Abstract
C-type lectins are one family of pattern recognition receptors (PRRs) that play important roles in innate immunity. In this work, cDNA and genomic sequences for a new C-type lectin (FcLec5) were obtained from the Chinese white shrimp Fenneropenaeus chinensis. FcLec5 cDNA contains an open reading frame of 1,008 bp and its genomic sequence is 1,137 bp with 4 exons and 3 introns. The predicted FcLec5 protein contains a signal peptide and two carbohydrate recognition domains (CRDs). The N-terminal CRD of FcLec5 has a predicted carbohydrate recognition motif of Gln-Pro-Asp (QPD), while the C-terminal CRD contains a motif of Glu-Pro-Gln (EPQ). Northern blot analysis showed that FcLec5 mRNA was specifically expressed in hepatopancreas. FcLec5 protein was expressed in hepatopancreas and secreted into hemolymph. Real-time PCR showed that FcLec5 transcript exhibited different expression profiles after immune-challenged with Vibrio anguillarum or White Spot Syndrome Virus (WSSV). Recombinant FcLec5 and its two individual CRDs could agglutinate most bacteria tested, and the agglutinating activity was Ca2+-dependent. Besides, the agglutinating activity to gram-negative bacteria is higher than that to gram-positive bacteria. Direct binding assay showed that recombinant FcLec5 could bind to all microorganisms tested (five gram-positive and four gram-negative bacteria, as well as yeast) in a Ca2+-independent manner. Recombinant FcLec5 also directly bound to bacterial peptidoglycan, lipopolysaccharide and lipoteichoic acids. These results suggest that FcLec5 may act as a PRR for bacteria via binding to bacterial cell wall polysaccharides in Chinese white shrimp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invertebrates lack an adaptive immune system and mainly rely on innate immunity. The innate immune system distinguishes nonself from the conserved molecules on the surface of invading microorganisms. These molecules are so-called pathogen-associated molecular patterns (PAMPs) (Janeway 1989), which can be recognized by several groups of proteins termed pattern recognition receptors (PRRs) (Medzhitov and Janeway 2002). In invertebrates, several groups of PRRs, including C-type lectins (CTLs), galactoside-binding lectins (galectins), peptidoglycan-recognition proteins (PGRPs), thioester-containing proteins (TEPs), gram-negative binding proteins (GNBPs), multidomain scavenger receptors (SCRs), fibrinogen-like domain immunolectin, and Down syndrome cell adhesion molecule (DSCAM), have been identified (Christophides et al. 2004; Watson et al. 2005). These PRRs play important roles in innate immunity. C-type lectins are one of the major PRRs that participate in many immune responses, such as recognition of PAMPs, clearance of bacteria, and enhancing phagocytosis (Zhang et al. 2009a; Wang et al. 2009a; Luo et al. 2006; Sun et al. 2008; Ling and Yu 2006).
C-type lectins belong to a large superfamily of carbohydrate binding proteins and are distributed from Caenorhabditis elegans to Homo sapiens. Vertebrate C-type lectins are conserved, and most members contain only one carbohydrate recognition domain (CRD) (Drickamer 1993). These vertebrate C-type lectins can be divided into 17 subgroups, although most members lose the primary carbohydrate recognition activity (Zelensky and Gready 2005).
C-type lectins are abundant in invertebrates, and they are ‘versatile’ compared to vertebrate C-type lectins. In C. elegans, the number of C-type lectin-like domains (CTLDs) is up to 183 (Drickamer and Dodd 1999; Dodd and Drickamer 2001). In insects, C-type lectins have been reported in several species and their functions are diverse. In Drosophila melanogaster, 32 CTLDs are detected in the genome. In Manduca sexta, four C-type lectins named immulectins have been discovered. Immulectin-1 is inducible by gram-negative, gram-positive bacteria and yeast, and it can stimulate prophenoloxidase activation (Yu et al. 1999). Immulectin-2 can also stimulate prophenoloxidase activation and enhance encapsulation and melanization (Yu and Kanost 2000, 2004). Immulectin-3 and -4 are involved in encapsulation and melanization (Yu et al. 2005, 2006). In Bombyx mori, several C-type lectins have been characterized, including Bm-LBP and Bm-MBP (Koizumi et al. 1999; Watanabe et al. 2006). Bm-LBP can recognize lipid-A portion of LPS and participate in Escherichia coli clearance (Koizumi et al. 1999). Bm-MBP can mediate nodule formation and hemagglutination (Watanabe et al. 2006). C-type lectin of Helicoverpa armigera is up-regulated after challenging with microorganisms, and it is involved in clearance of bacterial pathogens (Chai et al. 2008; Tian et al. 2009).
C-type lectins have also been reported in recent years in several shrimp species, including Penaeus monodon, Litopenaeus vannamei, Penaeus japonicus and Fenneropenaeus chinensis. PmAV from P. monodon exhibits antiviral activity (Luo et al. 2003), and PmLec can enhance hemocyte phagocytosis (Luo et al. 2006). LvLT from L. vannamei is up-regulated after injection of White Spot Syndrome Virus (WSSV) (Ma et al. 2007), LvCTL1 can bind to WSSV and protect shrimp from WSSV infection (Zhao et al. 2009), and LvLec can agglutinate E. coli in Ca2+-dependent manner (Zhang et al. 2009b). A P. japonicus C-type lectin is an essential component in the antiviral process (He et al. 2005). Fc-hsl (FcLec1) from F. chinensis has antimicrobial activity (Sun et al. 2008), Fclectin is up-regulated by LPS and a mixture of Vibrio anguillarum and Staphylococcus staphylolyticus (Liu et al. 2007), FcLec2 acts as a PRR and can bind to LPS, LTA and peptidoglycan directly (Zhang et al. 2009a), FcLec3 may bind to VP28, a major envelop protein of WSSV (Wang et al. 2009b). FcLec4 facilitates the clearance of Vibrio anguillarum (Wang et al. 2009a). These F. chinensis C-type lectins may serve as a reservoir of PRRs for a variety of pathogens. In this paper, a new C-type lectin named FcLec5 from F. chinensis was characterized.
Materials and methods
Chemicals and microorganisms
Mannose, galactose, glucose, maltose, xylose, sucrose and lactose were purchased from Amresco (Solon, OH, USA). N-acetyl-d-glucosamine, N-acetyl-d-galactose, N-acetyl-d-mannosamine, trehalose, LPS (Escherichia coli Serotype 055:135), peptidoglycan (Staphylococcus staphylolyticus), lipoteichoic acid, muramic acid, mannan and dextran were obtained from Sigma (St. Louis, MO, USA). Biozol total RNA extraction reagent was from Bioflux (Japan). SMART PCR cDNA synthesis kit was obtained from BD Bioscience Clontech (Mountain View, CA, USA). Taq Polymerase and DNA markers were from TaKaRa Biotech (Dalian, China). pET-30a(+) vector and E. coli BL21 (DE3) were the products from Novagen company (Germany).Genomic DNA extraction kit was purchased from Toyobo company (Japan).
Bacillus subtilis, Bacillus cereus, Bacillus megaterium, Bacillus thuringiensis, Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa were obtained from Shandong Agricultural University. Vibrio anguillarum was obtained as a gift from the Institute of Oceanology, Chinese Academy of Sciences. Escherichia coli and Saccharomyces cerevisiae were maintained in our lab.
Immune challenge of shrimps
F. chinensis (approximately 10–20 g each) were obtained from Xiaogang market in Qingdao, Shandong Province, China, and cultured temporarily in 500-L tanks filled with air-pumped sea water. For bacteria-challenged experiments, V. anguillarum (2 × 107 cells) were injected into the abdominal segment of each shrimp. For the WSSV-challenged groups, WSSV was injected to abdominal segment (3.2 × 107 virions per shrimp). WSSV inoculum was prepared as described previously (Wang et al. 2009a). Hemolymph was taken from the ventral sinus using a 1-ml sterile syringe preloaded with 100 μl anticoagulant (10% sodium citrate, pH 7.0), and centrifuged immediately at 800 × g for 5 min (4°C) to isolate hemocytes. Other tissues, such as heart, hepatopancreas, gill, stomach, intestine and spermary were also collected for RNA extraction and tissue fixation. Hemocytes and tissues were collected at 0, 2, 6, 12 and 24 h after injection of bacteria or WSSV.
Total RNA isolation
Total RNAs from hemocytes and tissues (heart, hepatopancreas, gills, stomach, intestine and spermary) were extracted according to the protocol of Biozol reagent (Bioflux, Japan). Total RNA (5 μg) from each tissue was reverse transcribed to first-strand cDNA using the oligo anchor R and SMART F primers (Table 1) with a first-strand cDNA synthesis kit (Sangon, Shanghai).
cDNA cloning of the full-length FcLec5
Based on the EST sequence of FcLec5 obtained in our lab, one pair (R and F) of specific primers (Table 1) was designed to amplify FcLec5, the PCR fragment was sequenced by Sangon company (Shanghai, China) and confirmed to be a C-type lectin sequence using the online BLAST programs. Then 3′ and 5′ rapid amplification of cDNA ends (RACE) were performed to obtain the full-length sequence of FcLec5. For 3′ RACE, F and 3′ anchor R primers were used. For 5′ RACE, 5′ primer and R primer were used.
Phylogenetic and sequence analyses
Phylogenetic analysis of some selected C-type lectins was performed with MEGA 4.0. (Tamura et al. 2007). Phylogenetic tree was constructed to analyze evolutionary relationship. Comparison of the predicted domains, repeats, motifs and features of lectins from different animals were performed with the Sample Modular Architecture Research Tool (SMART) program (http://smart.embl-heidelberg.de/).
Genomic sequence cloning
Muscle (10 mg) was collected from healthy shrimp for preparation of genomic DNA according to the protocol of Genomic DNA extraction kit. FcLec5-specific primers mR and mF (Table 1) were used in PCR to amplify the genomic sequence. PCR reactions were performed as follows: 98°C for 5 min, 10 cycles of 94°C for 30 s, 60–50°C for 45 s (temperature was decreased 1°C every cycle), 72°C for 2 min, then 25 cycles of 94°C for 30 s, 50°C for 45 s, 72°C for 2 min, and finally 72°C for 10 min to complete the extension.
Northern blot
To prepare RNA probe, FcLec5 cDNA was subcloned into a pGEM-T-Easy vector (Promega). The recombinant plasmid was linearized by PstI. DIG-RNA labeling kit (Roche) was used to transcribe Dig-tagged probe with Sp6 RNA polymerase in vitro.
Northern blot analysis was performed as described previously (Zhao et al. 2004). Briefly, 5 μg RNA (from normal and bacteria-challenged shrimp) was denatured and electrophoresed on a formaldehyde-containing agarose gel, and then transferred to a nylon membrane (IMMOBILON-NY+, Millipore). Target mRNA was hybridized with antisense probe (100 ng/ml) in 50% formamide at 68°C and then washed with 2× wash solution (2× SSC containing 0.1% SDS) and 0.1× wash solution (0.1× SSC containing 0.1% SDS) at 68°C. Anti-Dig-phosphatase AB was used to detect the probe, which was visualized with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium chloride. Prehybridization, hybridization, washing and color development were performed according to the manufacturer’s protocols.
Quantitative real-time PCR
Five micrograms of DNase-treated total RNAs (TaKaRa RNase-free DNase I) were used for reverse transcription of the first-strand cDNAs. Transcription levels of FcLec5 at different times after immune challenge were evaluated by qRT-PCR following the protocol of SYBR Premix Ex Taq (Takara, Japan). The qRT-PCR was performed using real-time thermal cycler (Bio-Rad, USA) in a total volume of 10 μl containing 5 μl of 2× Premix Ex Taq, 1 μl of the 1:100 diluted cDNA, 2 μl each of the forward and reverse primer (realF and realR, 1 μM). The amplification procedure consisted of an initial denaturation step at 95°C for 3 min and then 40 cycles of 95°C for 30 s, 62°C for 50 s followed by performing melting curve from 60 to 95°C. Actin was also amplified with specific primers Actin F and Actin R using the same conditions (Table 1). All samples were repeated in triplicates for real-time PCR analysis. The expression level of FcLec5 was analyzed using the comparative C T method that the discrepancy between the C T for FcLec5 and β-actin (ΔC T) was calculated to normalize the variation in the amount of cDNA in each reaction. The FcLec5 expression level was calculated by \( 2^{{ - \Updelta C_{\text{T}} }}. \) The normalized data were subjected to the statistical analysis followed by an unpaired sample t test. Significant difference was accepted at P < 0.05.
Recombinant expression and purification of FcLec5
Primers (mF, mR, d1mF, d1mR, d2mF and d2mR) for protein expression were used to amplify the coding sequence of FcLec5 and its two individual CRD domains. The fragments were subcloned into pET30a (+) at the EcoRI and SalI sites. Recombinant plasmids were sequenced and then transformed into E. coli BL21 (DE3) cells for recombinant protein expression.
FcLec5 and the two CRD domains were expressed as inclusion bodies. Purification of recombinant proteins was performed as described previously (Du et al. 2006). Purified recombinant proteins were dialyzed using buffer A (50 mM Tris–HCl, pH 8.0) to remove the imidazole in the elution buffer.
Preparation of antiserum and Western blot
Purified recombinant mature FcLec5 was used as an antigen for antiserum preparation in New Zealand rabbits following the method described by Du et al. (2007).
Shrimp tissues were homogenized in lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM PMSF, 3 mM EDTA). The homogenates were centrifuged at 12,000 × g for 10 min at 4°C and supernatants were collected. Protein concentrations were determined by the Bradford method (Bradford 1976) using BSA as standards. Total proteins (200 μg) from each tissue or purified recombinant proteins were run on 12.5% (for FcLec5) or 15% (for the two CRD domains) SDS-PAGE by the Laemmli method (Laemmli 1970), and proteins were then transferred onto a nitrocellulose membrane. The membrane was blocked with 2% non-fat dry milk in TBS (10 mM Tris–HCl, pH 7.5, 150 mM NaCl) and then incubated with polyclonal rabbit antiserum to FcLec5 (1:500 dilution). Band was visualized by a colorimetric reaction catalyzed by peroxidase-conjugated goat anti-rabbit IgG (1:10,000 in TBS).
Localization of FcLec5 by immunohistochemistry
Hepatopancreas from shrimp were fixed immediately after dissection by incubating for 24 h at 4°C in PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 8.0) solution containing 4% paraformaldehyde. After dehydration, hepatopancreas were embedded in paraffin, sectioned at a thickness of 7 μm and rehydrated in water. The sections were blocked with 1% BSA in PBS and then incubated with polyclonal rabbit antiserum to FcLec5 (1:100 dilution) overnight at 4°C. Hybridization signals were visualized by fluorescence microscope using FITC-conjugated goat anti-rabbit IgG (1:1,000 in PBS). DAPI was also used to stain the nuclei of hepatopancreas cells.
Hemagglutination and microbial agglutination assays
Blood from rabbits was collected in sterile TBS and erythrocytes were collected by centrifugation at 800 × g for 10 min. Erythrocytes were then washed four times with TBS and resuspended in TBS. Hemagglutinating assay was performed by incubating rabbit erythrocytes (25 μl) with recombinant FcLec5 (200 μg/ml; 25 μl) in the presence or absence of 10 mM CaCl2. Hemagglutination was examined 1 h after incubation at room temperature by microscopy. Phytohemagglutinin was used as a positive control (200 μg/ml), and BSA (200 μg/ml) was used as a negative control.
Gram-positive bacteria (B. subtilis, B. cereus, B. megaterium, B. thuringiensis and S. aureus), gram-negative bacteria (E. coli, K. pneumoniae and P. aeruginosa) and yeast (S. crevisiae) were used for agglutination assays. Bacteria in mid-logarithmic phase were collected by centrifugation at 6,000 × g for 5 min and resuspended in TBS to OD600 of 0.4. Bacteria (25 μl) were added to 25 μl TBS containing different concentrations (12.5–200 μg/ml) of recombinant FcLec5 and its two CRD domains in the presence or absence of 10 mM CaCl2. The mixture was incubated at room temperature for 1 h, and agglutination was observed by microscopy. BSA (200 μg/ml) was used as a negative control.
To determine the sugar binding specificity of FcLec5 and its two CRD domains, an inhibitory agglutination assay was performed. Recombinant proteins (200 μg/ml; 25 μl) were incubated with different concentrations of sugars (25 μl) for 1 h at room temperature. Then E. coli cells (OD600 of 0.4 in TBS; 25 μl) were added, and inhibition of agglutination was detected. Inhibitory capacity was shown as the minimum concentration of the sugar that can inhibit agglutination. Assays were performed in triplicates.
Binding of recombinant FcLec5 and its two CRD domains to microorganisms
Gram-positive bacteria (B. subtilis, B. cereus, B. megaterium, B. thuringiensis and S. aureus), gram-negative bacteria (E. coli, K. pneumonia, P. aeruginosa and V. anguillarum) and yeast (S. crevisiae) were used to test binding activity of recombinant FcLec5 and its two CRD domains using a method previously described by Sun et al. (2008) with slight modification. Briefly, bacterial or yeast cells were cultured in 9-ml LB medium or YPD medium (10 g yeast extract, 20 g peptone, 20 g glucose in 1 l distilled water) overnight, and the cells were pelleted by centrifugation at 6,000 × g for 5 min. Bacterial and yeast cells were washed with TBS, and then thoroughly resuspended in TBS to OD600 of 1.0. The purified recombinant FcLec5 and its two CRD domains in buffer A (50 mM Tris–HCl, pH 8.0) were incubated with microorganisms with rotation for 20 min at room temperature. Microorganisms were pelleted, washed four times with TBS, and then washed with 7% SDS for 1 min. Then microorganisms were washed with TBS again to remove the remaining SDS. As a control, bacterial cells were incubated with TBS and subjected to the same treatments without adding recombinant proteins. Finally, microorganisms were resuspended in SDS-PAGE loading buffer, and run on 12.5% SDS-PAGE under reducing conditions. Recombinant FcLec5 and its two CRD domains were detected by immunoblotting using polyclonal rabbit antiserum.
Binding of recombinant FcLec5 and its two CRD domains to microbial components
The binding activity of recombinant FcLec5 and its two CRDs was determined by an enzyme-linked immunosorbent assay (ELISA) described previously (Yu and Kanost 2000) with slight modifications. Peptidoglycan, LPS, lipoteichoic acid, mannan and dextran were dissolved in water at 80 μg/ml and sonicated for 3 × 15 s, and 50 μl (4 μg) of each microbial component were used to coat a well of a microtiter plate. The plate was then incubated at 37°C until the solution was evaporated. The plates were heated to 60°C for 30 min and then blocked with 200 μl/well of BSA (1 mg/ml) in TBS for 2 h at 37°C. The plates were washed four times with TBS (200 μl/well), then 50 μl recombinant proteins (0–20 μg/ml FcLec5 diluted with TBS containing 0.1 mg/ml BSA) were added to the wells. After incubating for 3 h at room temperature, the plates were then washed four times with TBS (200 μl/well), and rabbit anti-FcLec5 antiserum (1:300 dilution with TBS containing 0.1 mg/ml BSA) was added to the wells (100 μl/well). After incubation for 1 h at 37°C, the wells were washed four times with TBS. Peroxidase-conjugated goat anti-rabbit IgG (1:3,000 dilution) was added to each well and incubated for 1 h at 37°C. The plates were washed four times as described above. After adding 0.01% 3,3′,5,5′-tetramethylbenzidine (Sigma) liquid substrate in citric acid-Na2HPO4 buffer, and the absorbance was measured at 450 nm. Sample without FcLec5 was used as a negative control. The assay was repeated three times. The dissociation constants (K d) and the maximum binding (B max) parameters were calculated with GraphPad Prism version 5.00 for Windows, GraphPad Software (San Diego, California, USA).
Result
cDNA sequence of FcLec5
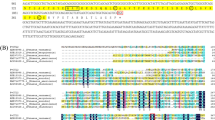
The full-length cDNA sequence of FcLec5 was obtained by 5′ and 3′ RACE, and it is 1,137 bp long (accession number: EU834290) encoding 335 amino acids with a signal peptide of 20 residues (Fig. 1a). The predicted FcLec5 protein contains two carbohydrate recognition domains (CRDs), each consists of ~130 amino acids. BLASTx search showed that FcLec5 is similar to other animal C-type lectins, with 53% identity to two C-type lectins from the shrimp P. monodon (accession numbers: ABI97373, ABI97374). FcLec5 contains two sugar binding motifs. One is QPD (Gln-Pro-Asp) predicted for galactose in the N-terminal CRD, and the other is EPQ (Glu-Pro-Gln), which is altered from EPN predicted for mannose/glucose in the C-terminal CRD (Fig 1a). We also cloned genomic sequence of FcLec5, and it contains three introns of 241, 149 and 99 bp, respectively (Fig 1b).
Phylogenetic analysis of FcLec5
Phylogenetic tree was constructed to analyze the relationship of FcLec5 with some selected animal C-type lectins (Fig. 2). These C-type lectins clustered into three groups. Group 1 contained lectins from shrimp and some fishes, FcLec1, 2, 3 and 5 belonged to this group. Group 2 contained lectins mainly from insects, and FcLec4 was clustered to the group. Group 3 contains a lectin from crayfish, Pacifastacus leniusculus. The P. leniusculus lectin was like an ancestral genotype. Group I can be divided into three subgroups. FcLec 2, 5 and four other shrimp C-type lectins, including FcLecAAX63905 (Liu et al. 2007) also from Chinese white shrimp, all contain two CRDs, formed the subcluster II. Shrimp C-type lectins containing a single CRD, including FcLec1 and 3 formed the subcluster III.
Phylogenetic analysis of FcLec5 with other animal C-type lectins. The NJ tree was obtained using MEGA4.0. FcLec1-5 were indicated by box. The other C-type lectin from F. chinensis reported by Liu et al. (2007) was underlined
Tissue distribution and transcription levels of FcLec5 mRNA
Northern blot was performed to assess tissue distribution of FcLec5 mRNA using 18S rRNA as a reference (Fig. 3). FcLec5 mRNA was detected only in the hepatopancreas of healthy shrimp, but not in other tissues such as haemocyte, heart, gill, stomach, intestine and ovary, and its expression in the hepatopancreas was induced after V. anguillarum-challenge slightly (Fig. 3). Then, expression of FcLec5 mRNA in the hepatopancreas after being immune-challenged was determined by quantitative real-time PCR (Fig. 4). The expression level of FcLec5 mRNA in hepatopancreas was significantly increased at 2 h after injection of V. anguillarum, and then quickly dropped to the original level at 6 h post-injection (Fig. 4a). The expression level of FcLec5 transcript was also increased significantly in the hepatopancreas at 2 h after injection of WSSV, peaked at 6 h post-injection, and then returned to the original level at 12 h post-injection (Fig. 4b).
Distribution of FcLec5. Northern blot in seven tissues (normal and 24 h after bacteria injection). Expression of FcLec5 was detected in hepatopancreas in both normal and bacteria-challenged shrimp (− for normal and + for bacteria-challenge), and FcLec5 expression increased at 24 h post-infection. 18S rRNA was used as a control
Time course expression of FcLec5 mRNA after immune-challenge by qRT-PCR. a mRNA levels of FcLec5 in hepatopancreas after challenged with bacteria. b mRNA levels of FcLec5 in hepatopancreas after challenged with WSSV. The asterisks indicate significant differences (P < 0.05) from the unchallenged shrimp. Bars represent mean ± SD from three independent PCR amplifications and quantifications
Expression and purification of recombinant FcLec5 and its two CRD domains
Recombinant FcLec5 and its two individual CRD domains were expressed in E. coli BL21 (DE3) as inclusion bodies with extra 52 residues (from the expression vector pET30a (+), about 5.7 kDa mainly containing a His-tag and an S-tag) to their N-termini, so the predicted molecular masses for recombinant FcLec5 and its two CRD domains are 43, 20 and 20 kDa, respectively. Figure 5 showed the expression and purification of recombinant proteins, and the molecular masses of recombinant FcLec5 and C-terminal CRD2 were consistent with the predicted masses (Fig. 5, lanes 1 and 7). However, the mass of the N-terminal CRD1 appeared at ~30 kDa, which was larger than the predicted mass of 20 kDa (Fig. 5, lane 4). After digestion with thrombin, which removes the fusion partner of 5.7 kDa, the mass of CRD1 was consistent with the predicted mass of 15 kDa (Fig. 5, lane 5).
FcLec5 protein in shrimp
To detect FcLec5 protein in shrimp, a Western blot was performed. FcLec5 protein was detected only in the hepatopancreas and hemolymph, but not in other tissues (Fig. 6a), indicating that FcLec5 is specifically expressed in the hepatopancreas and the protein is secreted into the hemolymph. Then an immunohistochemistry assay was also performed to further localize FcLec5 protein in the hepatopancreas. Hepatopancreas is composed of many hepatopancreas tubules that contain five main cell types: embryonic (E)-cells, resorptive (R)-cells, fibrillar (F)-cells, blister-like (B)-cells and midget (M)-cells (Lehnert and Johnson 2002). FcLec5 protein was detected only in the F-cells (Fig. 6b), which synthesize secreted proteins (Lehnert and Johnson 2002).
a Western blot of FcLec5 in different tissues of normal shrimp. b Localization of FcLec5 protein in hepatopancreas by immunohistochemistry. i Negative control, ii hepatopancreas stained by DAPI, iii hepotapancreas stained by FITC-conjugated goat anti-rabbit IgG showed distribution of FcLec5. Bar 5 μm. Deep color in (ii) indicates the nucleus of F-cells. The arrows show the F-cells and the location of FcLec5. c Expression change of FcLec5 at 12 h post-pathogen infection. Upper panel Western blot analysis, lower panel SDS-PAGE to quantify the loading proteins. N normal, B bacteria-challenge, V virus-challenge
We also detected the change of FcLec5 level in hemolymph after pathogen infection. The results showed that the protein level was increased at 12 h post V. anguillarum or WSSV infection, suggesting that more secretion, and possibly enhanced expression of FcLec5 in hepatopancreas upon infection (Fig. 6c).
Agglutinating activity of FcLec5
C-type lectins have agglutinating activity. We first tested the hemagglutinating activity of recombinant FcLec5 with rabbit and mouse erythrocytes, and found that FcLec5 did not agglutinate erythrocytes in the presence or absence of Ca2+ (data not shown). Then agglutinating activity of FcLec5 and its two CRD domains with ten microorganisms (five gram-positive bacteria, four gram-negative bacteria, and yeast) was performed. FcLec5 and its two CRD domains could agglutinate four gram-positive bacteria and four gram-negative bacteria but not B. megaterium or yeast (Fig. 7; Table 2), and the agglutinating activity was Ca2+-dependent. Table 2 listed minimal agglutinating concentrations of FcLec5 and its two CRDs for microorganisms. FcLec5 had higher affinity for microorganisms compared to its two individual CRD domains, and FcLec5 and its two CRD domains had higher agglutinating activity against gram-negative bacteria than gram-positive bacteria (Table 2).
Sugar specificity
FcLec5 has two CRDs, the N-terminal CRD1 contains a binding motif of QPD (Gln-Pro-Asp) with predicted specificity for galactose, while the C-terminal CRD2 contains a motif of EPQ (Glu-Pro-Gln) possibly with binding specificity for mannose/glucose. To test binding specificity of FcLec5 and its two CRDs for carbohydrates, an inhibitory agglutinating assay was performed with E. coli, and the minimal inhibitory concentrations of carbohydrates were listed in Table 3. Agglutinating activity of FcLec5 and its two CRD domains was inhibited by three polysaccharides (LPS, LTA and peptidoglycan), but not by monosaccharides such as galactose and mannose (Table 3), suggesting that binding of FcLec5 and its two CRD domains to monosaccharides may be too weak to be detected.
Direct binding of FcLec5 and its two CRDs to microorganisms
Agglutination assay showed that FcLec5 and its two CRDs could agglutinate microorganisms, and the agglutinating activity was inhibited by LPS, LTA and peptidoglycan (Fig. 7; Table 3). Then, direct binding of recombinant FcLec5 and its two CRDs to ten microorganisms was performed. FcLec5 bound to all ten microorganisms tested, while both individual CRD domains bound to seven microorganisms but not to S. aureus, K. pneumoniae or yeast (Fig. 8). Direct binding of FcLec5 and its two CRDs to microorganisms was Ca2+-independent.
FcLec5 binds to polysaccharides
In order to confirm the sugar binding specificity of the recombinant proteins, direct binding to polysaccharides was performed by ELISA (Fig. 9). FcLec5 and its two CRDs all bound to LPS, LTA and peptidoglycan with different binding activities (Fig. 9). The apparent dissociation constants (K d) and the maximum binding (B max) parameters were calculated and listed in Table 4. Besides, the recombinant proteins could also bind to mannan and dextran (Fig. 9). Compared to those of CRD1 and FcLec5, the binding activity of CRD2 to mannan and dextran was lower.
Discussion
C-type lectins exist in many animals. They are involved in nonself-recognition, binding to specific carbohydrates in a Ca2+-dependent manner, and clearance of invading microorganisms (Drickamer 1993; Drickamer and Dodd 1999; Yu and Kanost 2004; Wang et al. 2009a). In this study we cloned a novel C-type lectin (FcLec5) in Chinese white shrimp by using ESTs and RACE. FcLec5 contains two CRDs with sugar binding motifs of QPD and EPQ, respectively. We also obtained a genomic sequence of FcLec5 with three introns and the boundary of introns was confirmed to be GT-AG.
Expression profiles of FcLec1-5
Many lectins have been identified in shrimps, and most are tissue-specific. In Chinese white shrimp, several C-type lectins have been reported in different tissues. Fclectin is hemocyte-specific (Liu et al. 2007), FcLec1, FcLec3 (Wang et al. 2009b) and FcLec5 are hepatopancreas-specific, FcLec1 mRNA is detected in bacteria-challenged stomach (Sun et al. 2008), FcLec2 mRNA is only detected in hepatopancreas, but the protein is also present in gill and stomach in addition to hepatopancreas (Zhang et al. 2009a), FcLec4 mRNA exists in hepatopancreas, gill, stomach and intestine, and the protein is detected in gills and stomach (Wang et al. 2009a). FcLec5 mRNA was increased at 2 h after shrimp were challenged with V. anguillarum, but returned to normal level quickly. This result is similar to that of FcLec2, which also contain two CRD domains (Zhang et al. 2009a). FcLec5 transcript was also up-regulated after WSSV challenge and reached its peak level at 6 h post-challenge, while FcLec2 mRNA reached its maximum at 12 h post-challenge (Zhang et al. 2009a). Besides, more FcLec5 protein could be detected in the hemolymph after V. anguillarum or WSSV infection, suggesting that more FcLec5 was induced and secreted into the circulating hemolymph to defend the invading pathogens.
Agglutinating and binding activity of FcLec1-5
In vitro experiments showed that FcLec5 and its two CRDs could agglutinate gram-negative and gram-positive bacteria, but not gram-positive B. megaterium. Compared to the two CRDs, FcLec5 had lower minimal agglutinating concentrations suggesting that FcLec5 might have higher agglutinating activity. This result is consistent with that of FcLec2 (Zhang et al. 2009a). FcLec1, FcLec2, FcLec3 and FcLec4 have similar agglutinating activity against gram-negative and gram-positive bacteria, but FcLec5 showed higher agglutinating activity against gram-negative bacteria than gram-positive bacteria.
Characteristics of binding and agglutinating activity of FcLec5
FcLec5 bound to yeast but did not agglutinate yeast cells (Fig. 8, and Table 2), whereas FcLec5 and its two CRDs had weak binding activity to B. megaterium but did not agglutinate the bacterium. Agglutinating activity is one basic action for C-type lectins to resist the invading pathogens, and binding is the first step for this course. Some co-factors (calcium for instance) may be also needed for agglutination. FcLec5, like immulectin-4, showed Ca2+-independent binding activity but Ca2+-dependent agglutinating activity. Ca2+ is required for agglutinating activity, this may be because Ca2+ can promote formation of lectin oligomers (Yu et al. 2006). We could detect the agglutinating, but not the strong binding of two CRDs to S. aureus. This phenomenon has also been observed for FcLec2 (Zhang et al. 2009a). We think that weak binding activity may be enough for the following agglutination.
We also performed an inhibitory agglutination assay to determine sugar binding activity of FcLec5 and its two CRDs. CRD1 showed weak affinity for d-galactose and N-acetyl-d-galactose (200 mM), consistent with its QPD binding motif predicted for galactose. CRD2 did not have affinity for monosaccharides. This may be due to its altered sugar binding motif from EPN to EPQ. Generally EPN motif is predicted to bind mannose/glucose. Some altered motifs exhibit different binding tendencies. For example, FcLec3 with EPS motif (Wang et al. 2009b) lost the binding activity towards mannose and glucose, while a Pleurodeles waltl lectin with the same motif could recognize glucose (Tiffoche et al. 1993). Besides, Cf-Lec2 with an EPD motif could still bind to mannose (Zheng et al. 2008).
It was well known that one single CRD has weak affinity for polysaccharides, and C-type lectins with multiple CRDs have higher affinity for polysaccharides. FcLec5 had lower minimal agglutinating concentrations for bacteria and higher binding affinity for bacteria compared to its two CRDs, suggesting that the two CRDs may synergistically contribute to binding affinity of FeLec5. Recombinant FcLec5 and its two CRDs all could bind to bacterial polysaccharides (Fig. 9). FcLec5 might target bacteria via binding to bacterial polysaccharides. Shrimps have many C-type lectins that can bind to microbial polysaccharides. For example, PmLec can bind to LPS (through the O-antigen) and its agglutinating activity can be inhibited by LPS (Luo et al. 2006). FcLec1 (hsL), a C-type lectin reported in our lab, has affinity for LPS and peptidoglycan (Sun et al. 2008), and FcLec2 can recognize LPS, LTA and peptidoglycan (Zhang et al. 2009a). FcLec3 and FcLec4 have affinity for peptidoglycan (Wang et al. 2009a, b).
Besides, all three recombinant proteins (FcLec5, CRD1 and 2) could bind to mannan and dextran, the multimers of mannose and glucose. The binding activity of CRD2 is lower than those of FcLec5 and CRD1. It seems that there exists conflict between the indirect and direct assays. We suppose that the formation from monomer to multimers would facilitate the recognition of lectins. Moreover, the case that the ELISA assay is much more precise than the indirect assay should not be ignored.
Because many C-type lectins are present in animals, each lectin may have its own functions. For example, PmAV exhibits antiviral activity in fish cell line (Luo et al. 2003). Injection of antibody of immulectin-2 can affect clearance of bacteria and survival of larvae (Yu and Kanost 2003). Immulectin-1 and -2 can activate phenol oxidase activity in plasma (Yu et al. 1999; Yu and Kanost 2000). So in vivo experiments are necessary to elucidate the functions of C-type lectins in shrimps.
In conclusion, a new C-type lectin named FcLec5 was studied in this work. Expression of FcLec5 was up-regulated in responses to bacteria and WSSV injection. FcLec5 has a broad binding spectrum for microorganisms and could bind to LPS, LTA and peptidoglycan. Our results suggest that FcLec5 may serve as one PRR for bacteria in Chinese white shrimp F. chinensis.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Chai LQ, Tian YY, Yang DT, Wang JX, Zhao XF (2008) Molecular cloning and characterization of a C-type lectin from the cotton bollworm, Helicoverpa armigera. Dev Comp Immunol 32:71–83
Christophides GK, Vlachou D, Kafatos FC (2004) Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol Rev 198:127–148
Dodd RB, Drickamer K (2001) Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology 11:71R–79R
Drickamer K (1993) Ca2+-dependent carbohydrate-recognition domains in animal proteins. Curr Opin Struct Biol 3:393–400
Drickamer K, Dodd RB (1999) C-type lectin-like domains in Caenorhabditis elegans: predictions from the complete genome sequence. Glycobiology 9:1357–1369
Du XJ, Wang JX, Liu N, Zhao XF, Li FH, Xiang JH (2006) Identification and molecular characterization of a peritrophin-like protein from fleshy prawn (Fenneropenaeus chinensis). Mol Immunol 43:1633–1644
Du XJ, Zhao XF, Wang JX (2007) Molecular cloning and characterization of a lipopolysaccharide and β-1, 3-glucan binding protein from fleshy prawn (Fenneropenaeus chinensis). Mol Immunol 44:1085–1094
He NH, Qin QW, Xu X (2005) Differential profile of genes expressed in hemocytes of White Spot Syndrome Virus-resistant shrimp (Penaeus japonicus) by combining suppression subtractive hybridization and differential hybridization. Antivir Res 66:39–45
Janeway CA Jr (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54:1–13
Koizumi N, Imamura M, Kadotani T, Yaoi K, Iwahana H, Sato R (1999) The lipopolysaccharide-binding protein participating in haemocyte nodule formation in the silkworm Bombyx mori is a novel member of the C-type lectin superfamily with two different tandem carbohydrate-recognition domains. FEBS Lett 443:139–143
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lehnert SA, Johnson SE (2002) Expression of hemocyanin and digestive enzyme messenger RNAs in the hepatopancreas of the Black Tiger Shrimp Penaeus monodon. Comp Biochem Physiol B Biochem Mol Biol 133:163–171
Ling E, Yu XQ (2006) Cellular encapsulation and melanization are enhanced by immulectins, pattern recognition receptors from the tobacco hornworm Manduca sexta. Dev Comp Immunol 30:289–299
Liu YC, Li FH, Dong B, Wang B, Luan W, Zhang XJ, Zhang LS, Xiang JH (2007) Molecular cloning, characterization and expression analysis of a putative C-type lectin (Fclectin) gene in Chinese shrimp Fenneropenaeus chinensis. Mol Immunol 44:598–607
Luo T, Zhang X, Shao Z, Xu X (2003) PmAV, a novel gene involved in virus resistance of shrimp Penaeus monodon. FEBS Lett 551:53–57
Luo T, Yang H, Li F, Zhang X, Xu X (2006) Purification, characterization and cDNA cloning of a novel lipopolysaccharide-binding lectin from the shrimp Penaeus monodon. Dev Comp Immunol 30:607–617
Ma TH, Tiu SH, He JG, Chan SM (2007) Molecular cloning of a C-type lectin (LvLT) from the shrimp Litopenaeus vannamei: early gene down-regulation after WSSV infection. Fish Shellfish Immunol 23:430–437
Medzhitov R, Janeway CA Jr (2002) Decoding the patterns of self and nonself by the innate immune system. Science 296:298–300
Sun YD, Fu LD, Jia YP, Du XJ, Wang Q, Wang YH, Zhao XF, Yu XQ, Wang JX (2008) A hepatopancreas-specific C-type lectin from the Chinese shrimp Fenneropenaeus chinensis exhibits antimicrobial activity. Mol Immunol 45:348–361
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tian YY, Liu Y, Zhao XF, Wang JX (2009) Characterization of a C-type lectin from the cotton bollworm, Helicoverpa armigera. Dev Comp Immunol 33:772–779
Tiffoche C, Chesnel A, Jego P, Le Pennec JP (1993) Isolation and characterization of a cDNA clone encoding a Pleurodeles lectin. Eur J Biochem 213:901–907
Wang S, Zhao XF, Wang JX (2009a) Molecular cloning and characterization of the translationally controlled tumor protein from Fenneropenaeus chinensis. Mol Biol Rep 36:1683–1693
Wang XW, Zhang XW, Xu WT, Zhao XF, Wang JX (2009b) A novel C-type lectin (FcLec4) facilitates the clearance of Vibrio anguillarum in vivo in Chinese white shrimp. Dev Comp Immunol 33:1039–1047
Wang XW, Xu WT, Zhang XW, Zhao XF, Yu XQ, Wang JX (2009c) A C-type lectin is involved in the innate immune response of Chinese white shrimp. Fish Shellfish Immunol 27:556–562
Watanabe A, Miyazawa S, Kitami M, Tabunoki H, Ueda K, Sato R (2006) Characterization of a novel C-type lectin, Bombyx mori multibinding protein, from the B mori hemolymph: mechanism of wide-range microorganism recognition and role in immunity. J Immunol 177:4594–4604
Watson FL, Püttmann-Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, Rebel VI, Schmucker D (2005) Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 309:1874–1878
Yu XQ, Kanost MR (2000) Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to gram-negative bacteria. J Biol Chem 275:37373–37381
Yu XQ, Kanost MR (2003) Manduca sexta lipopolysaccharide-specific immulectin-2 protects larvae from bacterial infection. Dev Comp Immunol 27:189–196
Yu XQ, Kanost MR (2004) Immulectin-2, a pattern recognition receptor that stimulates haemocyte encapsulation and melanization in the tobacco hornworm, Manduca sexta. Dev Comp Immunol 28:891–900
Yu XQ, Gan H, Kanost MR (1999) Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenol oxidase. Insect Biochem Mol Biol 29:585–597
Yu XQ, Tracy ME, Ling E, Scholz FR, Trenczek T (2005) A novel C-type immulectin-3 from Manduca sexta is translocated from hemolymph into the cytoplasm of haemocytes. Insect Biochem Mol Biol 35:285–295
Yu XQ, Ling E, Tracy ME, Zhu Y (2006) Immulectin-4 from the tobacco hornworm Manduca sexta binds to lipopolysaccharide and lipoteichoic acid. Insect Mol Biol 15:119–128
Zelensky AN, Gready JE (2005) The C-type lectin-like domain superfamily. FEBS J 272:6179–6217
Zhang XW, Xu WT, Wang XW, Mu Y, Zhao XF, Yu XQ, Wang JX (2009a) A novel C-type lectin with two CRD domains from Chinese shrimp Fenneropenaeus chinensis functions as a pattern recognition protein. Mol Immunol 46:1626–1637
Zhang Y, Qiu LM, Song LS, Zhang H, Zhao JM, Wang LL, Yu YD, Li CH, Li FM, Xing KZ, Huang BX (2009b) Cloning and characterization of a novel C-type lectin gene from shrimp Litopenaeus vannamei. Fish Shellfish Immunol 26:183–192
Zhao XF, Wang JX, Xu XL, Li ZM, Kang CJ (2004) Molecular cloning and expression patterns of the molt-regulating transcription factor HHR3 from Helicoverpa armigera. Insect Mol Biol 13:407–412
Zhao ZY, Yin ZX, Xu XP, Weng SP, Rao XY, Dai ZX, Luo YW, Yang G, Li ZS, Guan HJ, Li SD, Chan SM, Yu XQ, He JG (2009) A novel C-Type lectin from the shrimp Litopenaeus vannamei possesses anti-white spot syndrome virus activity. J Virol 83:347–356
Zheng P, Wang H, Zhao J, Song L, Qiu L, Dong C et al (2008) A lectin (CfLec-2) aggregating Staphylococcus haemolyticus from scallop Chlamys farreri. Fish Shellfish Immunol 24:286–293
Acknowledgments
Mannan and dextran (Sigma) were gifted by Professor Weifeng Liu, Shandong University, China. This work was supported by grants from the National Natural Science Foundation of China (No. 30728001, 30770282) and the National High Technology Research and Development Program of China (863 Program) (No. 2007AA09Z425).
Author information
Authors and Affiliations
Corresponding authors
Additional information
W.-T. Xu and X.-W. Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, WT., Wang, XW., Zhang, XW. et al. A new C-type lectin (FcLec5) from the Chinese white shrimp Fenneropenaeus chinensis . Amino Acids 39, 1227–1239 (2010). https://doi.org/10.1007/s00726-010-0558-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0558-7