Abstract
Apoptosis of vascular smooth muscle cells (VSMCs) plays an important role in regulating vascular remodeling during cardiovascular diseases. Apelin is the endogenous ligand for the G-protein-coupled receptor APJ and plays an important role in the cardiovascular system. However, the mechanisms of apelin on apoptosis of VSMCs have not been elucidated. Using a culture of human VSMCs as a model for the study of apoptosis, the relationship between apelin and apoptosis of human VSMCs and the signal pathway involved were investigated. Using western blotting, we confirmed that VSMCs could express APJ. To evaluate the possible role of apelin in VSMC apoptosis, we assessed its effect on apoptosis of human VSMCs. The results showed that apelin inhibited human VSMCs apoptosis induced by serum deprivation. Suppression of APJ with small-interfering RNA (siRNA) abolished the anti-apoptotic activity of apelin. Apelin increased Bcl-2 protein expression, but decreased Bax protein expression. An increase in activation of extracellular signal-regulated protein kinase (ERK) and Akt (a downstream effector of phosphatidylinositol 3-kinase) was shown after apelin stimulation. Suppression of APJ with siRNA abolished the apelin-induced activation of ERK and Akt. LY294002 (a PI3-K inhibitor) blocked apelin-induced activation of Akt and abolished the apelin-induced antiapoptotic activity. Our study suggests that apelin suppresses serum deprivation-induced apoptosis of human VSMCs, and that the anti-apoptotic action is mediated through the APJ/PI3-K/Akt signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis of vascular smooth muscle cells (VSMCs) occurs in normal vessel development and is crucial for regulating vascular remodeling during a variety of cardiovascular pathologies including hypertension, restenosis after angioplasty or stenting, aneurysms, and atherosclerosis (Bauriedel et al. 1999; Henderson et al. 1999; Isner et al. 1995; Okura et al. 2002). Apoptosis accelerates atherosclerosis, promotes plaque calcification and medial degeneration, prevents expansive remodeling, and promotes stenosis in atherosclerosis (Clarke et al. 2008).
Apelin was first identified in the gastrointestinal tract as an endogenous ligand of the G-protein-coupled receptor APJ (Tatemoto et al. 1998). Apelin peptides (active in forms that range in length from 13 to 36 residues) are produced from the C-terminal portion of the pre-proprotein consisting of 77 amino acid residues. The full-length apelin (1–36) peptide is metabolically less active than the C-terminal fragments, apelin-13 (Hosoya et al. 2000) and apelin-17 (De Mota et al. 2004). Apelin-13 is the variant most active in signal transduction and consequently is most frequently studied; however, the 36-peptide could be the most active apelin in the blockade of virus entry (Zou et al. 2000). Apelin receptor (APJ), first described as a receptor similar to AT1 angiotensin-I receptor by O’Dowd et al. (1993), is a member of the rhodopsin family (Fredriksson et al. 2003) of heptahelical G-protein-coupling receptors [also known as family A (Horn et al. 1998)].
Apelins and APJ have wide ranges of expression in mammalian tissues. However, the cardiovascular system could be the main target of apelin. Apelin induces an increase in myocardial contractility and a reduction of vasomotor tone (Ishida et al. 2004; Farkasfalvi et al. 2007). It has fast effects on the propagation of action potential and contractility in cardiomyocytes (Farkasfalvi et al. 2007), which may include activity at the level of transporters precoupled to APJ. Apelin expression is induced by hypoxia in endothelial and vascular smooth muscle cells (Eyries et al. 2008), and is involved in the regulation of blood vessel diameter during angiogenesis (Kidoya et al. 2008). Apelin is also required for normal vascular development in the frog embryo and has properties consistent with a role during normal and pathological angiogenesis (Cox et al. 2006).
Apelin induces VSMC proliferation by regulating cyclin D1 (Li et al. 2008). More recently, apelin has been shown to prevent aortic aneurysm formation by inhibiting macrophage infiltration (Leeper et al. 2009). Apoptosis, the generation of reactive oxygen species, malonaldehyde content, and lactate dehydrogenase leakage are all lowered by apelin in rat cardiomyocytes (Zeng et al. 2009). Apelin was shown to suppress human osteoblasts apoptosis via APJ/PI3-K/Akt signaling pathway (Xie et al. 2006). However, there is no information on the anti-apoptotic effects of apelin in human VSMCs. The present study was undertaken to investigate the mechanisms of apelin effects in these cells, and also included a supplementary verification using APJ expression in CHO cells.
Materials and methods
Reagents
Synthetic apelin-13 peptide was purchased from American Peptide Company Inc. (Sunnyvale, CA, USA). The amino acid sequence of apelin-13 is Gln-Arg-Pro-Arg-Leu-Ser-His-Lys-Gly-Pro-Met-Pro-Phe. Anti-human apelin receptor APJ antibody was purchased from Novus Biologicals, Inc. (Littleton, CO, USA). β-Actin, anti-Bcl-2, Bax, ERK, p-ERK, P38, p-P38, JNK, p-JNK, Akt, p-Akt antibodies, anti-mouse, and rabbit IgG peroxidase conjugate antibodies were purchased from Santa Cruz Biotechnology Inc. (Waltham, MA, USA). PD98059, SB202190, SP600125, and LY294002 were purchased from Calbiochem Corp. (San Diego, CA, USA).
Cell cultures
Human VSMCs were cultured from umbilical artery explants in MCDB131 medium (Life Technologies, Barcelona, Spain) supplemented with 10% fetal bovine serum at 37°C in a 5% CO2 atmosphere. Cells were confirmed as smooth muscle by their typical “hill-and-valley” morphological features and by positive staining for smooth muscle α-actin. VSMCs between passages 3 and 5 were used in all experiments. Chinese hamster ovary (CHO) cells were maintained in modified alpha essential medium and transfected with the 1,290-bp msr/APJ receptor gene fragment (see supplementary data). For the positive control, subcutaneous adipose tissue was obtained with informed consent from patients undergoing mammoplastic surgery and after approval by the local research ethics committee. Samples were snap-frozen and stored in liquid nitrogen until protein extraction.
Cell apoptosis measurement
Cell death ELISA detection
Apoptosis was assessed directly by measuring cytoplasmic nucleosomes (i.e. DNA complexed with histone in the cytoplasm) using a Cell Death Detection ELISA kit (Roche Diagnostics GmbH, Roche Molecular Biochemicals, Mannheim, Germany), according to the kit protocol. Briefly, cells were plated at a density of 10,000 cells/well in 24-well plates for 1 day followed by culture in serum-free medium for 48 h in the absence or presence of 10 pM–10 nM apelin. The cell layers were rinsed with PBS and incubated with 0.5 ml of lysis buffer at 4°C for 30 min and then extracted and centrifuged for 10 min at 15,000 rpm. Aliquots of the supernatant were tested for apoptosis using the Cell Death Detection kit. To investigate the effects of inhibitors, cells were pretreated with 10 μM PD98059 (an ERK inhibitor), SB202190 (a p38 inhibitor), SP600125 (a JNK inhibitor), or LY294002 (a PI3-K 14 inhibitor) for 3 h prior to 48 h of apelin treatment.
TUNEL staining
Terminal deoxynucleotidyl transferase-mediated deoxyribonucleotide triphosphate nick end-labeling (TUNEL) was used to evaluate in situ apoptosis in human VSMCs. Cells were plated at a density of 10,000 cells/well in 6-well plates for 1 day followed by culture in serum-free medium for 48 h in the absence or presence of 1 nM apelin. The cells were then stained with in situ cell death detection reagent (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer’s instructions. The nuclei were counterstained with 4, 6-diamidino-2-phenylindole (DAPI, blue fluorescence) and TUNEL-positive cells were visualized under the fluorescence microscope (100×).
Western blotting
Cells were plated in 6-well plates for 1 day followed by culture in serum-free medium for 48 h in the absence or presence of 10 pM–1 nM apelin. Western blotting was done as previously described (Yuan et al. 2007, 2009). The cell layers were homogenized in Triton lysis buffer (50 mM Tris–HCl, pH 8.0 containing 150 mM NaCl, 1% Triton X-100, 0.02% sodium azide, 10 mM EDTA, 10 μg/ml aprotinin, and 1 μg/ml aminoethylbenzenesulfonyl fluoride). The lysates were centrifuged for 15 min at 12,000g to remove debris. Protein concentrations were determined using the Bradford protein assay. Forty micrograms of protein from each cell layer extract was loaded onto a 10% polyacrylamide gel and transferred to a PVDF membrane. After blocking with 5% nonfat milk, membranes were incubated with anti-Bcl-2 monoclonal antibody and anti-Bax monoclonal antibody. The membrane was re-probed with peroxidase-conjugated secondary antibodies. Blots were processed using an ECL kit, exposed to film and then analyzed by densitometry. To investigate the expression of APJ protein in human VSMCs, western blotting with anti-APJ monoclonal antibody was performed as above in human VSMCs extracts. Similar procedures were employed for APJ–CHO expression.
Detection of MAPK and PI3-K/Akt activation
Cells were first treated with 1 nM apelin for 1–60 min. The monolayers were then washed quickly with cold PBS containing 5 mM EDTA and 0.1 mM Na3VO4, and lysed with a buffer consisting of 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 10 mM NaH2PO4, 10% glycerol, 2 mM Na3VO4, 10 mM NaF, 1 mM ABSF, 10 µg/ml leupeptin, and 10 µg/ml aprotinin. Western blotting was done as above. Proteins were then transferred to a nitrocellulose membrane. The membrane was incubated with ERK, p-ERK, p38, p-p38, JNK, p-JNK, Akt, and p-Akt antibodies at 1:500 dilution in PBS for 2 h. The membrane was then incubated with goat anti-mouse IgG antibody or rabbit IgG/horseradish peroxidase conjugate at 1:1,000 dilution in PBS for 1 h. Blots were processed using an ECL kit and apposed to X-ray film.
RNA interference for APJ
Two pairs of small-interfering RNAs (siRNAs) and scramble siRNAs were synthesized by Genesil Biotechnology Co (Wuhan, China) as previously described (Xie et al. 2006). The targets were GGUGCAGUGCUACAUGGACdTdT (for human APJ siRNA) and AUGCUGCGAGCUAGAUCGGdTdT (for scramble human APJ siRNA). Human VSMCs were plated in 60 mm diameter dish and cultured for 24 h in medium without antibiotics. Cells were transfected with siRNAs (1 nM per well) using Lipofectamine 2000 (Invitrogen Inc, USA) according to the manufacturer’s instructions. After 24 h of culture, cells were retransfected with siRNAs and then recultured for another 48 h. Protein expression was analyzed by immunoblotting of cell lysates.
Statistical analyses
Data are presented as mean ± SD. Comparisons were made using a one-way ANOVA. All experiments were repeated at least three times, and representative experiments are shown.
Results
Expression of APJ in human VSMCs
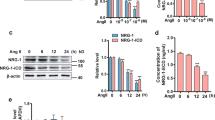
Using western blotting, we detected APJ expression in human VSMCs, human subcutaneous adipose tissue, and CHO cells transfected with the 1,290-bp msr/APJ receptor gene fragment (supplementary Fig. S1). Treatment with siRNA-APJ blocked the expression of APJ protein in human VSMCs since no bands were detected. No blockade was observed upon treatment with the scramble APJ siRNA (Fig. 1).
Expression of APJ in human VSMCs. Representative results of immunoblot analysis using an antibody to APJ. Total cellular protein was subjected to immunoblot analysis. The anti-APJ antibody identified a band at 41 kDa. Representative results are shown. Lane 1 human VSMCs lysate, lane 2 human subcutaneous adipose tissue as a positive control, lane 3 lysate from VSMCs treated with scramble APJ siRNA, lane 4 lysate from human VSMCs treated with APJ siRNA
Apelin protected human VSMCs against serum deprivation-induced apoptosis
Cell death ELISA assay showed that apoptotic human VSMCs at 10 pM (2.05 ± 0.14 ELISA absorbance units), 100 pM (1.62 ± 0.15 ELISA absorbance units), 1 nM (1.21 ± 24 0.13 ELISA absorbance units), and 10 nM (1.33 ± 0.13 ELISA absorbance units) of apelin were less than that of controls (2.41 ± 0.16 ELISA absorbance units, all p < 0.05), reaching the maximal anti-apoptotic effect at 1 nM (Fig. 2). CHO cells transfected with the 1,290-bp msr/APJ receptor gene fragment also showed a decrease in cell apoptosis after apelin treatment (Supplementary Fig. S2). The TUNEL assay indicated that serum deprivation caused significant apoptosis, while 1 nM apelin decreased apoptosis of VSMCs. Apoptotic nuclei were observed upon serum deprivation, but decreased in cells treated with 1 nM apelin (Fig. 3).
Effect of apelin on human VSMCs apoptosis followed by cell death ELISA detection assay. Cells were exposed to 10 pM–10 nM apelin in serum-free medium for 48 h. Apoptosis was assessed using a cell death detection kit (see “Materials and methods”) and are expressed as ELISA absorbance units. The bars represent the mean ± SD (n = 6). Asterisks indicate differences significant at the level of 95% confidence compared to the control
Effect of apelin on apoptosis of human VSMCs detected by TUNEL assay. Cells were exposed to 1 nM apelin for 48 h. Apoptotic nuclei were visualized by TdT-mediated dUTP nick end-labeling (TUNEL) (see “Materials and methods”). White arrows indicate apoptotic VSMCs. Original magnification ×100
Effect of apelin on Bcl-2 and Bax protein expression in human VSMCs
Western blotting was performed to determine the role of apelin on Bcl-2 and Bax 7 protein expression in human VSMC cultures. Apelin upregulated Bcl-2 protein expression and down-regulated Bax protein expression in human VSMCs in a dose-dependent way. A typical western blotting is shown in the top panel of Fig. 4 and the Bax/Bcl-2 ratios are presented in the bottom panel. The Bax/Bcl-2 ratio in control cells was arbitrarily set to 1. Apelin decreased the Bax/Bcl-2 ratio with a maximal decrease to 0.08 at 1 nM.
Effects of apelin on Bcl-2 and Bax protein expression in human VSMCs. Cells were exposed to 10 pM–1 nM apelin for 48 h. Western blot analysis was performed using anti-Bcl-2, -Bax, β-action antibodies. The levels of Bcl-2 and Bax were quantitated by densitometry of autoradiographs and the relative mean ratio of Bax/Bcl-2 was determined
Apelin activated ERK and PI3-K/Akt signaling pathway in human VSMCs
Apelin enhanced the levels of phosphorylated ERK after 1 min incubation (Fig. 5a). The peak activation of ERK occurred at 30 min (Fig. 5a). Apelin enhanced the levels of phosphorylated Akt after 5 min incubation (Fig. 5a). The peak activation of Akt occurred at 15 min (Fig. 5a). However, apelin showed no effect on the activation of p38 and JNK signaling in human VSMCs (Fig. 5a). These data demonstrated that apelin activated ERK and PI3-K/Akt signaling pathways in human VSMCs. Apelin treatment also activated ERK and Akt in CHO cells expressing APJ (Supplementary Fig. S3). This is in accordance with previous reports (Masri et al. 2002, 2004). The activation of ERK and Akt by apelin was abolished by PD98059 (an ERK 24 inhibitor) and LY294002 (a PI3-K inhibitor) (Fig. 5b). Suppression of APJ with siRNA blocked the activation of ERK and Akt by apelin (Fig. 5c). These data indicate that apelin stimulates the ERK and PI3-K/Akt activation via APJ in human VSMCs.
Effects of apelin on MAKP and PI3-K/Akt activation in human VSMCs. Cell lysates were subjected to western blotting and incubated with p-ERK, ERK, p-P38, P38, p-JNK, JNK, p-Akt, and Akt antibodies. Shown are the representative results. a Cells exposed to 1 nM apelin for 1–60 min. b Cells incubated with PD98059 (10 μM) or LY294002 (10 μM) for 3 h prior to treatment with 1 nM apelin for 15 min. c Cells treated with scramble APJ siRNA or APJ siRNA in the presence of 1 nM apelin
APJ/PI3-K/Akt signaling mediates the anti-apoptotic effect of apelin in human VSMCs
After finding that apelin treatment activated the ERK and PI3-K/Akt signaling pathway in human VSMCs, we examined whether the apelin-induced activation of the ERK and PI3-K/Akt signaling pathways plays a role in cell apoptosis. This was examined by the cell death ELISA assay. Pretreatment of human VSMCs with the PI3-K inhibitor LY294002 abolished the anti-apoptotic effect of apelin, but not that of the ERK inhibitor PD98059, the p38 inhibitor SB202190, or the JNK inhibitor SP600125 (Fig. 6). Moreover, suppression of APJ with siRNA also blocked apelin’s anti-apoptotic effect in human VSMCs (Fig. 6). Pretreatment of CHO cells expressing APJ with the PI3-K inhibitor LY294002 abolished the anti-apoptotic effect of apelin (Supplementary Fig. S4). These data indicate that the anti-apoptotic effect of apelin was mediated by the APJ/PI3-K/Akt pathway.
APJ/PI3-K/Akt signaling pathways mediate the anti-apoptotic effect of apelin in human VSMCs. Cells were incubated with PD98059 (10 μM), SB202190 (10 μM), SP600125 (10 μM), or LY294002 (10 μM) for 3 h prior to treatment with 1 nM apelin for 48 h. Cells were also treated with scramble APJ siRNA or APJ siRNA in the presence of 1 nM apelin. Cell apoptosis was determined by Cell Death Detection kit (see “Materials and methods”) and expressed as ELISA absorbance units. The bars represent the mean ± SD (n = 6). Asterisks indicate differences significant at the level of 95% confidence compared to the apelin-treated control
Discussion
Apelin along with its specific receptor, APJ, has been identified in various tissues including gastrointestinal tract, adipose tissue, brain, kidney, liver, lung, bone, and at various sites within the cardiovascular system (Boucher et al. 2005; De Falco et al. 2002; De Mota et al. 2004; Edinger et al. 1998; Habata et al. 1999; Hosoya et al. 2000; Jaszberenyi et al. 2004; Kawamata et al. 2001; Kasai et al. 2004; Kleinz and Davenport 2004; Losano et al. 2005; Masri et al. 2004; Reaux et al. 2001; SorhedeWinzellM et al. 2005; Sunter et al. 2003; Tatemoto et al. 1998; Wang et al. 2004; Wei et al. 2005; Xie et al. 2006). Apelin and APJ are expressed in human VSMCs and apelin can stimulate VSMCs proliferation (Li et al. 2008). However, the effect of apelin on apoptosis of human VSMCs remains unclear. The present study shows that the administration of synthetic apelin peptide suppresses serum deprivation-induced apoptosis of human VSMCs, indicating an anti-apoptotic role for apelin in addition to its mitogenic effect in human VSMCs. We also found that PI3-K/Akt signaling mediated apelin’s protective effect via APJ against serum deprivation-induced apoptosis in human VSMCs.
Apoptosis is a multi-step process characterized by both morphological changes including cell shrinkage, chromatin condensation, membrane blebbing, and finally nuclear fragmentation and activation of a sequence of cysteine protease enzymes (Degterev et al. 2003). The Bcl-2 family proteins form heterodimers between apoptosis-inhibiting proteins such as Bcl-2, Bcl-XL, and A1, and inducing proteins such as Bax, Bad, and Bid. These heteromers participate in the regulation of cell survival (Oltvai et al. 1993). The ratio of anti-apoptotic to proapoptotic proteins, and especially the Bcl-2/Bax ratio, determines susceptibility to apoptosis (Korsmeyer et al. 1993). The present study showed that apelin induced the expression of Bcl-2 protein and downregulated the expression of Bax protein in human VSMCs, resulting in a dramatic decrease in the Bax/Bcl-2 ratio. This suggests that apelin inhibits human VSMCs apoptosis through regulating Bax/Bcl-2 expression.
Apelin exerts its effect on regulating body fluid homeostasis, cardiovascular functions, and osteoblast proliferation through the APJ receptor (De Mota et al. 2004; Ishida et al. 2004; Xie et al. 2006). We investigated whether apelin affects human VSMCs apoptosis through APJ. Our results show that suppression of APJ with siRNA inhibits the anti-apoptotic effect of apelin in human VSMCs. This suggests that apelin protects human VSMCs against apoptosis through APJ. Apelin activates ERK, MAPK and PI3-K/Akt and promotes cellular growth. These stimulations are generally recognized as characteristics of cardioprotective agents (Masri et al. 2002; Wang et al. 2004). The ERK cascade functions in cellular proliferation, differentiation, and survival. The serine/threonine kinase Akt [also known as protein kinase B (PKB)] is essential for cell proliferation, migration, and protection against apoptosis induced by oxidative stress (Cantley 2002; Whiteman et al. 2002). In the cardiovascular system, Akt plays an important role in the regulation of cardiac hypertrophy, angiogenesis, and apoptosis (Ackah et al. 2005; Shiojima et al. 2005; Somanath et al. 2006). Absence of Akt reduces VSMC migration and survival, and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis (Fernández-Hernando et al. 2009). Apelin was reported to enhance superoxide dismutase activity and phosphorylation of ERK and Akt after hypoxia/re-oxygenation (Zeng et al. 2009). To gain further insight into the mechanisms by which apelin suppresses human VSMC apoptosis, we examined intracellular signaling pathways. We show that apelin induces activation of ERK and Akt in human VSMCs. Treatment with the PI3-K inhibitor LY294002 abolishes the activation of Akt in human VSMCs, indicating that the phosphorylation of Akt is dependent on PI3-K. Suppression of APJ with siRNA blocks the effects of apelin on ERK and Akt suggesting that the activation of ERK and PI3-K/Akt is mediated through APJ. To elucidate the signal pathway involved in the apelin-reduced VSMCs apoptosis, we examined the effect of MAPK and PI3-K/Akt inhibitors on human VSMCs apoptosis. The PI3-K inhibitor LY294002 abolishes the effects of apelin on human VSMCs apoptosis, but not that of the ERK inhibitor PD98059, indicating that ERK is not involved in apelin’s anti-apoptotic effect. siRNA of APJ also blocks apelin’s anti-apoptotic effect. These results indicate that apelin-induced inhibition of apoptosis in human VSMCs is mediated through APJ/PI3-K/Akt pathways. In conclusion, the present study illustrates that apelin protects human VSMC against apoptosis through APJ/PI3-K/Akt signaling, which could support vascular remodeling during cardiovascular diseases.
References
Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC (2005) Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 115:2119–2127
Bauriedel G, Hutter R, Welsch U, Bach R, Sievert H, Lüderitz B (1999) Role of smooth muscle cell death in advanced coronary primary lesions: implications for plaque instability. Cardiovasc Res 41:480–488
Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpéné C, Audigier Y, Saulnier-Blache JS, Valet P (2005) Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146:1764–1771
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657
Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, Bennett MR (2008) Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res 102:1529–1538
Cox CM, D’Agostino SL, Miller MK, Heimark RL, Krieg PA (2006) Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol 296:177–189
De Falco M, De Luca L, Onori N, Cavallotti I, Artigiano F, Esposito V, De Luca B, Laforgia V, Groeger AM, De Luca A (2002) Apelin expression in normal human tissues. In Vivo 16:333–336
De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C (2004) Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci USA 101:10464–10469
Degterev A, Boyce M, Yuan J (2003) A decade of caspases. Oncogene 22:8543–8567
Edinger AL, Hoffman TL, Sharron M, Lee B, Yi Y, Choe W, Kolson DL, Mitrovic B, Zhou Y, Faulds D, Collman RG, Hesselgesser J, Horuk R, Doms RW (1998) An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol 72:7934–7940
Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, Soubrier F (2008) Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res 103:432–440
Farkasfalvi K, Stagg MA, Coppen SR, Siedlecka U, Lee J, Soppa GK, Marczin N, Szokodi I, Yacoub MH, Terracciano CM (2007) Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem Biophys Res Commun 357:889–895
Fernández-Hernando C, József L, Jenkins D, Di Lorenzo A, Sessa WC (2009) Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiacd ysfunction during atherosclerosis. Arterioscler Thromb Vasc Biol 29:2033–2040
Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63:1256–1272
Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M (1999) Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta 1452:25–35
Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P (1999) Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation 99:96–104
Horn F, Weare J, Beukers MW, Horsch S, Bairoch A, Chen W, Edvardsen O, Campagne F, Vriend G (1998) GPCRDB: an information system for G protein-coupled receptors. Nucleic Acids Res 26:275–279
Hosoya M, Kawamata Y, Fukusumi S (2000) Molecular and functional characteristics of APJ: tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem 275:21061–21067
Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, Okunishi H, Kihara M, Umemura S, Sugiyama F, Yagami K, Kasuya Y, Mochizuki N, Fukamizu A (2004) Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem 279:26274–26279
Isner JM, Kearney M, Bortman S, Passeri J (1995) Apoptosis in human atherosclerosis and restenosis. Circulation 91:2703–2711
Jaszberenyi M, Bujdoso E, Telegdy G (2004) Behavioral, neuroendocrine and thermoregulatory actions of apelin-13. Neuroscience 129:811–816
Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Hinuma S, Baba A (2004) Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem Biophys Res Commun 325:395–400
Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O, Fujino M (2001) Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta 1538:162–171
Kidoya H, Ueno M, Yamada Y, Mochizuki N, Nakata M, Yano T, Fujii R, Takakura N (2008) Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J 27:522–534
Kleinz MJ, Davenport AP (2004) Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept 118:119–125
Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN (1993) Bcl-2/bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol 4:327–332
Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL, Quertermous T (2009) Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol 296:H1329–H1335
Li F, Li L, Qin X, Pan W, Feng F, Chen F, Zhu B, Liao D, Tanowitz H, Albanese C, Chen L (2008) Apelin-induced vascular smooth muscle cell proliferation: the regulation of cyclin D1. Front Biosci 13:3786–3792
Losano G, Penna C, Cappello S, Pagliaro P (2005) Activity of apelin and APJ receptors on myocardial contractility and vasomotor tone. Ital Heart J Suppl 6:272–278
Masri B, Lahlou H, Mazarguil H, Knibiehler B, Audigier Y (2002) Apelin (65–77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem Biophys Res Commun 290:539–545
Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y (2004) Apelin (65–77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J 18:1909–1911
O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T (1993) A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136:355–360
Okura T, Watanabe S, Jiang Y, Nakamura M, Takata Y, Yang ZH, Kohara K, Kitami Y, Hiwada K (2002) Soluble Fas ligand and atherosclerosis in hypertensive patients. J Hypertens 20:895–898
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programed cell death. Cell 74:609–619
Reaux A, de Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, Corvol P, Palkovits M, Llorens-Cortès C (2001) Phisiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem 77:1085–1096
Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K (2005) Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115:2108–2118
Somanath PR, Razorenova OV, Chen J, Byzova TV (2006) Akt1 in endothelial cell and angiogenesis. Cell Cycle 5:512–518
SorhedeWinzell M, Magnusson C, Ahren B (2005) The APJ receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept 131:12–17
Sunter D, Hewson AK, Dickson SL (2003) Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neurosci Lett 353:1–4
Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251:471–476
Wang G, Anini Y, Wei W, Qi X, OCarroll AM, Mochizuki T, Wang HQ, Hellmich MR, Englander EW, Greeley GH Jr (2004) Apelin, a newenteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology 145:1342–1348
Wei L, Hou X, Tatemoto K (2005) Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3–L1 adipocytes. Regul Pept 132:27–32
Whiteman EL, Cho H, Birnbaum MJ (2002) Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab 13:444–451
Xie H, Tang SY, Cui RR, Huang J, Ren XH, Yuan LQ, Lu Y, Yang M, Zhou HD, Wu XP, Luo XH, Liao EY (2006) Apelin and its receptor are expressed in human osteoblasts. Regul Pept 134:118–125
Yuan LQ, Liu YS, Luo XH, Guo LJ, Xie H, Lu Y, Wu XP, Liao EY (2007) Recombinant tissue metalloproteinase inhibitor-3 protein induces apoptosis of murine osteoblast MC3T3–E1. Amino Acids 35:123–127
Yuan LQ, Liu W, Cui RR, Wang D, Meng JC, Xie H, Wu XP, Zhou HD, Lu Y, Liao EY (2009) Taurine inhibits osteoclastogenesis through the taurine transporter. Amino Acids [Epub ahead of print]
Zeng XJ, Zhang LK, Wang HX, Lu LQ, Ma LQ, Tang CS (2009) Apelin protects heart against ischemia/reperfusion injury in rat. Peptides 30:1144–1152
Zou MX, Liu HY, Haraguchi Y, Soda Y, Tatemoto K, Hoshino H (2000) Apelin peptides block the entry of human immunodeficiency virus (HIV). FEBS Lett 473(1):15–18
Acknowledgments
This work was supported by funding from The National Natural Science Foundation of China (No. 30801174 and 30900622), the Ph. D. Programs Foundation of Ministry of Education of China (No.200805331017), and clinic medicine special research fund-endocrinology disease research fund (10020040226).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Rong-Rong Cui, Ding-An Mao and Lu Yi contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, RR., Mao, DA., Yi, L. et al. Apelin suppresses apoptosis of human vascular smooth muscle cells via APJ/PI3-K/Akt signaling pathways. Amino Acids 39, 1193–1200 (2010). https://doi.org/10.1007/s00726-010-0555-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0555-x