatic properties of LAB GADs
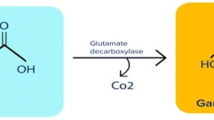
GAD is responsible for converting l-glutamate to GABA. The decarboxylation of l-glutamate to GABA catalyzed by GAD takes the following general form:
LAB GAD is an intracellular enzyme (Huang et al. 2007a; Komatsuzaki et al. 2008; Ueno et al. 1997) and induction of it is one of the acid stress responses in LAB (Sanders et al. 1998; Small and Waterman 1998). GAD is produced as a mature form which consists of identical subunits with molecular mass ranging from 54 to 62 kD, not as a precursor protein, and has highly conserved catalytic amino acid residues containing a lysine residue (Hiraga et al. 2008; Komatsuzaki et al. 2008; Park and Oh 2004; 2007a). GADs have been isolated from a variety of LAB and their biochemical properties have been characterized. Although decarboxylation reaction for LAB GADs is identical, primary structure especially the N-terminal and C-terminal regions are significantly different (Fig. 1). Differences in primary structure might affect the GABA-producing ability of LAB (Komatsuzaki et al. 2008). In LAB, the dimer formation of GAD might be conserved. However, the active form of the GAD from Lb. brevis IFO12005 was proved to be a tetramer (Hiraga et al. 2008). This is the first report of a tetramer form of GAD from microorganisms.
Comparison of the amino acid sequence of GADs from Lc. lactis subsp. lactis 01-7, Lb. brevis IFO12005, Lb. paracasei NFRI 7415, Lb. brevis OPK-3 and Lb. plantarum KCTC3015. Asterisk indicates identical amino acid residues for all the GADs. Boxed amino acid residues are catalytic amino acid residues. The first four GADs correspond to GenBank accession nos: BAA24584 (gene AB010789), BAF99137 (gene AB258458), BAG12190 (gene AB295641) and AAZ95185 (gene DQ168031), respectively. The amino acid sequence of GAD of Lb. plantarum KCTC3015 is identical to that of Lb. plantarum WCFS1, which corresponds to GenBank accession no. CAD65520 (gene AL935262)
The Lb. brevis GAD activity could be increased by the addition of sulfate ions in a dose-dependent manner. The order of effect was as follows: ammonium sulfate > sodium sulfate > magnesium sulfate, indicating that the increase of hydrophobic interaction between subunits causes the increase of GAD activity (Ueno et al. 1997). The addition of ammonium sulfate did not cause any significant structural changes, but did induce subtle structural changes at the active site, probably in the vicinity of the catalytic residues (Hiraga et al. 2008). The optimum pH values for maintaining the activity of the GADs were in the range of 4.0–5.0. In high GABA-producing strains Lb. paracasei NFRI 7415 (Komatsuzaki et al. 2008) and Lb. brevis IFO 12005 (Ueno et al. 1997), the GAD activity was still observed at pH 4.0 or above pH 5.5, but very low levels of GAD activity were observed at pH 4.0 and no activity was detected above pH 5.5 in a low GABA-producing strain L. lactis (Nomura et al. 1998). These results suggest that low-pH GAD activity and broad-pH GAD activity might be important for producing high levels of GABA in LAB. The optimal temperatures of LAB GADs range from 30 to 50°C.
The substrate specificity of GAD from Lb. brevis was tested by using 22 kinds of amino acids (l-alanine, ε-aminocaproic acid, l-arginine, l-aspartic acid, l-citrulline, l-cysteine, l-glutamic acid, l-glutamine, glycine, l-histidine, l-homoserine, l-isoleucine, l-leucine, l-lysine, l-methionine, l-ornithine, l-tyrosine, and l-valine). The Decarboxylated product was observed only for l-glutamic acid (Ueno et al. 1997). The Lc. lactis GAD also reacted only with l-glutamate among the 20 α-amino acids (Nomura et al. 1999b). These results indicate that GADs from LAB are specific for l-glutamic acid. The properties of the reported LAB GADs are shown in Table 2.
Cloning of GAD genes and their regulators
The full-length GAD genes from Lb. paracasei NFRI 7415 (Komatsuzaki et al. 2008), Lb. plantarum KCTC3015 (Park and Oh 2004), Lb. brevis OPK-3 (Park and Oh 2007a), Lb. brevis IFO12005 (Hiraga et al. 2008) and Lc. Lactis 01-7 (Nomura et al. 1999b), and the core fragments of gadBs from L. paracasei PF6 (accession number EF174473), L. delbrueckii subsp. bulgaricus PR1 (accession number EF174472), L. lactis PU1 (accession number EF174474), and L. plantarum C48 (accession number EF174475) were cloned and sequenced (Siragusa et al. 2007). In addition, the GAD genes from Lb. plantarum KCTC3015 (Park and Oh 2004) and Lb. brevis OPK-3 (Park and Oh 2007a) were successfully expressed in E. coli, and the GAD gene from Lb. brevis OPK-3 was successfully expressed in Bacillus subtilis (Park and Oh 2006a).
Lactococcus lactis contains only one GAD gene (gadB) (Nomura et al. 1999b). gadB and gadC (encoding GadC, an antiporter which is highly hydrophobic and contains 12 putative membrane-spanning domains and is responsible for the antiport of glutamate and GABA) form an operon gadCB. This operon is transcribed from the chloride-dependent promoter Pgad and the expression of it is glutamate-dependent. The GadR is the activator of the gadCB operon and is encoded by a gene located in the immediate upstream of the Pgad (Sanders et al. 1998). L. brevis also has only a single copy gene gadB for GAD, as does L. lactis. A gene similar to gadC (42.5% identity with Lc. lactisgadC) is located very close to the 5′-side of the gadB gene. These results suggest that L. brevis has an acid tolerance mechanism similar to Lc. Lactis (Hiraga et al. 2008). Komatsuzaki et al. (2008) cloned gadB of Lb. paracasei and found a ribosome binding sequence (GGAGG) in the conserved sequence upstream of gadB, but did not find any possible promoter sequences. They speculated that gadB and the other genes located upstream of it might also form an operon structure. Up to date, it is unknown whether gadC exists as an upstream of gadB in Lb. paracasei.
Why some LAB strains can not produce GABA
It is well known that some LAB strains produce GABA while others do not (Nomura et al. 1999a). A study focused on Lc. lactis subsp. lactis (a GABA-producing strain) and Lc. lactis subsp. cremoris (a GABA-negative strain) has given us some hints to understand the reasons. Nomura et al. (2000) verified that gadCB genes are also present in Lc. lactis subsp. cremoris and that they are not grossly rearranged by insertions or deletions of large fragments. However, a one-base deletion of adenine and a one-base insertion of thymine were detected within the coding region, resulting in frame shift mutations. Because of the frame shift resulting from a one-base insertion or deletion within the coding region, the translated protein was not functional. The regions around these two mutations were subsequently sequenced in other L. lactis subsp. cremoris strains to confirm that the mutations are common. These results suggest that it is infeasible to develop polymerase chain reaction (PCR)-based methods for rapid detection of GABA-producing LAB.
Potential applications of GABA-producing LAB
As functional starter cultures
Nowadays, the consumer pays a lot of attention to the relation between food and health. As a consequence, the market for foods with health-promoting properties, so-called functional foods, has shown a remarkable growth over the last few years. GABA has many bioactivities and hence has a great application potential in functional foods. However, the direction addition of chemical GABA to food is regarded as unnatural and unsafe and is still illegal in Korea (Kim et al. 2009; Seok et al. 2008). LAB play a central role in fermentation processes, and have a long and safe history of application and consumption in the production of fermented foods and beverages (Leroy and Vuyst 2004). The use of GABA-producing LAB strains as starter cultures in fermentation processes can help to achieve bio-synthetic production of the GABA. This provides a way of replacing chemical GABA by natural GABA, at the same time offering the consumer with new, attractive food products. This also reduces the production cost because of the omission the extra addition of GABA. Currently, the bio-synthetic production of natural GABA produced by LAB for the manufacturing of dairy products (Hayakawa et al. 2004; Inoue et al. 2003; Park and Oh 2007b; Skeie et al. 2001), of black raspberry juice (Kim et al. 2009), of soymilk (Tsai et al. 2006), of kimchi (Seok et al. 2008), and of cheese (Nomura et al. 1998) is being explored. However, the GABA formation is restricted by the GABA-producing ability of LAB and l-glutamic acid concentration in the food matrices. To increase the GABA content of the fermented products, strains with a high GAD activity should be selected for fermentation use. Meanwhile, the concentration of free l-glutamic acid in the food matrices should be enough high. The concentration of l-glutamic acid could be enhanced by (1) adding exogenous l-glutamic acid (Kim et al. 2009; Nomura et al. 1998; Park and Oh 2005; Seok et al. 2008); (2) adding protease to hydrolyze proteins and produce l-glutamic acid (Zhang et al. 2006); (3) using LAB having protein hydrolyzing ability as co-cultures for the fermentation processes (Inoue et al. 2003).
As probiotics
Probiotics can only be effective if they remain viable as they pass through the stomach and colonize the intestine (Chou and Weimer 1999; Nishida et al. 2008). Decarboxylation of glutamate within the LAB cell consumes an intracellular proton. This helps maintain a neutral cytoplasmic pH when the external pH drops. Considering their role in pH resistance, LAB with a high GAD activity have potential as probiotics. Siragusa et al. (2007) isolated three lactobacillus strains which could survive and synthesize GABA under simulated gastrointestinal conditions. This shows that GABA-producing LAB as probiotics could colonize in the gastrointestinal tract and produce GABA in situ. Hence, GABA-producing LAB will show promise potentials as a probiotics through exploitation of the health-promoting properties of GABA and LAB themselves.
To make full use of by-products in food industry
Some by-products in food industry can be used as cheap substrates to synthesize GABA by LAB for the manufacture of functional foods or beverages. It seems to be an economical process of natural GABA production. For instance, Yokoyama et al. (2002) applied Lb. brevis IFO-12005 to produce GABA from distillery lees. Almost all of the free glutamic acid (10.50 mM) in the distillery lees was converted to GABA (10.18 mM). After centrifugation, flocculation, and removal of the yellow pigment and undesired flavors, the GABA-containing solution is suitable for the production of liquors and beverages. Di Cagno et al. (2010) manufactured a functional grape must beverage enriched GABA by a fermentation of Lb. plantarum DSM19463. This beverage was reported to have potential anti-hypertensive effect and dermatological protection. Hence, the full use of the by-products based on LAB capacity for synthesizing GABA may open new perspectives on production of GABA-enriched products.
Conclusions
GABA-producing LAB offer the opportunity of developing naturally fermented health-oriented products. Although some GABA-producing LAB have been isolated to find strains suitable for different fermentations, further screening of various GABA-producing strains from LAB, especially high-yielding strains, is necessary. The high-throughput screening methods enable us to isolate GABA-producing LAB rapidly and conveniently. The elucidation of molecular mechanism and roles of GABA production, knowledge of the regulation aspects of GABA production, and profound comprehension of GABA-producing cell physiology would offer us the theory and tools to increase GABA yield at genetic and metabolic levels. On other hand, the expression of LAB GAD genes in other microbes will further expand the application area of GABA-producing LAB.
References
Abe Y, Umemura S, Sugimoto K, Hirawa N, Kato Y, Yokoyama N, Yokoyama T, Iwai J, Ishii M (1995) Effect of green tea rich in gamma-aminobutyric acid on blood pressure of Dahl salt-sensitive rats. Am J Hypertens 8:74–79
Adeghate E, Ponery AS (2002) GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 34:1–6
Aoki H, Furuya Y, Endo Y, Fujimoto K (2003) Effect of gamma-aminobutyric acid-enriched tempeh-like fermented soybean (GABA-tempeh) on the blood pressure of spontaneously hypertensive rats. Biosci Biotechnol Biochem 67:1806–1808
Ben Omar N, Ampe F, Raimbault M, Guyot JP, Tailliez P (2000) Molecular diversity of lactic acid bacteria from cassava sour starch (Colombia). Syst Appl Microbiol 23:285–291
Capitani G, De Biase D, Aurizi C, Gut H, Bossa F, Grutter MG (2003) Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J 22:4027–4037
Cho YR, Chang JY, Chang HC (2007) Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol 17:104–109
Choi SI, Lee JW, Park SM, Lee MY, Ji GE, Park MS, Heo TR (2006) Improvement of gamma-aminobutyric acid (GABA) production using cell entrapment of Lactobacillus brevis GABA 057. J Microbiol Biotechnol 16:562–568
Chou LS, Weimer B (1999) Isolation and characterization of acid- and bile-tolerant isolates from strains of Lactobacillus acidophilus. J Dairy Sci 82:23–31
Di Cagno R, Mazzacane F, Rizzello CG, De Angelis M, Giuliani G, Meloni M, De Servi B, Gobbetti M (2010) Synthesis of gamma-aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: functional grape must beverage and dermatological applications. Appl Microbiol Biotechnol 86:731–741
Gardner NJ, Savard T, Obermeier P, Caldwell G, Champagne CP (2001) Selection and characterization of mixed starter cultures for lactic acid fermentation of carrot, cabbage, beet and onion vegetable mixtures. Int J Food Microbiol 64:261–275
Hagiwara H, Seki T, Ariga T (2004) The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 68:444–447
Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, Yamori Y (2004) Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar–Kyoto rats. Br J Nutr 92:411–417
Higuchi T, Hayashi H, Abe K (1997) Exchange of glutamate and gamma-aminobutyrate in a Lactobacillus strain. J Bacteriol 179:3362–3364
Hiraga K, Ueno Y, Oda K (2008) Glutamate decarboxylase from Lactobacillus brevis: activation by ammonium sulfate. Biosci Biotechnol Biochem 72:1299–1306
Horie H, Rechnitz GA (1995) Enzymatic flow-injection determination of gamma-aminobutyric-acid. Anal Lett 28:259–266
Huang J, Mei L, Sheng Q, Yao S, Lin D (2007a) Purification and characterization of glutamate decarboxylase of Lactobacillus brevis CGMCC 1306 isolated from fresh milk. Chinese J Chem Eng 15:157–161
Huang J, Mei L, Wu H, Lin D (2007b) Biosynthesis of γ-aminobutyric acid (GABA) using immobilized whole cells of Lactobacillus brevis. World J Microbiol Biotechnol 23:865–871
Hwanhlem N, Watthanasakphuban N, Riebroy S, Benjakul S, H-Kittikun A, Maneerat S (2010) Probiotic lactic acid bacteria from Kung-Som: isolation, screening, inhibition of pathogenic bacteria. Intel J Food Sci Technol 45:594–601
Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H (2003) Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr 57:490–495
Jakobs C, Jaeken J, Gibson KM (1993) Inherited disorders of GABA metabolism. J Inherit Metab Dis 16:704–715
Junter GA, Jouenne T (2004) Immobilized viable microbial cells: from the process to the proteome… or the cart before the horse. Biotechnol Adv 22:633–658
Kagan IA, Coe BL, Smith LL, Huo CJ, Dougherty CT, Strickland JR (2008) A validated method for gas chromatographic analysis of gamma-aminobutyric acid in tall fescue herbage. J Agric Food Chem 56:5538–5543
Karahan AG, Kilic GB, Kart A, Aloglu HS, Oner Z, Aydemir S, Erkus O, Harsa S (2010) Genotypic identification of some lactic acid bacteria by amplified fragment length polymorphism analysis and investigation of their potential usage as starter culture combinations in Beyaz cheese manufacture. J Dairy Sci 93:1–11
Kim SH, Shin BH, Kim YH, Nam SW, Jeon SJ (2007) Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus brevis BH2. Biotechnol Bioprocess Eng 12:707–712
Kim JY, Lee MY, Ji GE, Lee YS, Hwang KT (2009) Production of gamma-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int J Food Microbiol 130:12–16
Komatsuzaki N, Shima J, Kawamoto S, Momose H, Kimura T (2005) Production of gamma-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol 22:497–504
Komatsuzaki N, Nakamura T, Kimura T, Shima J (2008) Characterization of glutamate decarboxylase from a high gamma-aminobutyric acid (GABA)-producer, Lactobacillus paracasei. Biosci Biotechnol Biochem 72:278–285
Kono I, Himeno K (2000) Changes in gamma-aminobutyric acid content during beni-koji making. Biosci Biotechnol Biochem 64:617–619
Lee JY, Kim CJ, Kunz B (2006) Identification of lactic acid bacteria isolated from kimchi and studies on their suitability for application of as starter culture in the production fermented sausages. Meat Sci 72:437–445
Leroy F, Vuyst LD (2004) Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Technol 15:67–78
Li H, Cao Y, Gao D, Xu H (2008) A high γ-aminobutyric acid-producing ability Lactobacillus brevis isolated from Chinese traditional paocai. Ann Microbiol 58:649–653
Li H, Qiu T, Cao Y, Yang J, Huang Z (2009a) Pre-staining paper chromatography method for quantification of gamma-aminobutyric acid. J Chromatogr A 1216:5057–5060
Li H, Qiu T, Gao D, Cao Y (2009b) Medium optimization for production of gamma-aminobutyric acid by Lactobacillus brevis NCL912. Amino Acids. doi:10.1007/s00726-009-0355-3
Lu X, Chen Z, Gu Z, Han Y (2008) Isolation of gamma-aminobutyric acid-producing bacteria and optimization of fermentative medium. Biochem Eng J 41:48–52
Lu X, Xie C, Gu Z (2009) Optimisation of fermentative parameters for GABA enrichment by Lactococcus lactis. Czech J Food Sci 27:433–442
Masuda K, Guo XF, Uryu N, Hagiwara T, Watabe S (2008) Isolation of marine yeasts collected from the Pacific Ocean showing a high production of gamma-aminobutyric acid. Biosci Biotechnol Biochem 72:3265–3272
Nagaoka H (2005) Treatment of germinated wheat to increase levels of GABA and IP6 catalyzed by endogenous enzymes. Biotechnol Prog 21:405–410
Nishida S, Michinaka A, Nakashima K, Iino H, Fujii T (2008) Evaluation of the probiotic potential of Lactobacillus paracasei KW3110 based on in vitro tests and oral administration tests in healthy adults. J Gen Appl Microbiol 54:267–276
Nomura M, Kimoto H, Someya Y, Furukawa S, Suzuki I (1998) Production of gamma-aminobutyric acid by cheese starters during cheese ripening. J Dairy Sci 81:1486–1491
Nomura M, Kimoto H, Someya Y, Suzuki I (1999a) Novel characteristic for distinguishing Lactococcus lactis subsp. lactis from subsp. cremoris. Int J Syst Bacteriol 49:163–166
Nomura M, Nakajima I, Fujita Y, Kobayashi M, Kimoto H, Suzuki I, Aso H (1999b) Lactococcus lactis contains only one glutamate decarboxylase gene. Microbiology 145:1375–1380
Nomura M, Kobayashi M, Ohmomo S, Okamoto T (2000) Inactivation of the glutamate decarboxylase gene in Lactococcus lactis subsp. cremoris. Appl Environ Microbiol 66:2235–2237
Oh SH (2003) Stimulation of gamma-aminobutyric acid synthesis activity in brown rice by a chitosan/glutamic acid germination solution and calcium/calmodulin. J Biochem Mol Biol 36:319–325
Park KB, Oh SH (2004) Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus plantarum. J Food Sci Nutr 9:324–329
Park KB, Oh SH (2005) Production and characterization of GABA rice yogurt. Food Sci Biotech 14:518–522
Park KB, Oh SH (2006a) Enhancement of gamma-aminobutyric acid production in Chungkukjang by applying a Bacillus subtilis strain expressing glutamate decarboxylase from Lactobacillus brevis. Biotechnol Lett 28:1459–1463
Park KB, Oh SH (2006b) Isolation and characterization of Lactobacillus buchneri strains with high gamma-aminobutyric acid producing capacity from naturally aged cheese. Food Sci Biotechnol 15:86–90
Park KB, Oh SH (2006c) Isolation and characterization of Lactobacillus buchneri strains with high gamma-aminobutyric acid producing capacity from naturally aged cheese. Food Sci Biotechnol 15:86–90
Park KB, Oh SH (2007a) Cloning, sequencing and expression of a novel glutamate decarboxylase gene from a newly isolated lactic acid bacterium, Lactobacillus brevis OPK-3. Bioresour Technol 98:312–319
Park KB, Oh SH (2007b) Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour Technol 98:1675–1679
Rhyu MR, Kim EY, Kim HY, Ahn BH, Yang CB (2000) Characteristics of the red rice fermented with fungus Monascus. Food Sci Biotechnol 9:21–26
Rizzello CG, Cassone A, Di Cagno R, Gobbetti M (2008) Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and gamma-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J Agric Food Chem 56:6936–6943
Rossetti V, Lombard A (1996) Determination of glutamate decarboxylase by high-performance liquid chromatography. J Chromatogr B 681:63–67
Sanders JW, Leenhouts K, Burghoorn J, Brands JR, Venema G, Kok J (1998) A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbiol 27:299–310
Satokari RM, Vaughan EE, Smidt H, Saarela M, Matto J, de Vos WM (2003) Molecular approaches for the detection and identification of bifidobacteria and lactobacilli in the human gastrointestinal tract. Syst Appl Microbiol 26:572–584
Schuller HM, Al-Wadei HAN, Majidi M (2008) Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis 29:1979–1985
Seidl R, Cairns N, Singewald N, Kaehler ST, Lubec G (2001) Differences between GABA levels in Alzheimer’s disease and Down syndrome with Alzheimer-like neuropathology. Naunyn-Schmiedeberg’s Arch Pharmacol 363:139–145
Seok JH, Park KB, Kim YH, Bae MO, Lee MK, Oh SH (2008) Production and characterization of kimchi with enhanced levels of gamma-aminobutyric acid. Food Sci Biotechnol 17:940–946
Sethi ML (1999) Enzyme inhibition XI: glutamate decarboxylase activity relationship with the reaction products as determined by the colorimetric and radioisotopic methods. J Pharm Biomed Anal 19:847–854
Shizuka F, Kido Y, Nakazawa T, Kitajima H, Aizawa C, Kayamura H, Ichijo N (2004) Antihypertensive effect of gamma-amino butyric acid enriched soy products in spontaneously hypertensive rats. Biofactors 22:165–167
Siragusa S, De Angelis M, Di Cagno R, Rizzello CG, Coda R, Gobbetti M (2007) Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol 73:7283–7290
Skeie S, Lindberg C, Narvhus J (2001) Development of amino acids and organic acids in Norvegia, influence of milk treatment and adjunct Lactobacillus. Inter Dairy J 11:399–411
Small PL, Waterman SR (1998) Acid stress, anaerobiosis and gadCB: lessons from Lactococcus lactis and Escherichia coli. Trends Microbiol 6:214–216
Song Y, Shenwu M, Dhossche DM, Liu YM (2005) A capillary liquid chromatographic/tandem mass spectrometric method for the quantification of gamma-aminobutyric acid in human plasma and cerebrospinal fluid. J Chromatogr B 814:295–302
Su YC, Wang JJ, Lin TT, Pan TM (2003) Production of the secondary metabolites gamma-aminobutyric acid and monacolin K by Monascus. J Ind Microbiol Biotechnol 30:41–46
Sun TS, Zhao SP, Wang HK, Cai CK, Chen YF, Zhang HP (2009) ACE-inhibitory activity and gamma-aminobutyric acid content of fermented skim milk by Lactobacillus helveticus isolated from Xinjiang koumiss in China. Eur Food Res Technol 228:607–612
Svensson E, Skoog A, Amend JP (2004) Concentration and distribution of dissolved amino acids in a shallow hydrothermal system, Vulcano Island (Italy). Org Geochem 35:1001–1014
Tannock GW (2004) A special fondness for lactobacilli. Appl Environ Microbiol 70:3189–3194
Tsai JS, Lin YS, Pan BS, Chen TJ (2006) Antihypertensive peptides and gamma-aminobutyric acid from prozyme 6 facilitated lactic acid bacteria fermentation of soymilk. Process Biochem 41:1282–1288
Tsukatani T, Higuchi T, Matsumoto K (2005) Enzyme-based microtiter plate assay for gamma-aminobutyric acid: application to the screening of gamma-aminobutyric acid-producing lactic acid bacteria. Anal Chim Acta 540:293–297
Tsushida T, Murai T (1987) Conversion of glutamic-acid to gamma-aminobutyric-acid in tea leaves under anaerobic conditions. Agric Biol Chem 51:2865–2871
Tujioka K, Ohsumi M, Horie K, Kim M, Hayase K, Yokogoshi H (2009) Dietary gamma-aminobutyric acid affects the brain protein synthesis rate in ovariectomized female rats. J Nutr Sci Vitaminol 55:75–80
Tuohy KM, Probert HM, Smejkal CW, Gibson GR (2003) Using probiotics and prebiotics to improve gut health. Drug Discov Today 8:692–700
Ueno H (2000) Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B Enzyme 10:67–79
Ueno Y, Hayakawa K, Takahashi S, Oda K (1997) Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Biosci Biotechnol Biochem 61:1168–1171
Wong CG, Bottiglieri T, Snead OC 3rd (2003) GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol 54:S3–12
Yan PM, Xue WT, Tan SS, Zhang H, Chang XH (2008) Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control 19:50–55
Yang SY, Lu ZX, Lu FX, Bie XM, Sun LJ, Zeng XX (2006) A simple method for rapid screening of bacteria with glutamate decarboxylase activities. J Rapid Methods Autom Microbiol 14:291–298
Yang SY, Lu FX, Lu ZX, Bie XM, Jiao Y, Sun LJ, Yu B (2008) Production of gamma-aminobutyric acid by Streptococcus salivarius subsp thermophilus Y2 under submerged fermentation. Amino Acids 34:473–478
Yokoyama S, Hiramatsu J, Hayakawa K (2002) Production of gamma-aminobutyric acid from alcohol distillery lees by Lactobacillus brevis IFO-12005. J Biosci Bioeng 93:95–97
Zhang H, Yao HY, Chen F (2006) Accumulation of gamma-aminobutyric acid in rice germ using protease. Biosci Biotechnol Biochem 70:1160–1165
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Cao, Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 39, 1107–1116 (2010). https://doi.org/10.1007/s00726-010-0582-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0582-7