Abstract
Alpine-type fissure vein mineralization in the Hiddenite area of western North Carolina, USA consists mostly of quartz, but locally contains Cr-bearing beryl (emerald) or Cr-bearing spodumene (hiddenite). These gem minerals occur in mineral-lined cavities and may be accompanied by euhedral crystals of quartz, calcite, muscovite, rutile, albite, pyrite, siderite and dolomite. Chabazite-Ca occurs as a late stage phase in spodumene-bearing veins, but is absent in emerald-bearing veins. Chabazite-Ca occurs as simple penetrating twins of pseudocubic rhombohedra and as the lens-shaped variety, phacolite. Chabazite-Ca from Hiddenite contains minor amounts of Na, Mg, Fe and K. Phacolitic chabazite-Ca shows Fe-enriched but Mg-depleted cores relative to the rims. Chemical zoning is absent in rhombohedral chabazite. The Hiddenite chabazite apparently precipitated under low temperature (< 250°C) and low pressure (< 2 kbar) conditions during the waning stages of crystallization of an alkaline hydrothermal fluid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the immediate vicinity of the town of Hiddenite, North Carolina (latitude 35.90N, longitude 81.09W) are quartz veins which host gem- and specimen-grade Cr-bearing beryl and Cr-bearing spodumene, better known in the gem trade as emerald and hiddenite, respectively. According to Wise and Anderson (2006), both gem minerals occur as part of an assemblage that formed from hydrothermal fluids. The Hiddenite area is particularly interesting because emeralds are extremely rare in North America and their association with Cr-bearing spodumene is not known from other worldwide emerald deposits. The unusual geochemical features of the deposits, (e.g., presence of Li, Be and Cr in a non-pegmatitic environment) has led to an ongoing investigation into the origin of the gem deposits (Wise and Anderson 2006). Previous studies of the Hiddenite deposits consisted mainly of descriptions of the various mineral species present in quartz veins (Davidson 1927; Palache et al. 1930; Sinkankas 1976; Brown and Wilson 2001). More recently, additional mineral species including graphite, monazite-(Ce) and molybdenite have been recognized (Wise and Anderson 2006). During the present study of quartz veins from the North American Emerald Mine (NAEM) property (part of the former Rist Emerald mine), chabazite was found as part of a late stage assemblage. Subsequent to this discovery, examination of spodumene samples that reside in the mineral collection of the National Museum of Natural History, Smithsonian Institution, revealed the presence of chabazite crystals from the Adams property (formerly the Warren Farm), the site of the original discovery of hiddenite. To the best of the author’s knowledge, chabazite has never been reported previously from the Adams property, and was only recently described by Wise and Anderson (2006) from the NAEM property. Furthermore, the occurrence of chabazite with spodumene in a fissure vein setting has not been described elsewhere in the world. The present paper reports on this rare and unusual association and discusses the conditions in which chabazite is stable with spodumene.
Geology
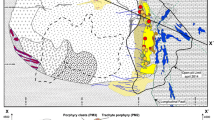
The Hiddenite deposits are located in the intensely deformed and metamorphosed Inner Piedmont Belt of western North Carolina (Fig. 1). This area is nearly 100 km wide and extends approximately 700 km southwest of the Sauratown Mountain window from North Carolina to Alabama. The Inner Piedmont Belt is bounded on the west by the northeast-trending Brevard Fault Zone which separates the Inner Piedmont Belt from the Blue Ridge, and is bounded on the east by the Carolina Piedmont Suture which separates it from the Carolina Terrane. The Inner Piedmont Belt consists of sillimanite-grade rocks that include 500 to 750 Ma old gneisses, migmatites and schists that have been intruded by numerous deep- and shallow-level granitic to gabbroic plutons.
The bedrock geology of the Hiddenite area consists of Precambrian migmatitic schists and gneisses that are cross-cut by an extensive set of steeply dipping, NE trending quartz veins. The schists are interlayered with discontinuous lenses of calc-silicate rocks consisting of calcite, grossular, diopside, titanite and quartz. The area is locally intruded by the medium-grained leucocratic Rocky Face Granite. Prolonged weathering of the migmatitic bedrock has resulted in the formation of a thick red lateritic saprolite horizon. Quartz veins may be identified near the surface in the upper part of the saprolitic zone and followed downward through the saprolite into the unweathered bedrock.
Details of the geology and mineralogy of the quartz veins were provided by Palache et al. (1930), Brown and Wilson (2001) and most recently by Wise and Anderson (2006). Sinkankas (1976) noticed a remarkable similarity in the structure and mineral assemblage of the Hiddenite quartz veins to the Alpine-type fissure veins found in the Swiss Alps. Classic Alpine-type fissure veins are mineralized infillings of brittle tensional fractures that develop in relatively competent rock-units during regional deformation and strain-related metamorphism. The fissure minerals which form in cavities result from the chemical leaching of surrounding rock by hydrothermal solutions and subsequent precipitation of low temperature minerals into localized open fractures. Minerals which are typical of Alpine-type veins include quartz, fluorite, calcite, adularia, chlorite, titanite anatase, rutile and brookite (Graeser 1998).
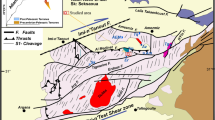
The quartz veins sampled in this study are from the same part of the NAEM property studied by Wise and Anderson (2006) and from the Adams property which lies approximately 3.2 km southwest of the NAEM property. All of the known Hiddenite emerald occurrences occur within the same migmatitic schist and gneiss unit. The Hiddenite quartz veins are typically 1 to 10 cm wide, but may reach widths of up to 1.5 m. Some quartz veins are characterized by rare-element mineralization in the form of Cr-bearing beryl (variety emerald) and Cr-bearing spodumene (variety hiddenite) which occur in voids within the veins. The beryl and spodumene are accompanied by quartz, carbonates (calcite, siderite and dolomite), sulfides (pyrite and pyrrhotite), rutile, graphite, muscovite and a clinochlore-like phase (Wise and Anderson 2006), although minor differences in accessory mineralogy between the vein types have been observed. Beryl and spodumene appear to be almost mutually exclusive, occurring only rarely together within an individual cavity. The origin of the gem-bearing veins has been debated over the past 75 years. Derivation of the emeralds, and presumably the hiddenite from a pegmatite source was favored by Palache et al. (1930) and Brown and Wilson (2001). Crystallization from hydrothermal fluids, however was proposed by Sterrett (1908), Sinkankas (1976, 1981) and Tacker (1999) and later confirmed by Wise and Anderson (2006).
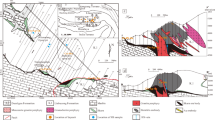
At the Adams and NAEM properties, chabazite occurs only in quartz veins that carry Cr-bearing spodumene and is always one of the last minerals to form (Fig. 2). Veins consist mainly of massive white quartz which locally develops cavities that contain euhedral crystals that protrude inward from the roof and walls. The cavities that contain hiddenite are relatively simple in their mineralogical makeup consisting mostly of quartz, muscovite, spodumene, calcite, rutile and pyrite. Euhedral quartz crystals are typically 1 to 2 cm in length, although crystals as long as 15 cm have also been recovered and generally exhibit the prismatic Tessin habit characterized by steep rhombohedral faces and a tapered termination. At the NAEM locality pale purple quartz sometimes occurs as small clusters or radiating aggregates of pale purple crystals, 0.3 to 0.5 cm long, typically forming on the terminations of colorless quartz prisms to form pale purple scepters. Muscovite ranges from silver- to bronze-colored crystals at the NAEM locality, but may sometimes display deep green cores or rims. Muscovite is conspicuously absent in the veins hosting chabazite at the Adams property. The carbonate mineralogy is dominated by rhombohedral crystals of calcite although dolomite may sometimes be present. Silver-gray, prismatic crystals of rutile are found attached to the vein walls, included in quartz and calcite or perched on quartz, calcite and muscovite. Crystals of Cr-bearing spodumene, up to 7 cm long, occur attached to the cavity walls or loose in the red saprolitic clay of weathered pockets. The most valued crystals are emerald green in color; but most crystals show pronounced color zonation with dark emerald-green tips and grading to yellowish-green along the length of the crystal. They are usually etched and commonly covered by thin layers of a dark to light green clinochlore-like mineral. Late-stage mineralization of the pocket includes the formation of sulfides and graphite in addition to chabazite. Pyrite is the dominant sulfide mineral present and forms 1 mm wide cubo-octahedrons which crystallize on quartz, calcite and muscovite. Other sulfides of note include rare molybdenite and pyrrhotite. Graphite crystals, 1 mm in diameter, occur in, and on the surfaces of calcite, muscovite and chabazite. Schorl and zircon were rarely observed in some hiddenite-bearing cavities.
Chabazite is rare in the quartz veins and represents the only zeolite mineral found to date in the Hiddenite deposits. Chabazite is typically found growing on the surfaces of quartz, calcite and muscovite, but very rarely on Cr-bearing spodumene. All chabazite collected from the NAEM property displays pseudocubic rhombohedral crystals that form transparent and colorless penetration twins (Fig. 3). Most crystals are 0.5 to 0.7 mm in diameter and rarely exceed 1 mm in length. At the Adams property, chabazite has been found only as pale yellow to honey-yellow colored crystals that show complex twinning to form the lens-shaped variety known as phacolite. This habit results from multiple penetration twins rotated 60º on the c-axis (Akizuki and Konno 1987). Crystals may show color zonation with yellow cores and colorless rims. The largest crystals found measure only 2.5 mm in length; most are typically < 1 mm long.
Compositional characteristics
X-ray powder diffraction data were obtained from finely ground chabazite that was attached to a glass fiber and mounted on a Rigaku D/MAX-RAPID microdiffractometer system operating at 50 kV and 40 mA using MoKα radiation (λ=0.7093Å). The X-ray beam was focused on the sample using a 0.3 mm collimator for an exposure time of 10 min. Samples were identified as chabazite using the search-match routine of the JADE program. The X-ray diffraction patterns of the two samples show a small shift in peak position and slightly different peak intensities (Fig. 4). Unit-cell parameters obtained from refinement of the powder data in the space group R -3 m, were a = 9.400(7) Ǻ, α = 94.36(3)º and V = 822.9(6) Ǻ3 for the NAEM chabazite and a = 9.395(7) Ǻ, α = 94.36(3)º and V = 821.8(6) Ǻ3 for the Adams chabazite. The unit-cell data suggest only a modest difference in the structure of the two chabazite samples.
Ten colorless and five yellow chabazite crystals were selected from samples that were collected in the Hiddenite area and obtained from the Mineralogy collection of the National Museum of Natural History, Smithsonian Institution for use in the current study. Quantitative chemical analyses of chabazite were obtained using a JEOL Model JXA-8900R electron microprobe in the wavelength-dispersion mode. Operating conditions were: 15kV and 15 nA using a defocused beam diameter of 10 μm to minimize alkali loss. Counting time for background and peak determinations were 5-10 s and 15-40 s, respectively. Natural mineral standards used for analyses were microcline (Si, Al, K), anorthoclase (Na), hornblende (Fe, Mg, Ti), and apatite (Ca). The structural formula for chabazite was calculated on the basis of 24 oxygens. Chabazite is slightly unstable under the electron beam and tends to suffer alkali loss thus resulting in poor analyses. The balance error function of Passaglia (1970) expressed by the following equation:
is a widely accepted criterion for evaluating analyses of zeolites. The calculated E% value should be <10% and preferably <5% for an acceptable analysis (Coombs et al. 1998; Coombs et al. 2005).
Representative analyses of chabazite from Hiddenite are listed in Table 1. The balance error function for the Hiddenite chabazite ranged from -13.2 to 8.6, but only those <10% are reported in this study. The composition of the chabazite from both Hiddenite localities is chabazite-Ca with Ca>(Na+K) and plots along the Ca-K join towards chabazite-K of the Na-K-Ca ternary diagram (Fig. 5). In general, the chabazite exhibits Si/Al ratios of 2.37 to 3.32 and contains variable amounts of Ca and K, but with low Na and moderate amounts of Mg. The K content varies from 2.00 to 3.76 wt.% K2O. Na content from 0.04 to 0.23 wt.% Na2O and Mg content varies from 0.00 to 1.48 wt.% MgO. Samples were checked for the presence of Sr and Ba, but none was detected.
Compositions of Hiddenite chabazite plotted on the chabazite group Na-K-Ca ternary diagram (Coombs et al. 1998). a NAEM property (open circles). b Adams property (open diamonds)
The analyses of phacolitic chabazite from the Adams property indicate slightly higher Si/Al ratios (2.37 to 3.32) than found in the rhombohedral chabazite of the NAEM site (2.42 to 2.83). The Adams property chabazite has similar K contents but slightly less Ca than chabazite from the NAEM property (Table 1). The Mg content of the Adams chabazite is consistently higher than the NAEM chabazite. The Adams phacolitic chabazite is also chemically zoned with respect to Fe2O3 and MgO unlike the rhombohedral chabazite from the NAEM property, which shows no chemical zonation and is essentially devoid of Fe. Analyses that correspond to the yellow core of the phacolitic chabazite show Fe2O3 contents of 0.44 to 2.17 wt.%. Rim compositions are significantly lower in Fe at 0.03 to 0.06 wt.% Fe2O3. By comparison MgO values are highest in the rim (0.44 to 1.46 wt.%) and decrease gradually to core (0.63 to 0.68 wt%).
Genetic implications
Conditions of chabazite formation
The observed mineral associations of the spodumene-bearing cavities show that chabazite was one of the last minerals to precipitate from the mineralizing fluid. Chabazite-Ca formed after the crystallization of spodumene, quartz and pyrite and may have been contemporaneous with the growth of calcite. The formation of chabazite known from volcanic rocks and sedimentary environments is influenced by a number of factors including: chemical composition of the starting material; chemical activities of CO2, H2O and SiO2; alkalinity of the fluid; Si/Al ratio; and temperature and pressure (Hay 1978; Passaglia and Vezzalini 1985; Barth-Wirsching and Höller 1989). In most cases, decomposition of a basaltic glass precursor favors chabazite formation, although rhyolitic glasses may produce chabazite-K in the presence of K-rich fluids. Thermodynamic modeling of a suite of zeolites found in hydrothermal environments shows that chabazite is unstable at temperatures >150 °C (Chipera and Apps 2001). In mafic volcanic rocks chabazite appears to be most stable at temperatures <100 °C. Chabazite has been recognized as part of the low temperature-low pressure mineral assemblage of some Alpine fissure-type veins (Weibel 1963; Graeser 1998; Hrazdil 2001; Bucher and Weisenberger 2006) but in general this occurrence is uncommon. Köngisberger (1901) estimated the crystallization temperature of chabazite at <130°C in quartz-orthoclase-calcite fissure veins from Aar, Switzerland. Chabazite from a fluorite-quartz assemblage in the Alpine fissures at Gibelsbach, Switzerland, was considered to have crystallized at temperatures <100 °C (Armbruster et al. 1996).
The conditions at which the Hiddenite chabazite crystallized were also likely at low temperatures and pressures. Preliminary fluid inclusion data of early generations of quartz crystals from the spatially associated emerald-bearing veins place the P-T conditions around 250ºC and 1 kbar (Lapointe et al. 2004; Alan Anderson, pers. comm. 2004). Since crystallization of chabazite occurred later than most quartz, we estimate that the temperatures of chabazite formation was probably <200ºC. This would be in general agreement with the range of crystallization temperatures (50 to 200ºC) determined by hydrothermal experiments and measured directly from geothermal areas (Kristmannsdóttir and Tómasson 1978; Cole and Ravinsky 1984; Höller and Wirsching 1988; Chipera and Apps 2001).
Crystallization of chabazite from the alteration of Ca-rich feldspars was unlikely at Hiddenite as feldspar is generally absent in the hiddenite assemblage and, when present, is albitic in composition. Chabazite apparently precipitated from a fluid that was highly enriched in Ca, with high CO2 fugacities as indicated by the Ca-rich nature of the chabazite, presence of abundant calcite, CO2-rich fluid inclusions (Lapointe et al. 2004) and the absence of associated Na or K zeolites. This, plus the absence of kaolinitic clays suggests that the mineralizing fluid was alkaline prior to crystallization of chabazite. Leaching and mobilization of Ca, K, Cr, Fe, Mg and Ti from the schistose and calc-silicate wallrock is believed to be the main mechanism responsible for the fluid chemistry and subsequent mineralization of chabazite and associated minerals in the fissures.
Significance of habit distribution
Although there is little difference in the mineral assemblage between the spodumene-bearing veins of the Adams and NAEM properties, the habit of chabazite is distinctive for each locality. Several workers have recognized that development of chabazite habit was sensitive to changes in the growth conditions of its environment. Walker (1951) suggested that the temperature of formation was the overriding factor responsible for variations in chabazite morphology based on his observations of chabazite crystallization in a series of lava flows in the Garron Plateau area, Ireland. Although Walker (1951) did not determine actual crystallization temperatures for the Garron chabazite, he concluded that chabazite with simple rhomobohedral habits, including those displaying penetration twins, crystallized at lower temperatures than phacolitic chabazite. According to Akizuki and Konno (1987), the phacolitic habit reflects a sudden change in the growth rate of chabazite. Under slow and constant growth rate, penetration twins predominate whereas a sudden increase in the growth rate due to changes in the chemical composition and Al-Si ordering causes the development of the phacolitic habit. Rapid crystallization in a Ca-rich solution, for example, appears to favor the development of the phacolitic habit over the rhombohedral form which requires slow growth rates (Akizuki et al. 1989). Experiments on the formation of chabazite conducted by Höller and Wirsching (1988) demonstrated the effects of the alkali concentrations in the mineralizing fluid on chabazite morphology. In their experiments, high concentrations of Na caused the formation of rhombohedral chabazite while K-rich solutions resulted in the phacolitic habit. Passaglia (1970), Birch (1989) and Graham et al. (2003) showed that there was no correlation between crystal morphology and chemical composition in natural samples; Na-rich chabazite can form rhombohera, phacolitic twins or flattened crystals. Based on the discussion above, the phacolitic chabazite from the Adams property probably crystallized under slightly different conditions (possibly higher temperatures, faster growth rates and higher concentration of K in solution) than the rhombohedral chabazite from the NAEM site.
Spodumene-chabazite association
The natural occurrence of zeolite minerals with spodumene is generally uncommon and develops mainly in granitic pegmatites as a result of the hydrothermal alteration of spodumene or eucryptite to form bikitaite (Hurlbut 1958; Vidal and Goffé 1989) or as the decomposition of associated pollucite to produce Cs-rich analcime (Černý 1972). The association of spodumene and chabazite is extremely rare in granitic pegmatites and not known to occur at all in Alpine fissure-type veins. The only documented spodumene-chabazite association is from the Branchville pegmatite, Connecticut, where chabazite occurs as part of a late alteration assemblage within the albite-spodumene zone of the pegmatite (Brush and Dana 1879). The chabazite occurs as crystals and irregular masses in rhodochrosite that resulted from the alteration of associated primary lithiophilite. Chabazite is known to occur at other pegmatite localities such as at Strzegom, Poland (Michell 1941; Janeczek 1985) and the Speranza vein near San Piero in Campo, Elba, Italy (Orlandi and Scortecci 1985) but none of these contain spodumene.
The estimated pressures and temperatures of crystallization for the Hiddenite deposits are well within the range of stability for bikitaite, yet spodumene appears as the stable Li phase (London 1984). The presence of Fe and Cr in the spodumene may be responsible for stabilizing it to lower temperatures and pressures and may also make it less susceptible to alteration to bikitaite (Brown 1971; Drysdale 1975; London 1984). In all probability, the crystallization of chabazite instead of bikitaite at Hiddenite may be due mainly to the removal of Li from the solution as a result of prior spodumene crystallization coupled with the overall Ca-rich nature of the hydrothermal fluid.
References
Akizuki M, Konno H (1987) Growth twinning in phacolite. Mineral Mag 51:427–430
Akizuki M, Nishido H, Fujimoto M (1989) Herschelite: morphology and growth sectors. Am Mineral 74:1337–1342
Armbruster Th, Kohler Th, Meisel Th, Nägler ThF, Götzinger MA, Stadler HA (1996) The zeolite, fluorite, quartz assemblage of the fissures at Gibelsbach, Fiesch (Valais, Switzerland): crystal chemistry, REE patterns, and genetic speculations. Schweiz Mineral Petrogr Mitt 76:131–146
Barth-Wirsching U, Höller H (1989) Experimental studies on zeolite formation conditions. Eur J Mineral 1:489–506
Birch WD (1989) Zeolites of Victoria. Mineralogical Society of Victoria Special Publication No. 2, Melbourne, Victoria, Australia, p 110
Brown DL, Wilson WE (2001) The Rist and Ellis Tract. Mineral Rec 32:129–140
Brown WL (1971) On lithium and sodium trivalent-metal pyroxenes and crystal field affects. Mineral Mag 38:43–48
Brush GJ, Dana ES (1879) On the mineral locality in Fairfield County, Connecticut. Am J Sci 18:45–50 3rd series
Bucher K, Weisenberger T (2006) Zeolites on fissures of crystalline basement rocks in the Swiss Alps. Geol Soc Am Abst Prog 38(7):113
Černý P (1972) The Tanco pegmatite at Bernic Lake, Manitoba: VIII: Secondary minerals from the spodumene-rich zones. Can Mineral 11:714–726
Chipera SJ, Apps JA (2001) Geochemical stability of natural zeolites. In Bish DL, MingDW (eds) Natural zeolites: occurrence, properties, applications. Rev. Mineral. Geochem 45, 117–161
Cole DR, Ravinsky LI (1984) Hydrothermal alteration zoning in the Beowave geothermal system, Eureka and Lander counties, Nevada. Econ Geol 79:759–767
Coombs DS, Alberti A, Armbruster T, Artioli G, Colella C, Galli E, Grice JD, Liebau F, Mandarino JA, Minato H, Nickel EH, Passaglia E, Peacor DR, Quartieri S, Rinaldi R, Ross M, Sheppard RA, Tilmanns E, Vezzalini G (1998) Recommended nomenclature for zeolite minerals: report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Mineral Mag 62:533–571
Coombs DS, Bosel CA, Kawachi Y, Paterson LA (2005) A silica-deficient, shallow-marine zeolite assemblage in the foveaux formation, Bluff Peninsula, New Zealand. Mineral Mag 69:137–144
Davidson SC (1927) The hiddenite occurrence in North Carolina. Am Mineral 12:305–307
Drysdale DJ (1975) Hydrothermal synthesis of various spodumenes. Am Mineral 60:105–110
Graeser S (1998) Alpine minerals: a review of the most famous localities of the Central Swiss Alps. Rocks Minerals 73:14–32
Graham IT, Pogson RE, Colchester DM, Baines A (2003) Zeolite crystal habits, Dunedin, New Zealand. Mineral Mag 67:625–637
Hrazdil Z (2001) Mineral assemblage of alpine-type veins from Templštejn near Jamolice, western Moravia (in Czech). Acta Mus Morav, Sci Geol 86:75–84
Hay R (1978) Geologic occurrences of zeolites. In: Sand LB, Mumpton FA (eds) Natural Zeolites: Occurrence, Properties, Use. Pergamon, Oxford, pp 135–144
Höller H, Wirsching U (1988) Experiments on the formation conditions and morphology of chabazite from volcanic glasses. In: Kallo D, Sherry HS (eds) Occurrence. Properties and Utilization of Natural Zeolites. Pergamon, Oxford, pp 171–191
Hurlbut CS (1958) Additional data on bikitaite. Am Mineral 43:768–770
Janeczek J (1985) Typomorphism of pegmatite minerals in the Strzegom-Sobotka granite massif (in Polish). Geol Sudet 20:1–81
Köngisberger J (1901) Die Minerallagerstätten im Biotitprotogin des Aarmassivs. Neues Jb Beil Bd 14:43–119
Kristmannsdóttir H, Tómasson J (1978) Zeolite zones in geothermal areas in Iceland. In: Sand LB, Mumpton FA (eds) Natural Zeolites: Occurrence, Properties, Use. Pergamon, Oxford, pp 277–284
Lapointe M, Anderson AJ, Wise MA (2004) Fluid inclusion constraints on the formation of emerald-bearing quartz veins at the Rist tract, Hiddenite, North Carolina. Atlantic Geoscience Society Colloquium and Annual General Meeting, Moncton, New Brunswick, Canada. Atlantic Geol 40:146
London D (1984) Experimental phase equilibria in the system LiAlSiO4-SiO2–H2O: a petrogenetic grid for lithium-rich pegmatites. Am Mineral 69:995–1004
Michell WD (1941) Paragenesis of the pegmatite minerals of Striegau, Silesia. Am Mineral 26:262–275
Orlandi P, Scortecci PB (1985) Minerals of the Elba pegmatites. Mineral Rec 16:353–363
Palache C, Davidson SC, Goranson EA (1930) The Hiddenite deposit in Alexander County, North Carolina. Am Mineral 15:280–302
Passaglia E (1970) The crystal chemistry of chabazites. Am Mineral 55:1278–1301
Passaglia E, Vezzalini G (1985) Crystal chemistry of diagenetic zeolites in volcanoclastic deposits of Italy. Contrib Mineral Petrol 90:190–198
Sinkankas J (1976) Gemstones of North America, vol 2. Van Nostrand Reinhold, New York
Sinkankas J (1981) Emerald and other Beryls. Chilton, Radnor, Pennsylvania
Sterrett DB (1908) Precious stones. In: United States Geological Survey Mineral. Reports of the U.S. for 1907, 7 pp 95-842
Tacker CR (1999) Preliminary observations of the emerald deposits of Hiddenite, North Carolina, USA. Geol Soc Am Abst Prog, 306
Vidal O, Goffé B (1989) Étude expérimentale préliminaire de l’équilibre : bikitaïte = spoduméne + eau. CR Acad Sci Paris 309:233–238
Walker GPL (1951) The amygdale minerals in the Tertiary lavas of Ireland. I. The distribution of chabazite habits and zeolites in the Garron plateau area, County Antrim. Mineral Mag 29:773–791
Weibel M (1963) Chabasit vom Chruezlistock (Tavetsch). Schweiz Mineral Petrogr Mitt 43:361–366
Wise MA, Anderson AJ (2006) The emerald- and spodumene-bearing quartz veins of the Rist emerald mine, Hiddenite, North Carolina. Can Mineral 44:1529–1541
Acknowledgements
The author extends his appreciation and thanks to James Hill, Jr. and North America Emerald Mines for allowing access to their emerald deposit and to Lynn Hill for providing logistical support while in the field. Dr. Alan Anderson assisted in the field collecting of hiddenite specimens. Fieldwork for this study was supported in part by the Sprague Endowment of the Smithsonian Institution. Special thanks are extended to Paul Carr, Anton Chakhmouradian and G. Diego Gatta for their thorough reviews and many helpful suggestions for improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: A.R. Chahmouradian
Rights and permissions
About this article
Cite this article
Wise, M.A. Chabazite in spodumene-bearing Alpine-type fissure veins from Hiddenite, North Carolina, USA. Miner Petrol 96, 213–220 (2009). https://doi.org/10.1007/s00710-009-0052-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-009-0052-7