Abstract
A 2NvS chromosomal segment carrying bread wheat variety, BARI Gom 33 (‘BG33’), showed tolerance to terminal heat stress and higher yield over a heat-tolerant non-2NvS BARI Gom 26 (‘BG26’) and a heat-susceptible Pavon 76 (‘Pavon’). This study aimed to ascertain the potential of the 2NvS ‘BG33’ in terminal heat-induced oxidative stress tolerance compared to non-2NvS ‘BG26’ and heat-susceptible ‘Pavon’ under two heat regimes at the reproductive stages viz. control (optimum sowing time) and heat stress (late sowing). We found that both ‘BG26’ and ‘BG33’ showed significantly higher tolerance to oxidative stress by limiting the generation of reactive oxygen species (ROS), methylglyoxal under heat stress. During terminal heat stress, both ‘BG33’ and ‘BG26’ exhibited greater cellular homeostasis than heat-susceptible ‘Pavon’, which was maintained by the increased accumulation of osmolytes, nonenzymatic antioxidants, and enzymes associated with ROS scavenging, ascorbate–glutathione cycle, and glyoxalase system. Lesser cellular damage in ‘BG26’ and ‘BG33’ was eventually imitated in a smaller reduction in grain yield (15 and 12%, respectively) than in ‘Pavon’, which had a 33% reduction owing to heat stress. Collectively, our findings revealed that the chromosomal segment 2NvS provides yield advantage to ‘BG33’ under terminal heat stress by lowering oxidative damage. As 2NvS translocation contains multiple nucleotide-binding domain leucine-rich repeat containing, cytochrome P450, and other gene families associated with plant stress tolerance, further studies are warranted to dissect the underlying molecular mechanisms associated with higher heat stress tolerance of 2NvS carrying ‘BG33’.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bread wheat (Triticum aestivum L.) ranks second among cereals in terms of production both globally and in Bangladesh (BBS 2019; FAO 2021). However, numerous environmental stressors are presently limiting wheat yields, with heat stress being one of the most significant ones. According to current research, most of the world’s wheat-growing regions are experiencing spells of above-optimal temperatures, resulting in a substantial reduction in grain yield (Coffel et al. 2018). Late harvesting of preceding rice crop forces late sowing of wheat in the Gangetic plains of India and Bangladesh, exposing the crop to terminal heat stress and limiting grain yield (Dubey et al. 2020).
The reproductive stages of wheat is the most susceptible to various biotic and abiotic stresses that severely impacts on global wheat production (Farooq et al. 2011; Asseng et al. 2015; Islam et al. 2016, 2020). It is proven that heat stress during reproductive stages has a significant detrimental influence on crops, including wheat (Jagadish 2020). Temperatures of 23 °C and 21.3 °C were found to be optimum for the anthesis and grain filling stages, respectively (Farooq et al. 2011; Narendra et al. 2021). Wheat yield is reduced by ~ 10% for every 1 °C increase over a mean temperature of 23 °C (Gibson and Paulsen 1999; Asseng et al. 2015). It is estimated that worldwide wheat production will decrease by 6% for every 1 °C increase in current mean temperature (Asseng et al. 2011; Zhao et al. 2017; Demelash et al. 2022). This predicted decrease in wheat yield poses a threat to future global food security. As a result, a deliberate effort should be made to minimize yield loss by selecting or developing heat-tolerant wheat varieties. Although some genotypes and cultivars of wheat display yield advantage under heat stress conditions, the underlying physiological and molecular mechanisms of their tolerance to heat are largely unknown.
Heat stress severely affects photosynthesis, affecting plant growth and development (Wahid et al. 2007), and stimulates the breakdown of the thylakoid membrane, resulting in electrolyte leakage (Djanaguiraman et al. 2018), and disruption of all photochemical reactions, especially, in photosystem II (Pastenes and Horton 1999). Terminal heat stress during the anthesis stage decreased floret fertility and at the grain filling stage decrease the grain yield through individual grain weight (Prasad and Djanaguiraman 2014), which is associated with early leaf senescence, and decreased grain-filling duration (Asseng et al. 2011; Lobell et al. 2012). Heat stress also induces oxidative damage by increasing the generation of reactive oxygen species (ROS), such as singlet oxygen (1O2), superoxide radicals (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and lipid peroxidation, which leads to enhanced membrane damage (Suzuki et al. 2012; Narayanan et al. 2016; Djanaguiraman et al. 2018).
Heat-tolerance attributes are linked to overall antioxidant activities, which enable plants to maintain cellular homeostasis under heat stress (Mohi-Ud-Din et al. 2021b). Plants possess both enzymatic and non-enzymatic antioxidants that play important role in scavenging ROS generated under stress. The enzymatic antioxidant includes superoxide dismutase (SOD); enzymes of the ascorbate–glutathione cycle viz. ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR); catalase (CAT), glutathione peroxidase (GPX), and glutathione S-transferase (GST) (Noctor et al. 2014). Ascorbate (AsA), glutathione (GSH), tocopherol, flavanones, carotenoids, anthocyanins, phenolic compounds, etc., act as nonenzymatic antioxidants (Gill and Tuteja 2010; Hasanuzzaman et al. 2020). The methylglyoxal (MG) is a highly reactive substance generated under various stresses including high temperature (Hasanuzzaman et al. 2017). Plants have variable mechanisms to detoxify the MG level by glyoxalase system consisting of glyoxalase I (Gly I) and glyoxalase II (Gly II) where GSH plays a central role (Hasanuzzaman et al. 2017).
Since the early nineties, the Aegilops ventricose (Zhuk.) 2NvS translocation segment has been used in the breeding of disease-resistant wheat varieties (Cruz et al. 2016; Gao et al. 2021). Several important genes have been identified in this segment that were associated with the resistance against various wheat diseases including root-knot nematode (Williamson et al. 2013), stripe rust, leaf rust, and stem rust (Bariana and McIntosh 1994). This segment has recently been found link to the resistance to the wheat blast fungus Magnaporthe oryze Triticum (MoT) pathotype, which is the causal agent of the devastating and emerging wheat blast disease (Cruz et al. 2016; Cruz and Valent 2017; Islam et al. 2020). Furthermore, new reports suggest that this translocation is contributing to lodging resistance in bread wheat (Singh et al. 2019). Recently, Gao et al. (2021) postulated that the physiological and yield benefits of 2NvS carrying wheat varieties are not only associated with disease resistance. The multiple gene families present in this chromosomal segment might be linked to the higher fitness of wheat variety under various stressful environments. These findings bring us to hypothesize that the 2NvS chromosomal segment may possess loci/genes that confer abiotic stress tolerance of bread wheat. To test this hypothesis, we assessed the potential of terminal heat-induced oxidative stress tolerance of a 2NvS chromosomal segment carrying bread wheat variety, BARI Gom 33 (‘BG33’) compared with a heat tolerant non-2NvS BARI Gom 26 (‘BG26’) (positive control) and a heat-susceptible wheat variety Pavon 76 (‘Pavon’) (negative control) under two heat regimes at the reproductive stages viz. control (optimum sowing time) and heat stress (late sowing). Here, we outlined the findings of the terminal heat-induced oxidative stress tolerance of ‘BG33’, carrying a 2NvS translocation segment from Aegilops ventricose (Zhuk.) compared with a non-2NvS heat-tolerant, ‘BG26’, and a heat-susceptible variety, ‘Pavon’, in a field experiment.
Materials and methods
Molecular detection of 2N v S translocation
Genomic DNA was extracted from 10-day-old seedlings using the modified cetyltrimethylammonium bromide (CTAB) method (Allen et al. 2006) to amplify the 2NvS translocation segment. PCR amplification was performed with two 2NvS specific primers VENTRIUP-F (5′-AGG GGC TAC TGA CCA AGG CT-3′), LN2-R (5′-TGC AGC TAC AGC AGT ATG TAC ACA AAA-3′) (Helguera et al. 2003; Cruz et al. 2016) and Yr17-F (5′-TTA TTA CCT TGA TGA GAA ATA CAG-3′), Yr17-R (5′-CTG AAA TTG GGA CTA GCG AAA TTA-3′) (Helguera et al. 2003; Alam et al. 2021). PCR was conducted in a volume of 10 μL in a Verity Thermal Cycler (Applied Biosystems, USA). The reaction mixture consisted of a minimum 2 μL (100 ng) of genomic DNA, 0.5 μL of each forward and reverse primers (10 μM), 5 μL PCR master mix 2X (Promega Corporation, USA), and ddH2O up to 10 μL. The PCR cycling program for VENTRUIP-F/LN2-R was as follows: denaturing at 94 °C for 3 min; amplification at 94 °C for 45 s, 65 °C for 30 s, and 72 °C for 60 s repeated for 30 cycles; and extension at 72 °C for 7 min. The amplification program of Yr17-F/Yr17-R was as follows: 94 °C for 3 min (enzyme deactivation); 26 cycles of 94 °C for 45 s (melting), 57 °C for 45 s (annealing) and 72 °C for 45 s (extension); and a final extension at 72 °C for 8 min. The presence or absence of 262-bp and 383-bp 2NvS segment of DNA for VENTRUIP-F/LN2-R and Yr17-F/Yr17-R, respectively, was observed after electrophoresis on 1.5% agarose gels stained with ethidium bromide.
Experimental setup, design, and treatments
Three wheat varieties developed by Bangladesh Wheat and Maize Research Institute (BAMRI) were used in this study namely, BARI Gom 26 (‘BG26’), BARI Gom 33 (‘BG33’), and Pavon 76 (‘Pavon’). ‘BG26’ is a widely cultivated yield-stable wheat variety and recognized as heat-tolerant from previous studies (Supplementary Table S1; Hossain and Teixeira da Silva 2013; Khatun et al. 2015; Mohi-Ud-Din et al. 2021b). The wheat blast resistant variety ‘BG33’ was derived from a simple cross between ‘Kachu’ and ‘Solala’. ‘Kachu’ is a ‘Kauz’-derived high-yielding and zinc fortified wheat variety carrying a 2NvS segment for blast resistance (Mottaleb et al. 2019). ‘Pavon’ is widely used as heat-susceptible check variety in different experiments (Khatun et al. 2015; Mohi-Ud-Din et al. 2021b).
The experiment was laid out in a split-plot design with four replications. The two growing conditions — “control” (optimum sowing on November 23) and “heat stress” (late sowing on January 03) — were placed in the main plots, whereas wheat varieties were placed randomly in the sub-plots.
The experiment was conducted in the field laboratory of the Department of Crop Botany, Bangabandhu Sheikh Mujibur Rahman Agricultural University (24.038°N latitude, 90.397°E longitude), Gazipur, Bangladesh. The experimental soil was silt loam in texture (sand 26%, silt 50%, and clay 24%), having the full field capacity at 30.6% volumetric soil water content. The daily maximum, minimum, mean air temperatures, and rainfall at the reproductive stages of control (A) and heat stress (B) conditions are presented in Fig. 1. Ten-years’ averaged climatic data for the same duration of control and heat-stressed reproductive growth stages are summarized in Fig. 1C, D. Under the heat stress conditions, wheat varieties received 26.5 °C mean air temperature throughout the reproductive stages, which was higher than the optimum temperature required for the anthesis (23 °C) and grain filling phases (21.3 °C) (Farooq et al. 2011). Healthy seeds were sown, and all agronomic practices were performed as per the recommendation.
Daily maximum (Max), minimum (Min), mean air temperatures, and rainfall data recorded at the reproductive growth stages for control and heat stress conditions (A and B). Ten-year average climatic data for the same duration was presented in C and D. Source: Weather Station, Department of Agricultural Engineering, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
Canopy temperature
Canopy temperature (CT) was recorded using a hand-held infrared thermometer (Model-IR-720, Amprobe, USA; distance-spot ratio of 20:1) between 11.30 a.m. and 12.30 p.m. At a distance of 1 m from the spotted canopy, measurements were taken at an angle of approximately 30° to the horizontal line. Ten readings were taken from different areas of each plot and averaged.
Cell membrane stability
Cell membrane stability (CMS) was determined following the procedure of Sairam et al. (1997). Briefly, five uniform flag leaves were collected from each plot and leaf discs (0.7 cm in diameter) were prepared by using a leaf puncher. In two sets, 15 leaf discs were put in test tubes containing 10 mL of deionized water. One set was incubated at 40 °C for 30 min, and the other set was kept at 100 °C in a boiling water bath for 15 min, and then, their electrical conductivities, C1 and C2, respectively, were measured with a conductivity meter. CMS was calculated following the equation:
Biochemical assays
Oxidative stress indicators
The O2•− generation of leaf sample was determined spectrophotometrically at 530 nm following the procedure of Elstner and Heupel (1976) modified by Mohi-Ud-Din et al. (2021a) and calculated by comparing a standard curve of NaNO2. For H2O2 and malondialdehyde (MDA) assay, 0.5 g fresh flag leaf tissues were homogenized in 3 mL of 5% (w/v) trichloroacetic acid (TCA), centrifuged at 11,500 × g, and the supernatants were collected. The concentration of H2O2 and MDA was then measured spectrophotometrically using the methods of Yang et al. (2007) and Heath and Packer (1968), and quantified using the extinction coefficients of 0.28 µM−1 cm−1 and 155 mM−1 cm−1, respectively. Lipoxygenase (LOX) (EC: 1.13.11.12) activity was determined spectrophotometrically at 234 nm as the method reported by Doderer et al. (1992), using linoleic acid as substrate, and then calculated by applying an extinction coefficient of 25,000 M−1 cm−1. Methylglyoxal (MG) was measured spectrophotometrically at 288 nm by using N-acetyl-ʟ-cysteine following the method of Wild et al. (2012) and calculated using a standard curve of known concentration of MG.
Osmolytes and nonenzymatic antioxidants
The amount of free proline (Pro) in flag leaf tissues was measured spectrophotometrically using the acid ninhydrin assay, as described by Bates et al. (1973), and was estimated as µmol g−1 FW using a standard curve. Glycine betaine (GB) was appraised spectrophotometrically using the 1,2-dichloroethane technique, as reported by Valadez-Bustos et al. (2016), and expressed as µmol g−1 FW using a standard curve. Ascorbate and glutathione contents were determined spectrophotometrically following the methods outlined in Hasanuzzaman et al. (2014).
Extraction of soluble protein
Soluble protein from the fresh flag leaves (1:2) (w/v) was extracted in 0.5 M potassium–phosphate (K–P) buffer (pH 7.0) containing 1 mM ascorbic acid, 1 M KCl, β-mercaptoethanol, and glycerol in an ice-cold mortar. The homogenate was centrifuged for 15 min at 11,500 × g, and the supernatant was collected for enzyme activity assays. The protein content of each enzyme solution was determined using Bradford’s (1976) fast quantitative technique.
Enzyme activity assays
The enzyme activities were determined using the comprehensive techniques outlined by Mohi-Ud-Din et al. (2021b); briefly, the activity of SOD (EC: 1.15.1.1) was quantified using the Spitz and Oberley’s (1989) inhibition technique. Catalase (EC: 1.11.1.6) activity was determined at 240 nm after 1 min using the method of Noctor et al. (2016), and the extinction coefficient of 39.4 M−1 cm−1 was used to compute the activity. The method of Castillo et al. (1984) was used to determine the activity of guaiacol peroxidase (POD, EC: 1.11.1.7) by recording the absorbance at 470 nm after 1 min and estimated using an extinction value of 26.6 mM−1 cm−1. Glutathione peroxidase (EC: 1.11.1.9) activity was measured at 340 nm for 1 min, as described by Elia et al. (2003) and the activity was calculated using a 6.62 mM−1 cm−1 extinction coefficient. The activity of GST (EC: 2.5.1.18) was determined spectrophotometrically at 340 nm and using the extinction coefficient of 9.6 mM−1 cm−1, as described by Hossain et al. (2006).
The activities of GR (EC: 1.6.4.2), APX (EC: 1.11.1.11), MDHAR (EC: 1.6.5.4), and DHAR (EC: 1.8.5.1) were determined at 340, 290, 340, and 265 nm, respectively, as reported by Noctor et al. (2016). The absorbances were measured after 1 min, and extinction coefficients of 6.2 mM–1 cm–1, 2.8 mM−1 cm−1, 6.2 mM–1 cm–1, and 14 mM–1 cm–1 were used to calculate GR, APX, MDHAR, and DHAR, respectively. The activities of Gly I (EC: 4.4.1.5) and Gly II (EC: 3.1.2.6) were determined at 240 and 412 nm as per the methods of Hossain and Fujita (2009) and Principato et al. (1987) using extinction coefficients of 3.37 mM−1 cm−1 and 13.6 mM−1 cm−1, respectively.
Grain yield
At the physiological maturity, plants of four linear meters from the middle of the plot were cut at the ground level. From the harvested samples, spikes were separated and collected in a cloth bag and dried in sun. The spikes were threshed and cleaned manually, and the weight of grain was taken and adjusted to 12% moisture, and grain yield was expressed in t ha−1.
Statistical analysis
Statistical analyses were performed using R-4.1.0 for win (http://CRAN.R-project.org/) (accessed on September 15, 2021). Physio-chemical and yield data were subjected to 3-factor (variety × growing condition × growth stage) and 2-factor (variety × growing condition) analysis of variance (ANOVA), respectively, in the general linear model using the package lme4 (Bates et al. 2015) and Tukey’s HSD test was used to compare mean differences using the library agricolae (de Mendiburu and Yaseen 2020). Differences at p ≤ 0.05 were deemed significant.
Results
Molecular screening of 2N v S and non-2N v S wheat varieties
The molecular detection revealed that ‘BG33’ was positive for 2NvS specific primers and, therefore, confirmed the presence of a 2NvS translocation segment in the variety (Fig. 2A, B). However, the negative reaction of the primers with heat-tolerant ‘BG26’ and heat-susceptible ‘Pavon’ indicated that these varieties did not carry a 2NvS segment.
Effect of variety, growing condition, and growth stage on the studied parameters
The main effect of variety (V), growing condition (C), and growth stage (S) in the general linear model (GLM) was highly significant for all studied parameters (Supplementary Table S2). Except for Gly II, the V × C was also significant for all traits, whereas V × S was significant for most of the studied traits excluding CMS, Pro, GPX, DHAR, and MDHAR. Apart from GR, C × S was significant for all studied parameters; however, V × C × S was significant only for O2•−, MDA, LOX, AsA, CAT, POD, GST, GR, and DHAR (Supplementary Table S2). Grain yield (GY) were estimated at the final harvest and subjected to two-factor analysis of variance and found that the main effects (V and C) for GY were highly significant, while interactions (V × C) were not significant in the GLM (Supplementary Table S2).
Canopy temperature, membrane stability, and oxidative stress indicators
With the progression of reproductive growth, the CT was increased significantly under heat stress conditions in all wheat varieties compared to control (Fig. 3A). Regarding growth stages, the CT increased by 26, 29, and 31% at anthesis, 7 DAA, and 15 DAA, respectively under heat stress. In terms of the varietal response to heat stress, ‘Pavon’ showed the highest increase (31%) in the CT in comparison with 27 and 28% increases in ‘BG26’ and ‘BG33’, respectively (Fig. 3A).
A Canopy temperature (CT), B cell membrane stability (CMS), C superoxide radicle (O2•−), D hydrogen peroxide (H2O2), E malondialdehyde (MDA), and F lipoxygenase (LOX) activity at the reproductive stages of wheat varieties grown under control and heat stress conditions. Vertical bars represent ± SE values. Asterisks over the bar indicate the mean value is significantly different from the corresponding mean value of control at p ≤ 0.05 by Tukey’s HSD test. FW fresh weight, DAA days after anthesis
Accumulation patterns of osmolytes and non-enzymatic antioxidants at the reproductive stages of wheat varieties grown under control and heat stress conditions: A proline, B glycine betaine (GB), C reduced glutathione (GSH), and D ascorbate (AsA) content. Vertical bars represent ± SE values. Asterisks over the bar indicate the mean value is significantly different from the corresponding mean value of control at p ≤ 0.05 by Tukey’s HSD test. FW fresh weight, DAA days after anthesis
Cell membrane stability was decreased under heat stress at all reproductive growth stages in the wheat varieties, but the magnitude was significant and higher in the variety ‘Pavon’ (Fig. 3B). Compared to control, CMS was decreased by 13, 16, and 24% at anthesis, 7 DAA, and 15 DAA, respectively, under heat stress. The highest 35% decrease in CMS due to heat stress was recorded in ‘Pavon’, while 9 and 10% for ‘BG26’ and ‘BG33’, respectively (Fig. 3B).
Under heat stress conditions, the generation of O2•− in all wheat varieties increased as reproductive growth progressed, with a greater extent of increase in ‘Pavon’ (Fig. 3C). Compared to the control, heat stress caused 79, 141, and 185% increases in O2•− at anthesis, 7 DAA, and 15 DAA, respectively, across 3 varieties. Regarding the varietal response to heat stress, ‘BG26’ and ‘BG33’ showed a relatively lesser increase in O2•− (88 vs. 82%) compared to the robust 235% increase in ‘Pavon’.
Hydrogen peroxide content increased sharply at all the reproductive growth stages in all wheat varieties, though the degree of increase was higher in ‘Pavon’ (Fig. 3D). Heat stress increased H2O2 content by 52, 59, and 92%, at anthesis, 7 DAA, and 15 DAA, respectively, when compared to the control. In response to heat stress, 54 and 46% increase in H2O2 content was recorded in ‘BG26’ and ‘BG33’, while ‘Pavon’ showed a 103% increase (Fig. 3D).
Due to heat stress, the MDA content and LOX activity were remarkably increased in wheat varieties at all reproductive growth stages (Fig. 3E, F). Irrespective of growing conditions and growth stages, MDA content and LOX activity were the lowest ‘BG33’ and the highest in ‘Pavon’. Regarding growth stages, MDA content increased by 39, 46, and 61% and the LOX activity by 88, 117, and 174% at anthesis, 7 DAA, and 15 DAA, respectively, under heat stress. In terms of varietal response to heat stress, ‘BG26’, ‘BG33’, and ‘Pavon’ showed 39, 34, and 74% increases in MDA content and 75, 82, and 221% increases in LOX activity, respectively (Fig. 3E, F).
Accumulation of osmolytes and non-enzymatic antioxidants
In response to heat stress, Pro and GB contents increased significantly in ‘BG26’ and ‘BG33’ at the time of anthesis; however, at 7 and 15 DAA, the osmolytes increased significantly in all three wheat varieties (Fig. 4A, B). Regarding growth stage response to heat stress, Pro content increased by 60, 86, and 94% at anthesis, 7 DAA, and 15 DAA, respectively, while GB increased by 84, 111, and 116%. ‘BG26’, ‘BG33’, and ‘Pavon’ exhibited 87, 100, and 54% increases in proline content and 109, 121, and 81% increases in GB content, respectively, in response to heat stress (Fig. 4A, B).
At the time of anthesis, GSH content increased substantially in ‘BG26’ and ‘BG33’ in response to heat stress; however, the increase was significant in all three wheat varieties at 7 and 15 DAA (Fig. 4C). GSH content elevated by 57, 63, and 67% at anthesis, 7 DAA, and 15 DAA, respectively, when heat stress was induced. In response to heat stress, the GSH content of ‘BG26’, ‘BG33’, and ‘Pavon’ increased by 87, 78, and 22%, respectively (Fig. 4C). Contrarily, at all the growth stages, AsA content decreased significantly only in ‘Pavon’ in response to heat stress; however, the decrease was significant for ‘BG26’, at 7 and 15 DAA (Fig. 4D). At anthesis, 7 DAA, and 15 DAA, AsA content decreased by 27, 33, and 35%, respectively, in response to heat stress. The AsA content of ‘BG26’, ‘BG33’, and ‘Pavon’ decreased by 17, 12, and 67%, respectively, in response to heat stress (Fig. 4D).
Activities of antioxidant enzymes
In response to heat stress, SOD activity increased substantially in all wheat varieties at the reproductive stages, with a greater extent in ‘BG26’ and ‘BG33’ than ‘Pavon’ (Fig. 5A). Regarding growth stage response to heat stress, SOD activity increased by 53, 74, and 92% at anthesis, 7 DAA, and 15 DAA, respectively. Regarding the varietal response to heat stress, ‘BG26’, ‘BG33’, and ‘Pavon’ exhibited 78, 94, and 47% increases in the SOD activity, respectively (Fig. 5A).
Specific activity of A superoxide dismutase (SOD), B catalase (CAT), C guaiacol peroxidases (POD), D glutathione peroxidase (GPX), and E glutathione S-transferase (GST) enzymes at the reproductive stages of wheat varieties grown under control and heat stress conditions. Vertical bars represent ± SE values. Asterisks over the bar indicate the mean value is significantly different from the corresponding mean value of control at p ≤ 0.05 by Tukey’s HSD test. DAA days after anthesis
Due to heat stress, ‘BG26’ and ‘BG33’ showed the increasing trend in the CAT and POD activities over the reproductive growth; however, except anthesis, both CAT and POD activities decreased at the later reproductive growth stages of ‘Pavon’ (Fig. 5B, C). Under heat stress conditions, CAT activity increased by 16, 16, and 7% at anthesis, 7 DAA, and 15 DAA, respectively, while POD activity increased by 9, 15, and 3%, respectively. In terms of varietal response to heat stress, ‘BG26’ and ‘BG33’ exhibited 24 and 22% increases in CAT activity and 20 and 19% increases in POD activity, respectively; however, the activities of both enzymes decreased by 7 and 12% in ‘Pavon’ (Fig. 5B, C).
Heat stress instigated a remarkable increase in the activity of GPX and GST in wheat varieties at the reproductive growth stages, with the greater extent of increase of GPX in ‘BG33’ and GST in ‘BG26’ (Fig. 5D, E). Under heat stress, GPX activity increased by 36, 73, and 43%, respectively, while GST activity increased by 30, 37, and 33% at anthesis, 7 DAA, and 15 DAA. In response to heat stress, the GPX activity of ‘BG26’, ‘BG33’, and ‘Pavon’ increased by 61, 67, and 25%, respectively, while the GST activity increased by 36, 49, and 15% compared to the control (Fig. 5D, E).
Ascorbate peroxidase activity increased markedly under heat stress at the reproductive growth stages in all wheat varieties, though the degree of increase was higher in ‘BG26’ (Fig. 6A). Regarding growth stages, heat stress increased APX activity by 47, 51, and 57%, at anthesis, 7 DAA, and 15 DAA, respectively, when compared to the control. As for varieties, 69 and 63% increase in APX activity was recorded in ‘BG26’ and ‘BG33’ under heat stress, while ‘Pavon’ showed only 24% increase (Fig. 6A).
Specific activity of A ascorbate peroxidase (APX), B glutathione reductase (GR), C dehydroascorbate reductase (DHAR), and D monodehydroascorbate reductase (MDHAR) enzymes at the reproductive stages of wheat varieties grown under control and heat stress conditions. Vertical bars represent ± SE values. Asterisks over the bar indicate the mean value is significantly different from the corresponding mean value of control at p ≤ 0.05 by Tukey’s HSD test. DAA days after anthesis
In response to heat stress, ‘BG26’ and ‘BG33’ showed an increasing trend in GR activity over reproductive growth. However, except for anthesis, GR activity decreased at the later reproductive growth stages of ‘Pavon’ (Fig. 6B). Under heat stress conditions, GR activity increased by 64, 61, and 66% at anthesis, 7 DAA, and 15 DAA, respectively. In terms of varietal response to heat stress, ‘BG26’ and ‘BG33’ exhibited 95 and 108% increases in GR activity, respectively; however, the activity of the enzyme decreased by 11% in ‘Pavon’ (Fig. 6B).
Due to heat stress, the MDHAR and DHAR activities were decreased in wheat varieties at all reproductive growth stages (Fig. 6C, D). Regarding growth stages, MDHAR activity decreased by 31, 37, and 39% and the DHAR activity by 18, 24, and 30% at anthesis, 7 DAA, and 15 DAA, respectively, under heat stress. When grown in the heat stress condition, ‘BG26’, ‘BG33’, and ‘Pavon’ showed 21, 30, and 54% decreases in MDHAR activity, respectively, whereas DHAR activity decreased by 11, 14, and 47%. (Fig. 6C, D).
Glyoxalase system
Under heat stress conditions, the MG content increased substantially in all wheat varieties as the reproductive growth progressed, with a greater extent of increase in ‘Pavon’ (Fig. 7A). Compared to the control, heat stress instigated 66, 67, and 76% increases in MG content at anthesis, 7 DAA, and 15 DAA, respectively across 3 varieties. As for varieties, ‘BG26’ and ‘BG33’ showed a lesser increase in MG content (64 and 54%, respectively) compared to the 91% increase in ‘Pavon’ (Fig. 7A).
A Methylglyoxal (MG) content; specific activity of B glyoxalase I (Gly I) and C glyoxalase II (Gly II) enzymes at the reproductive stages of wheat varieties grown under control and heat stress conditions. Vertical bars represent ± SE values. Asterisks over the bar indicate the mean value is significantly different from the corresponding mean value of control at p ≤ 0.05 by Tukey’s HSD test. FW fresh weight, DAA days after anthesis
Heat stress upregulated the activity of Gly I and Gly II in wheat varieties at the reproductive growth stages, with a comparatively lesser increase in ‘Pavon’ (Fig. 7B, C). Under heat stress, Gly I activity increased by 21, 36, and 34%, respectively, whereas Gly II activity increased by 17, 20, and 25% at anthesis, 7 DAA, and 15 DAA. Regarding the varietal response to heat stress, the Gly I activity of ‘BG26’, ‘BG33’, and ‘Pavon’ increased by 35, 45, and 10%, respectively, while the Gly II activity increased by 21, 29, and 13% compared to the control (Fig. 7B, C).
Effect of heat stress on grain yield
Under heat stress conditions, GY of the wheat varieties decreased remarkably but the decrease was significant only in ‘Pavon’ (Fig. 8). Irrespective of the growing conditions, 2NvS ‘BG33’ maintained a higher GY than that of ‘BG26’ and ‘Pavon’. Due to heat stress, the GY of ‘BG26’, ‘BG33’, and ‘Pavon’ decreased by 15, 12, and 33%, respectively (Fig. 8).
Discussion
Heat stress-induced damages are the result of multifaceted and interrelating plant physiological processes. Above crop- and phenological stage-specific threshold temperature can lead to plant tissue damage and stalled physiological processes. Relatively lower CT, was correlated with the final yield and other physiological processes under stress (Gautam et al. 2015). Our findings suggested that ‘BG33’ showed a comparatively lower increase in CT than heat-susceptible variety ‘Pavon’ under terminal heat stress which in turn reflected on the grain yield of the varieties. Both ‘BG26’ and ‘BG33’ also possess comparatively stable and functional CMS under heat stress and are regarded to be potential options for selecting or improving wheat varieties for heat tolerance (ElBasyoni et al. 2017).
Heat stress intensifies the generation of reactive oxygen species (ROS) (Mishra et al. 2011). MDA content and LOX activity are used to assess membrane lipid peroxidation and oxidative stress, as well as the effects of these molecules on proliferating membrane fluidity, leakiness, and damage to membrane proteins, enzymes, and ion channels (Alché 2019). In comparison with heat-tolerant ‘BG26’ and 2NvS ‘BG33’, heat-susceptible ‘Pavon’ showed a substantial increase in the levels of O2•−, H2O2, and MDA and the LOX activity under heat stress, indicating greater susceptibility of the variety to terminal heat-induced oxidative stress. Lower increases in these oxidative stress markers in heat-tolerant ‘BG26’ and 2NvS ‘BG33’ in response to heat stress suggest the improved heat tolerance of the varieties due to less inhibition or increased activation of antioxidant defense mechanisms compared to heat-susceptible ‘Pavon’.
As important osmolyte, accumulation of Pro is shown to alleviate osmotic stress (Majláth et al. 2012), protect some enzymes and protein complexes from heat-induced destabilization (Wang et al. 2010), and indirectly act as an antioxidant. Proline also increases the levels of AsA and GSH by activating the synthesis of these molecules (Iqbal et al. 2021). In the current research, in comparison to the heat-susceptible ‘Pavon’, the heat-tolerant ‘BG26’ and 2NvS ‘BG33’ had a higher accumulation of Pro, GB, GSH, and AsA, indicating less disruption of pathways synthesizing these antioxidants under heat stress. As a result, these varieties showed the fewer amount of ROS accumulation under heat stress.
Superoxide dismutase offers primary protection against O2•− in plant cells, which is then converted to H2O2 and then to H2O by CAT and peroxidases (POD, APX, and GPX), thus preventing cell damage (Gill and Tuteja 2010). In this study, SOD, CAT, and POD activity increased significantly in the heat-tolerant ‘BG26’ and 2NvS ‘BG33’, while CAT and POD activity decreased in the heat-susceptible ‘Pavon’ (Fig. 6A, B, C), perhaps owing to inactivation by the higher accumulation of H2O2 in the susceptible variety induced by heat stress. In tolerant varieties, the higher increase in SOD, CAT, and POD activity may effectively detoxify O2•− and H2O2 generated by heat stress, and vice versa in susceptible variety (Mohi-Ud-Din et al. 2021b). Increasing activity of SOD, CAT, and POD in heat-tolerant and -susceptible wheat varieties, but decreasing activity in CAT and POD in the heat-susceptible variety under terminal heat stress was also reported by Almeselmani et al. (2009). Glutathione peroxidases reduce H2O2 and lipid hydroperoxides (ROOHs) with GSH (Noctor et al. 2002), whereas GST catalyze the conjugation of electrophilic xenobiotic substrates with GSH (Dixon et al. 2010), and thus, protecting the plant cell membrane from oxidative damage. In comparison to ‘Pavon’, the results showed that ‘BG26’ and ‘BG33’ had a larger increase in GPX and GST activity in response to heat stress at all reproductive growth stages, indicating that the varieties had a greater ability to scavenge H2O2, ROOHs, and xenobiotics. In our study, enhanced GSH content in ‘BG26’ and ‘BG33’ provided enough substrate for these enzymes and took part in the protection again oxidative stress. Moreover, ‘BG26’ and 2NvS segment carrying ‘BG33’ counteracted ROS generation by maintaining a relatively higher increase in SOD, CAT, POD, GPX, and GST compared to a lower increase in SOD, GPX, and GST and a significant decrease in CAT and POD in heat-susceptible ‘Pavon’ under heat stress.
The AsA-GSH cycle is the primary metabolic pathway for the scavenging and/or detoxification of ROS and, therefore protecting plants from oxidative stress (Tiwari and Yadav 2019; Hasanuzzaman et al. 2019). Our findings revealed a substantial rise in GSH and a drop in AsA levels in all wheat varieties subjected to heat stress. However, ‘BG26’ and ‘BG33’ showed relatively higher accumulation of GSH and lower decrease in AsA contents compared to ‘Pavon’. The enhanced use of AsA to counteract the larger quantity of ROS generated during heat stress was shown by the greater drop in AsA concentration in heat-sensitive ‘Pavon’. Under heat stress, a greater rate of GSH synthesis was expedited by a significant rise in GR activity, resulting in a larger increase in GSH content in ‘BG26’ and ‘BG33’ than in ‘Pavon’.
Ascorbate peroxidase is the vital enzyme in the AsA–GSH cycle, and it plays a crucial role in plant defense against oxidative stress by catalyzing the conversion of H2O2 to water in the chloroplasts (Pandey et al. 2017). Heat-tolerant ‘BG26’ and ‘BG33’ showed a significant and higher relative increase in APX activity under heat stress conditions over the control at all reproductive growth stages compared to heat-susceptible ‘Pavon’, which is in agreement with Almeselmani et al. (2009) and Tiwari and Yadav (2019). In our study, the activities of DHAR and MDHAR were shown to be substantially reduced at all wheat varieties at the reproductive stages as a result of heat stress. Since DHAR and MDHAR both have a role in recycling AsA and its redox state under oxidative stress (Li et al. 2018), reductions in their activities were accompanied by a drop in AsA content in the current investigation and these reductions were more pronounced in the heat-sensitive variety ‘Pavon’. Collectively, our findings revealed that the AsA–GSH cycle is more efficient in the heat-tolerant variety ‘BG26’ and 2NvS translocation carrying ‘BG33’ than in the heat-susceptible variety ‘Pavon’. The differences in these varietal responses to heat stress are a result of the variations in the activities of enzymatic and non-enzymatic antioxidants associated with the AsA–GSH cycle. The augmented activity of the AsA–GSH cycle in ‘BG33’ might be due to the presence of oxidative stress-tolerant locus in the 2NvS segment.
Methylglyoxal damages cellular functions and can even destroy DNA (Hasanuzzaman et al. 2017). In comparison to ‘BG26’ and ‘BG33’, a higher relative increase in MG levels in ‘Pavon’ at all reproductive growth stages showed the degree of inactivation of the essential defense system and irreversible metabolic dysfunction under heat stress. Relatively, lower accumulation of MG and higher activities of Gly I and Gly II in the tolerant wheat varieties was reported earlier under heat-stress conditions (Mohi-Ud-Din et al. 2021b). Similarly, in the heat-tolerant ‘BG26’ and 2NvS ‘BG33’, a larger relative increase of Gly I and II imparts effective MG detoxification; additionally, these enzymes aid in the maintenance of GSH homeostasis and eventual ROS detoxification.
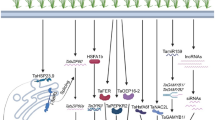
As a heat-tolerant variety, ‘BG26’ was able to maintain relatively lower generation of O2•−, H2O2, MDA, LOX and MG content, higher Pro, GB, GSH and AsA contents, and lessened oxidative stress with augmented antioxidant capacity and a lower reduction in grain yield under heat stress. Nonetheless, ‘BG33’, a variety that carries a 2NvS chromosomal segment, also exhibited a similar ability to tolerate terminal heat-induced oxidative stress. Our findings endorsed the postulation of Gao et al. (2021) that the 2NvS is actually conferring yield advantage, even in the absence of pathogen pressure. In our study, the higher heat tolerance of the ‘BG33’ variety containing cytochrome P450 (CYP) gene families in the 2NvS chromosomal segment might be linked with upregulation of these genes under heat stress (Fig. 9). The upregulation CYP genes for antioxidant enzymes is likely to maintain the levels of AsA and GSH. As a result, 2NvS chromosomal segment in ‘BG33’ mitigates the ROS-induced oxidative stress and confers heat tolerance. The CYP73A (trans-cinnamate 4-monooxygenase), CYP75A (flavonoid 3′,5′-hydroxylase), and CYP75B (flavonoid 3′-monooxygenase) genes have been significantly upregulated under heat and cold stress in Lolium perenne and Festuca arundinacea (Tao et al. 2017). A further study is needed to confirm the involvement of CYP gene families and/or other genes present in the 2NvS chromosomal segment of ‘BG33’ in terminal heat tolerance shown in this report. The molecular mechanism of heat tolerance of ‘BG26’ is also an interesting subject for further molecular study.
Conclusion
Comprehensive analysis of ROS, osmolytes, activities of antioxidant enzymes, the effectiveness of AsA–GSH cycle, and glyoxalase system reveal that both 2NvS translocation segment carrying ‘BG33’ and non-2NvS carrying heat-tolerant variety ‘BG26’ exhibited greater tolerance to terminal heat-induced oxidative stress compared to heat-susceptible ‘Pavon’. Both wheat varieties have potent antioxidant systems that serve in imparting tolerance throughout reproductive growth stages and, hence, compensate for yield losses due to heat stress. The substantial tolerance against heat-induced oxidative stress exhibited by ‘BG33’ might be linked with the presence of CYP and/or other genes present in the 2NvS chromosomal segment in ‘BG33’. Our findings serve as a foundation for further molecular research for dissecting the involvement of gene(s) in the 2NvS chromosomal segment of ‘BG33’ associated with heat stress tolerance in wheat.
References
Alam MA, Skalicky M, Kabir MR, Hossain MM, Hakim MA, Mandal MS, Islam R, Anwar MB, Hossain A, Hassan F, Mohammadein A (2021) Phenotypic and molecular assessment of wheat genotypes tolerant to leaf blight, rust and blast diseases. Phyton-Int J Exp Bot 90:1301–2132
Alché JD (2019) A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol 23:101136
Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF (2006) A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc 1:2320–2325
Almeselmani M, Deshmukh S, Sairam R (2009) High temperature stress tolerance in wheat genotypes: Role of antioxidant defense enzymes. Acta Agron Hung 57:1–14
Asseng S, Foster IAN, Turner NC (2011) The impact of temperature variability on wheat yields. Glob Chang Biol 17:997–1012
Asseng S, Ewert F, Martre P, Rötter RP, Lobell DB, Cammarano D, Zhu Y (2015) Rising temperatures reduce global wheat production. Nat Clim Chang 5:143–147. https://doi.org/10.1038/NCLIMATE2470
Bariana HS, McIntosh RA (1994) Characterisation and origin of rust and powdery mildew resistance genes in VPM1 wheat. Euphytica 76:53–61. https://doi.org/10.1007/BF00024020
Bates LS, Waldren RP, Teari D (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
BBS (2019) Yearbook of agricultural statistics of Bangladesh, 2019. Bangladesh Bureau of Statistics, Ministry of Planning, Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, pp. 78–79
Bradford MM (1976) A rapid and susceptible method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Castillo FI, Penel I, Greppin H (1984) Peroxidase release induced by ozone in Sedum album leaves. Plant Physiol 74:846–851
Coffel ED, Horton RM, Sherbinin A (2018) Temperature and humidity-based projections of a rapid rise in global heat stress exposure during the 21st century. Environ Res Lett 13:014001
Cruz CD, Peterson GL, Bockus WW (2016) The 2NS translocation from Aegilops ventricosa confers resistance to the Triticum pathotype of Magnaporthe oryzae. Crop Sci 56:990–1000. https://doi.org/10.2135/cropsci2015.07.0410
Cruz CD, Valent B (2017) Wheat blast disease: danger on the move. Trop Plant Pathol 42:210–222. https://doi.org/10.1007/s40858-017-0159-z
Demelash T, Amou M, Gyilbag A, Tesfay G, Xu Y (2022) Adaptation potential of current wheat cultivars and planting dates under the changing climate in Ethiopia. Agronomy 12:37. https://doi.org/10.3390/agronomy12010037
de Mendiburu F, Yaseen M (2020) Agricolae: Statistical procedures for agricultural research. R package version 1.4.0. https://myaseen208.github.io/agricolae/https://cran.r-project.org/package=agricolae. Accessed on 15 September 2021
Dixon DP, Skipsey M, Edwards R (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71:338–350
Djanaguiraman M, Boyle DL, Welti R, Jagadish SVK, Prasad PVV (2018) Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol 18:55
Doderer A, Kokkelink I, Van der Veen S, Valk B, Schram A, Douma A (1992) Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta 112:97–104
Dubey R, Pathak H, Chakrabarti B, Singh S, Gupta DK, Harit RC (2020) Impact of terminal heat stress on wheat yield in India and options for adaptation. Agric Syst 181:102826
ElBasyoni I, Saadalla M, Baenziger S, Bockelman H, Morsy S (2017) Cell membrane stability and association mapping for drought and heat tolerance in a worldwide wheat collection. Sustainability 9:1606
Elia AC, Galarini R, Taticchi MI, Dörr AJ, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55:162–167
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
FAO (2021) FAOSTAT, Food and Agriculture Organization, Rome. 2021. Available online: http://www.fao.org/faostat/en/#home. Accessed on 15 September 2021
Farooq M, Bramley H, Palta JA, Siddique KHM (2011) Heat stress in wheat during reproductive and grain-filling phases. Crit Rev Plant Sci 30:491–507
Gao L, Koo DH, Juliana P (2021) The Aegilops ventricosa 2NvS segment in bread wheat: cytology, genomics and breeding. Theor Appl Genet 134:529–542. https://doi.org/10.1007/s00122-020-03712-y
Gautam A, Sai Prasad SV, Jajoo A (2015) Canopy temperature as a selection parameter for grain yield and its components in durum wheat under terminal heat stress in late sown conditions. Agric Res 4:238–244. https://doi.org/10.1007/s40003-015-0174-6
Gibson LR, Paulsen GM (1999) Yield components of wheat grown under high temperature stress during reproductive growth. Crop Sci 39:1841–1846
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M (2014) Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res Int 2014:757219. https://doi.org/10.1155/2014/757219
Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M (2017) Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci 18:200. https://doi.org/10.3390/ijms18010200
Hasanuzzaman M, Bhuyan MHMB, Anee TI, Parvin K, Nahar K, Al Mahmud J, Fujita M (2019) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8:384
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Helguera M, Khan IA, Kolmer J, Lijavetzky D, Zhong-qi L, Dubcovsky J (2003) PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Sci 43:1839–1847
Hemantaranjan A (2014) Heat stress responses and thermotolerance. Adv Plants Agric Res 1:1–10
Hossain MA, Fujita M (2009) Purification of glyoxalase-I from onion bulbs and molecular cloning of its cDNA. Biosci Biotechnol Biochem 73:2007–2013
Hossain MZ, Hossain MD, Fujita M (2006) Induction of pumpkin glutathione S-transferase by different stresses and its possible mechanisms. Biol Plant 50:210–218
Hossain A, Teixeira da Silva JA (2013) Wheat production in Bangladesh: its future in the light of global warming. AoB Plants 5:pls042
Iqbal N, Umar S, Khan NA, Corpas FJ (2021) Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) Plants. Antioxidants 10:108
Islam MT, Croll D, Gladieux P, Soanes DM, Persoons A, Bhattacharjee P, Hossain MS, Gupta DR, Rahman MM, Mahboob MG, Cook N, Salam MU, Surovy MZ, Sancho VB, Maciel JLN, NhaniJúnior A, Castroagudín VL, de Assis Reges JT, Ceresini PC, Ravel S, Kellner R, Fournier E, Tharreau D, Lebrun M-H, McDonald BA, Stitt T, Swan D, Talbot NJ, Saunders DGO, Win J, Kamoun S (2016) Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol 14:84. https://doi.org/10.1186/s12915-016-0309-7
Islam MT, Gupta DR, Hossain A, Roy KK, He X, Kabir MR, Singh PK, Khan MAR, Rahman M, Wang GL (2020) Wheat blast: a new threat to food security. Phytopathol Res 2:28. https://doi.org/10.1186/s42483-020-00067-6
Jagadish SVK (2020) Heat stress during flowering in cereals-Effects and adaptation strategies. New Phytol 226:1567–1572
Khatun S, Ahmed JU, Mohi-Ud-Din M (2015) Variation of wheat cultivars in their relationship between seed reserve utilization and leaf temperature under elevated temperature. J Crop Sci Biotechnol 18:97–101
Li Q, Wang W, Wang W, Zhang G, Liu Y, Wang Y, Wang W (2018) Wheat F-box protein gene TaFBA1 is involved in plant tolerance to heat stress. Front Plant Sci 9:521
Lobell DB, Sibley A, Ortiz-Monasterio JI (2012) Extreme heat effects on wheat senescence in India. Nat Clim Chang 2:186–189
Majláth I, Szalai G, Soós V, Sebestyén E, Balázs E, Vanková R, Dobrev PI, Tari I, Tandori J, Janda T (2012) Effect of light on the gene expression and hormonal status of winter and spring wheat plants during cold hardening. Physiol Plant 145:296–314
Mishra A, Jha B, Dubey RS (2011) Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 248:565–577
Mohi-Ud-Din M, Siddiqui N, Rohman M, Jagadish SVK, Ahmed JU, Hassan MM, Hossain A, Islam T (2021b) Physiological and biochemical dissection reveals a trade-off between antioxidant capacity and heat tolerance in bread wheat (Triticum aestivum L.). Antioxidants 10:351
Mohi-Ud-Din M, Talukder D, Rohman M, Ahmed JU, Jagadish SVK, Islam T, Hasanuzzaman M (2021a) Exogenous application of methyl-jasmonate and salicylic acid mitigates drought-induced oxidative damages in french bean (Phaseolus vulgaris L.). Plants 10:2066. https://doi.org/10.3390/plants10102066
Mottaleb KA, Govindan V, Singh PK, Sonder K, He X, Singh RP, Joshi AK, Barma NCD, Kruseman G, Erenstein O (2019) Economic benefits of blast-resistant biofortified wheat in Bangladesh: the case of BARI Gom 33. Crop Prot 123:45–58
Narayanan S, Tamura PJ, Roth MR, Prasad PVV, Welti R (2016) Wheat leaf lipids during heat stress: I. high day and night temperatures result in major lipid alterations. Plant Cell Environ 39:787–803
Narendra MC, Roy C, Kumar S, Virk P, De N (2021) Effect of terminal heat stress on physiological traits, grain zinc and iron content in wheat (Triticum aestivum L.). Czech J Genet Plant Breed 57:43–50
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304
Noctor G, Mhamdi A, Foyer CH (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164:1636–1648
Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ 39:1140–1160
Pandey S, Fartyal D, Agarwal A, Shukla T, James D, Kaul T, Negi YK, Arora S, Reddy MK (2017) Abiotic stress tolerance in plants: myriad roles of ascorbate peroxidase. Front Plant Sci 8:581
Pastenes C, Horton P (1999) Resistance of photosynthesis to high temperature in two bean varieties (Phaseolus vulgaris L.). Photosynth Res 62:97–203
Prasad PVV, Djanaguiraman M (2014) Response of floret fertility and individual grain weight of wheat to high temperature stress: sensitive stages and thresholds for temperature and duration. Funct Plant Biol 41:1261–1269
Principato GB, Rosi G, Talesa V, Govannini E, Uolila L (1987) Purification and characterization of two forms of glyoxalase II from rat liver and brain of Wistar rats. Biochem Biophys Acta 911:349–355
Sairam RK, Deshmukh PS, Shukla DS (1997) Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci 178:171–178
Singh D, Wang X, Kumar U, Gao L, Noor M, Imtiaz M, Singh RP, Poland J (2019) High-throughput phenotyping enabled genetic dissection of crop lodging in wheat (Triticum aestivum). Front Plant Sci 10:394. https://doi.org/10.3389/fpls.2019.00394
Spitz DR, Oberley LW (1989) An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem 179:8–18
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Tao X, Wang M-X, Dai Y, Wang Y, Fan Y-F, Mao P, Ma X-R (2017) Identification and expression profile of CYPome in perennial ryegrass and tall fescue in response to temperature stress. Front Plant Sci 8:1519
Tiwari YK, Yadav SK (2019) Effect of high-temperature stress on ascorbate–glutathione cycle in maize. Agric Res 9:179–187
Valadez-Bustos MG, Aguado-Santacruz GA, Tiessen-Favier A, Robledo-Paz A, Munoz-Orozco A, Rascon-Cruz Q, Santacruz-Varela A (2016) A reliable method for spectrophotometric determination of glycine betaine in cell suspension and other systems. Anal Biochem 498:47–52
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang GP, Zhang XY, Li F, Luo Y, Wang W (2010) Overaccumulation of glycine betaine enhances tolerance to drought and heat stress in wheat leaves in the protection of photosynthesis. Photosynthetica 48:117–126
Wild R, Ooi L, Srikanth V, Munch G (2012) A quick convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal Bioanal Chem 403:2577–2581
Williamson VM, Thomas V, Ferris H, Dubcovsky J (2013) An Aegilops ventricosa translocation confers resistance against root-knot nematodes to common wheat. Crop Sci 53:1412–1418
Yang S-H, Wang L-J, Li S-H (2007) Ultraviolet-B irradiation-induced freezing tolerance in relation to antioxidant system in winter wheat (Triticum aestivum L.) leaves. Environ Exp Bot 60:300–307
Zhao C, Liu B, Piao S, Wang X, Lobell DB, Huang Y, Huang M (2017) Temperature increase reduces global yields of major crops in four independent estimates. Proc Nat Acad Sci U S A 114:9326–9331
Acknowledgements
We gratefully acknowledge the financial support of the Research Management Wing (RMW), Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh. We also extend our thanks to Molecular Breeding Laboratory, Bangladesh Agricultural Research Institute, Gazipur, Bangladesh for providing laboratory facilities during the research work.
Author information
Authors and Affiliations
Contributions
Mohammed Mohi-Ud-Din: investigation, visualization, software, writing — original draft preparation. Md. Motiar Rohman: data curation, methodology, investigation. Md. Ashraful Alam: investigation. Mirza Hasanuzzaman: methodology, writing — original draft preparation, writing — reviewing and editing. Tofazzal Islam: conceptualization, supervision, writing — reviewing and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Bhumi Nath Tripathi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohi-Ud-Din, M., Rohman, M.M., Alam, M.A. et al. Wheat variety carrying 2NvS chromosomal segment provides yield advantage through lowering terminal heat–induced oxidative stress. Protoplasma 260, 63–76 (2023). https://doi.org/10.1007/s00709-022-01759-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-022-01759-w