Carrot is a root crop consumed worldwide and has great nutritional qualities. It is considered as one of the ten most important vegetable crops. Cytokinins are an essential class of the plant hormones that regulate many processes of plant growth. Till now, the effects of cytokinin, BAP, on lignin biosynthesis and related gene expression profiles in carrot taproot is unclear. In order to investigate the effect of applied BAP on lignin-related gene expression profiles, lignin accumulation, anatomical structures, and morphological characters in carrot taproots. Carrot roots were treated with different concentrations of BAP (0, 10, 20, and 30 mg L−1). The results showed that the application of BAP significantly increased plant length, shoot fresh weight, root fresh weight, and taproot diameter. In addition, BAP at 20 mg L−1 or 30 mg L−1 enhanced the average number of petioles. BAP treatment led to increased number and width of xylem vessels. The parenchyma cell numbers of pith were significantly induced in taproots treated with the BAP at a concentration of 30 mg L−1. BAP significantly upregulated most of the expression levels of lignin biosynthesis genes, caused elevated lignin accumulation in carrot taproots. Our results indicate that BAP may play important roles in growth development and lignification in carrot taproots. Our results provide a valuable database for more studies, which may focus on the regulation of root lignification via controlling cytokinin levels in carrot taproots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carrot (Daucus carota L.), is a root vegetable crop of family Apiaceae, one of the most important vegetable crops based on the global production records of the main vegetables. Carrot taproot is rich in pro-vitamin A, carotenoids, anthocyanins, dietary fiber, and other nutrients (Que et al. 2019; Li et al. 2020; Wang et al. 2020c). Carrot crop is widely grown around the world. The global cultivated area of carrots and turnips was about 1.3 million hectares, while world production was around 40 million tons. China tops the world production rankings, with the production of both carrots and turnip about 17.9 million tons (FAO, 2018). Carrot taproots are used in various ways, particularly as raw or cooked vegetables, an alternative source of bioethanol and the food industries (Aimaretti et al. 2012; Que et al. 2019).
Plant hormones are a class of natural organic substances that regulate biological processes at low doses (Davies 2010; Wani et al. 2016). Cytokinins are important plant hormones that play a vital role in the regulation of cell division, proliferation and differentiation, seed germination, photomorphogenesis, shoot promoting, and pushing floral initiation (Werner and Schmulling 2009; Girgžde and Samsone 2017; Wang et al. 2020a, b). In the shoot of carrot, the main cytokinins were classified as 2-isopentenyladenosine and 2-isopentenyladenine, while, in the root, only 2-isopentenyladenin was observed in the root. The production of these two cytokinins increased with the growth of the taproot. The endogenous cytokinins increased with taproot development (Stiebeling and Neumann 1987). The fresh and dry weight of the carrot taproots appeared to be positively related to the activity of cytokinins. The increased growth of the carrot taproot diameter is positively correlated with the endogenous cytokinin level (Stiebeling and Neumann 1987). Cytokinins may play critical roles in the second growth phase (40 days after sowing) of carrot (Wang et al. 2015). BAP is very active and stable in aqueous solutions. In addition, it is simply absorbed into the plant tissues and speedily metabolized than the other cytokinins (Bertell and Eliasson 1992). In carrot and radish, BAP induced cortex cell division and promoted parenchyma cell numbers leading to the increased root diameter (Webster and Radin 1972; Stiebeling et al. 1987).
Lignin is considered as a complex polymer of phenylpropanoids, which is the second most popular biopolymer after the cellulose in vascular plants (Boerjan et al. 2003). Lignin biosynthesis in plants occurs through the two-stage process. Firstly, the synthesis of three hydroxycinnamyl alcohol monomers (p-coumaryl, coniferyl, and sinapyl alcohols) occurs via the phenylpropanoid pathway. After then, the peroxidase and laccase enzyme induce the formation of lignin polymers by using these three hydroxycinnamyl alcohol monomers as substrate. This process is termed as lignin polymerization. Throughout this process, the formation of p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units take place (Boerjan et al. 2003). Lignin is fundamentally accumulated in the secondary cell wall, which interacts with environmental conditions, including mechanical accidents and stresses (Rogers and Campbell 2004; Zhao and Dixon 2011). The primary role of lignin is providing mechanical support and water transport within plant tissues (Somerville et al. 2004). Nevertheless, the quality of the carrot gets affected by excessive lignin accumulation in carrot taproot (Que et al. 2019; Khadr et al. 2020). BAP elevated lignification and upregulate the expression level of several key genes related to lignin biosynthesis (Ogita et al. 2012, 2018).
The objective of the present study is to investigate the effect of BAP on carrot plant growth and also the lignin content and expression levels of related genes. This study provides a potential useful reference for using BAP to improve carrot taproots growth.
Materials and methods
Plant materials
In the present study, a carrot cultivar (Kurodagosun, deposited in State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University) was used as plant materials. The carrot seeds were germinated on wet filter paper for 48 h at 25 °C in full darkness for further experiments. The resulting plants were then moved into plastic pots (30 × 27 cm) in a greenhouse and maintained at 22 ± 3 °C and 18 ± 3 °C (day and night, respectively) with 65–75% humidity under a 16/8 h photoperiod. The plants were cultivated in a mixture of organic soil and vermiculite (1:1, v/v). Different concentrations of BAP solutions (0, 10, 20, and 30 mg L−1) (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) were prepared by dissolving in ethyl alcohol then diluted with distilled water. After the 40 days of sowing of carrot seeds, the soil mixture was sprayed with respective concentrations of BAP, four times at 3-day intervals. Control plants were sprayed with water without BAP. Carrot samples were harvested for analysis at 60 days after sowing.
Anatomical structure analysis
The BAP effects on the anatomical structure of carrot taproot were examined using the previously described method (Vazquez-Cooz and Meyer 2002; Wang et al. 2017; Zhou et al. 2018). Briefly, taproots cross-sections from control as well as BAP treated taproot (10, 20, and 30 mg L−1) were collected and kept in formalin-aceto-alcohol solution (FAA) for 24 h. Then they were washed three times (1 h each) with ethyl alcohol (75%) to remove FAA solution residues. Afterward, the root sections were dehydrated in a graded series of ethyl alcohol (75%, 85%, 95%, and 100%) at 1 h in each concentration. Then, root sections were cleared with ethanol: xylene (1:1, v/v) solution for 1 h, led by 2 stages of separate xylene baths for 30 min each. Fixed sections were placed in a xylene: paraffin (1:1, v/v) combination for 2 h, then two times in paraffin for 30 min each, heated to 58–65 °C, dropped in paraffin and cooled in water for 10 min. Thereafter, the sample-embedded paraffin block was placed on a glass plate and fixed on the sample stage with a heated dissection knife. Samples were sliced to (5 μm) sections using a Leica ultramicrotome cutting machine. Sample slices were spread on glass slides. Subsequently, the samples were prepared for staining with 1% safranin O for 2 h and counterstained with 0.5% fast green for 15 s. Sample slides were prepared and analyzed for then subjected to microscopy analysis using a light microscope (Olympus brand, CellSens software).
Quantitative determination of lignin content
The previously described methods by Cervilla et al. (2009) and Wang et al. (2017) were followed in lignin extraction and quantification. The carrot taproot samples were prepared with three independent biological replicates. Briefly, root sample (2.0 g) was grounded with a mortar and pestle in liquid nitrogen to be powder, then immediately homogenized in 6 mL of 99.7% ethanol in centrifuge tubes. The individual sample was then centrifuged for 20 min at 12,000×g. The supernatant was removed, and the sediment was collected and dried at room temperature overnight.10 mg of the air-dried residue was placed in a fresh centrifuge tube. Then, 1 mL of 2 M hydrochloric acid (HCl) and 0.1 mL of thioglycolic acid was added into the tube. The reaction tube was left in a water bath at 100 °C for 8 h and cooled on ice, then centrifuged at 12,000×g for 20 min at 4 °C. The precipitate was washed with 1 mL of sterile distilled water and dissolved in 1 mL of 1 M sodium hydroxide (NaOH), followed by 18 h in a shaker-incubator at 25 °C and then centrifuged for 20 min. The supernatant was collected and transferred to a clean tube with 1 mL of concentrated HCl and was incubated at 4 °C for 6 h to precipitate lignin thioglycolic acid. After the final 20 min of centrifuging, the sediment was resuspended in 1 mL of 1 M sodium hydroxide, also sodium hydroxide as blank. The absorption was detected at 280 nm using SpectraMax iD5, Soft Max Pro7 Software (Molecular Devices, Shanghai, China). The content of lignin is expressed as OD280 mg g−1 dry weight (DW).
Total RNA extraction and cDNA synthesis
Total RNA from individual carrot taproot samples of control, as well as samples treated with different concentrations of BAP was extracted using an RNA extraction kit (Tiangen, Beijing, China). Quantification of total RNA concentration in the sample was performed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA). The total RNA was treated with gDNAEraser (TaKaRa, Dalian, China) for 2 min at 42 °C, to remove the DNA contamination from samples. Synthesis of cDNA from total RNA obtained in respective samples was carried out using PrimeScriptRT reagent Kit (TaKaRa, Dalian, China).
Gene selection and expression levels analysis by RT-qPCR
The genes involved in lignin biosynthesis and polymerization were chosen according to the references from our published paper (Wang et al. 2017) based on a genomic and transcriptomic database Carrot DB (http://apiaceae.njau.edu.cn) (Xu et al. 2014). Real time quantitative PCR (RT-qPCR) experiment was performed using TaKaRa SYBR Premix Ex Taq (TaKaRa, Dalian, China) to quantify the transcript expression in control as well as BAP treated carrot plant samples. The primers used for RT-qPCR assays of DcPAL, DcC4H, Dc4CL, DcHCT, DcC3’H, DcCCoAOMT, DcF5H, DcCOMT, DcCCR, DcCAD, DcPER1, DcPER2, DcLAC1, and DcLAC2 were selected according to the references from our previously published articles (Wang et al. 2017). All PCR reaction mixtures comprised 10 μL of SYBR Premix Ex Taq, 7.4 μL of deionized water, 0.4 μL of each forward and reverse primer, and 2 μL of diluted cDNA strand. PCR cycle was set up to run initially 30 s at 95 °C followed by 40 cycles at 95 °C for 5 s, and 60 °C for 30 s. The relative mRNA was determined by following the method of Tian et al. (2015) trough normalizing the threshold cycle number of each gene to those of reference gene DcActin and DcTUB. Furthermore, utilizing delta-delta CT method, gene expressions were computed from the threshold cycle.
Protein-protein interaction network analysis
We analyzed the protein-protein interaction network based on the STRING database (https://string-db.org/) for understanding the interactions of enzymes relevant to lignin biosynthesis (Franceschini et al. 2013).
Statistical analysis
The experimental data were based on a completely randomized design set up with five replications. The recorded data was determined using one-way ANOVA. The difference in the mean data of the treatments and control was analyzed at the 0.05 significance level by Duncan’s method using the SPSS statistics software (Version 16, SPSS Inc. Chicago, USA). Correlations between lignin content and expression levels of genes involved in the lignin biosynthesis were determined by Pearson’s correlation coefficient (r) analysis. Significance was determined at P < 0.05 and P < 0.01.
Results
Morphological changes in carrot plant in response to BAP treatment
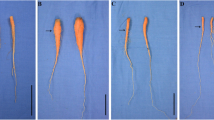
To determine the effect of BAP on carrot growth, 40-day-old carrot plants were treated with BAP. BAP enhanced the shoot growth and the taproot diameters of the treated carrot plants than the control (Fig. 1). Six morphological parameters, including plant length, root length, shoot fresh weight, root fresh weight, the number of petioles, and root diameter of carrot plants were determined for comparing amongst the different treatments. BAP was significantly increased the total plant length, but there was no significant effect on the root length of the carrot. The highest plant length was 77.5 cm, achieved in plants treated with 20 mg L−1 BAP (Fig. 2). Further, the treatment with BAP significantly increased the fresh weight of both the shoot and the taproot compared with the control. The highest shoot fresh weight was 15.34 g under treatment with 20 mg L−1.
Similarly, the highest average single fresh weight of taproot was 20 g at 20 mg L−1 BAP treatment. There is no significant variance between the treatment of 20 mg L−1 and 30 mg L−1 BAP (Fig. 3). Under 20 mg L−1 or 30 mg L−1 BAP treatments, the numbers of petioles were 1.45- and 1.23-fold than that of the control, respectively. There was no significant difference between the 10 mg L−1 BAP and the control (Fig. 4). The taproot diameter of the carrot was significantly increased under 10 mg L−1, 20 mg L−1, or 30 mg L−1 BAP treatments than that of control (Fig. 5).
Effect of BAP on the anatomical structure in carrot taproots
The transverse sections of taproots treated with different concentrations of BAP and the control were taken and stained with safranin O and fast green to highlight the basic anatomical structure of carrot taproots (Fig. 6). It was observed that the treatment with BAP enhanced the number and width of root xylem vessels comparing with the control. The parenchyma cell numbers in pith were significantly increased under 30 mg L−1 BAP treatment than control.
Effect of BAP on the lignin accumulation of carrot taproots
Lignin accumulation in carrot taproots under different treatments was determined. BAP significantly enhanced lignin content in carrot taproots (Fig. 7). The lignin content in the control was 18.52 mg g−1. The lignin contents were 20.61, 23.12, and 33.83 mg g−1 in taproots treated with 10, 20, and 30 mg L−1 BAP, respectively.
Influence of BAP on the expression level of key genes involved in lignin biosynthesis and polymerization
BAP involved in regulating lignin biosynthesis in the taproot of carrot. The accumulation of lignin in BAP-treated carrot taproots may be due to changed expression of the genes. To find out the molecular basis for the role of BAP in lignin biosynthesis and polymerization in carrot roots, the RT-qPCR was used for detecting the expression profiles of the selected genes. Nucleotide sequences of key enzymes related to lignin biosynthesis were obtained from the CarrotDB database (Xu et al. 2014). As shown in Figs. 8 and 9, BAP significantly upregulated most of lignin-related genes expression levels, including DcPAL, DcC4H, Dc4CL, DcHCT, DcF5H, DcCOMT, DcCCR, DcCAD, DcPER1, and DcLAC1. The expression levels of DcC3’H showed 1.64- and 14.68-fold increases as compared with the control under 20 mg L−1 or 30 mg L−1 BAP treatment, respectively. In the same trend, the expression levels of DcCCoAOMT increased due to 20 mg L−1 and 30 mg L−1 BAP, about 5.91- and 9.35-fold that of the control. There was no significant difference in DcLAC2 expression pattern under both 10 mg L−1 and 20 mg L−1 BAP treatments and the control. The expression pattern peaked at the carrot taproots-treated with 30 mg L−1 BAP. On the other hand, the expression pattern of DcPER2 showed dramatic downregulation, which exhibited the contrast trend of DcPER1.
The correlation between expression of genes related in the lignin biosynthesis polymerization and lignin accumulation in carrot taproots has been also analyzed (Table 1). DcCOMT, DcLAC2, DcC3’H, DcCCoAOMT, DcC4H, DcPAL, DcHCT, DcLAC1, and Dc4CL expression profiles showed significant positive correlations with the content of lignin in carrot taproots (P < 0.01; r = 0.992, 0.972, 0.969, 0.914, 0.908, 0.905, 0.887, 0.800, and 0.795, respectively). The expression profiles of DcF5H and DcCAD showed close correlations with the content of lignin in carrot taproots (P < 0.05; r = 0.651 and 0.645, respectively).
Protein-protein interaction analysis
String network analysis between lignin biosynthesis-involved proteins and polymerization-involved proteins was realized (Fig. 10). Arabidopsis thaliana has been selected as the basis for the orthologs. In the interaction network map, most lignin-involved proteins showed interactions with multiple Arabidopsis proteins, particularly OMT1, which interacted with all lignin-involved proteins except LAC3 and LAC11. Otherwise, LAC3 only interacted with RCI3. Moreover, it was found that PAL1 and C4H had the top score of interactions (0.997), whereas the interaction score of LAC3 and RCI3 was the lowest. Line numbers reflect the degree of closeness between the proteins. DcCOMT interacted with eleven lignin biosynthesis–related enzymes, including Dc4CL, DcF5H, DcC3’H, DcHCT, DcPAL, DcCCoAOMT, DcC4H, DcCAD, DcPER1, DcPER2, and DcCCR. In addition, DcPAL, Dc4CL, and DcHCT interacted with nine proteins. On the other hand, DcLAC1 only interacted with DcPER2.
Discussion
In our study, carrot plants were treated with different concentrations of BAP to examine the effects on lignin biosynthesis and related gene expression levels in carrot. The results of the current study exhibited that BAP increased plant length and shoot weight. Also, BAP enhanced the number of petioles under concentration 20 and 30 mg L−1 compared with the control. This result is consistent with the results of previous studies which observe that applied cytokinin to promote the shoot growth, shoot branching, the thickness of leaves, and shoot fresh weight (Stiebeling et al. 1987; Pathak et al. 2006; Shimizu-Sato et al. 2009; Sajjad et al. 2014; Yasmeen et al. 2014). BAP significantly enhanced the root diameter and the root fresh weight. It seems that BAP promotes the biosynthesis of endogenous cytokinin and stimulates the growth of the cortical cell, causing increased root diameter, thereby, increasing the root fresh weight of carrots (Stiebeling and Neumann 1987; Stiebeling et al. 1987). In addition, the increment of the taproot diameter may due to swelling in the root elongation zone. This swelling is owing to the extreme expansion of cortex cells in response to the exogenous cytokinin (Svensson 1972; Kappler and Kristen 1986; Bertell et al. 1990). On the other hand, no significant effect on the root length has been found. A similar result has been reported on the carrot taproot (Stiebeling et al. 1987).
Cytokinin alone does not induce the vascular tissue, but it promotes vascular differentiation and regeneration in the existence of the indole-3-acetic acid (IAA) (Aloni 1995). Cytokinin stimulates the division of cells into vascular tissues (Roberts et al. 2012), which is limiting and regulating factor in tissue culture, leading to increases in the number of xylem vessels (Aloni 1982). Cytokinins boost the activity of vascular cambium, thereby enhance vascular regeneration via raising the xylem vessels number (Baum et al. 1991; Aloni 1995; Aloni et al. 2006). BAP increased the number of parenchyma cells of storage tissue in both carrot and radish roots (Webster and Radin 1972; Stiebeling et al. 1987). In the same trend of these mention findings, we also observed an enhancement in the number and width of xylem vessels in the higher levels of BAP-treated roots compared with the control. Also, BAP at 30 mg L−1 promoted the parenchyma cells in the pith.
BAP increased lignin content of carrot in a dose-dependent manner. This result is in agreement with the findings of the previous study, which demonstrated that BAP promoted the lignification in bamboo (Ogita et al. 2012). Former studies have shown that alterations in lignin content are followed by a changed expression of the genes encoding the lignin pathway enzymes (Ali and McNear 2014; Wang et al. 2017; Wang et al. 2018; Duan et al. 2019). Therefore, we have studied the correlation between lignin content accumulation in carrot root tissue and the transcription pattern of genes involved in lignin biosynthesis and polymerization pathway. In the present study, most of the studied key genes involved in the lignin synthesis pathway, including DcPAL, DcC4H, Dc4CL, DcHCT, DcF5H, DcCOMT, DcCCR, DcCAD, DcLAC1, and DcPER1 showed significant upregulation in response to the BAP treatment as compared with control. In addition, DcC3’H and DcCCoAOMT expression profiles showed significantly increased under 20 mg L−1 and 30 mg L−1 BAP. DcLAC2 upregulated under 30 mg L−1 BAP, while downregulated at 10 mg L−1 and 20 mg L−1 BAP. However, the DcPER2 expression level significantly decreased, showing the opposite trend to DcPER1 (Fig. 11).
Our results confirm the earlier reports, which demonstrated that lignin accumulation increased owing to the expression level increase of key genes in the lignin synthetic pathway. The genes, PAL, 4CL, C4H, CAD, CCoAOMT, and CCR, were positively correlated with lignin accumulation in common buckwheat (Hu et al. 2015). In addition, PAL, C4H, 4CL1, F5H, and CCoAOMT were the potential key lignin-related genes in ramie (Tang et al. 2019), as well as CCoAOMT, 4CL, COMT, and F5H in the xylem of flax (Wang et al. 2009). Ogita and his colleges found that BAP application induced the lignification and the expression levels of lignin-involved genes (Ogita et al. 2012, 2018). In our study, we found positive and significant correlations between the expression of selected lignin-related genes and lignin content in carrot taproots. Similarly, prior studies have shown the physiological functions of most key genes related to lignin biosynthesis have a positive correlation with the accumulation and composition of lignin (Simmons et al. 2010; Vanholme et al. 2010).
In the current study, BAP treatment changed the morphological characters, anatomical structure, lignin content, and the expression profiles of genes related to lignin biosynthesis in carrot plants. The treatment of BAP at 20 mg L−1 could be recommended to enhance the growth parameters of the carrot taproots.
Abbreviations
- 4CL:
-
4-Coumarate-CoA ligase
- BAP:
-
6-Benzylaminopurine
- C3’H:
-
p-Coumaroyl shikimate/quinate 3-hydroxylase
- C4H:
-
Cinnamate 4-hydroxylase
- CAD:
-
Cinnamyl alcohol dehydrogenase
- CCoAOMT:
-
Caffeoyl-CoA O-methyltransferase
- CCR:
-
Cinnamoyl-CoA reductase
- COMT:
-
Caffeic acid O-methyltransferase
- DW:
-
Dry weight
- F5H:
-
Ferulate 5-hydroxylase
- HCT:
-
Hydroxycinnamoyl-CoA shikimate/quinate
- LAC:
-
Laccase
- PAL:
-
Phenylalanine ammonia lyase
- PER:
-
Peroxidase
- RT-qPCR:
-
Real-time quantitative PCR
References
Aimaretti NR, Ybalo CV, Rojas ML, Plou FJ, Yori JC (2012) Production of bioethanol from carrot discards. Bioresour Technol 123:727–732
Ali MB, McNear DH (2014) Induced transcriptional profiling of phenylpropanoid pathway genes increased flavonoid and lignin content in Arabidopsis leaves in response to microbial products. BMC Plant Biol 14(1):1–14
Aloni R (1982) Role of cytokinin in differentiation of secondary xylem fibers. Plant Physiol 70(6):1631–1633
Aloni R (1995) The induction of vascular tissues by auxin and cytokinin. Plant hormones. Springer, In, pp 531–546
Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot 97(5):883–893
Baum FS, Aloni R, Peterson CA (1991) Role of cytokinin in vessel regeneration in wounded Coleus internodes. Ann Bot 67(6):543–548
Bertell G, Bolander E, Eliasson L (1990) Factors increasing ethylene production enhance the sensitivity of root growth to auxins. Physiol Plant 79(2):255–258
Bertell G, Eliasson L (1992) Cytokinin effects on root growth and possible interactions with ethylene and indole-3-acetic acid. Physiol Plant 84(2):255–261
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54(1):519–546
Cervilla L, Rosales M, Rubio-Wilhelmi M, Sánchez-Rodríguez E, Blasco B, Ríos J, Romero L, Ruiz J (2009) Involvement of lignification and membrane permeability in the tomato root response to boron toxicity. Plant Sci 176(4):545–552
Davies PJ (2010) The plant hormones: their nature, occurrence, and functions. Plant hormones. Springer, In, pp 1–15
Duan AQ, Feng K, Wang GL, Liu JX, Xu ZS, Xiong AS (2019) Elevated gibberellin enhances lignin accumulation in celery (Apium graveolens L.) leaves. Protoplasma 256(3):777–788
Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C (2013) STRING v9. 1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res 41(D808)
Food and Agriculture Organization of the United Nations (FAO) (2018) FAOSTAT Statistic Database. http://www.fao.org/faostat/en/#data/QC/visualize
Girgžde E, Samsone I (2017) Effect of cytokinins on shoot proliferation of silver birch (Betula pendula) in tissue culture. Environ Exp Biol 15(1):1–5
Hu D, Liu X, Wang C, Yang H, Li H, Ruan R, Yuan X, Yi Z (2015) Expression analysis of key enzyme genes in lignin synthesis of culm among different lodging resistances of common buckwheat (Fagopyrum esculentum Moench). Sci Agric Sin 48:1864–1872
Kappler R, Kristen U (1986) Exogenous cytokinins cause cell separation and cell expansion in the root tip cortex of Zea mays. Bot Gaz 147(3):247–251
Khadr A, Wang Y, Que F, Li T, Xu Z, Xiong A (2020) Exogenous abscisic acid suppresses the lignification and changes the growth, root anatomical structure and related gene profiles of carrot. Acta Biochim Biophys Sin 52(1):97–100
Li T, Huang Y, Khadr A, Wang YH, Xu ZS, Xiong AS (2020) DcDREB1A, a DREB-binding transcription factor from Daucus carota, enhances drought tolerance in transgenic Arabidopsis thaliana and modulates lignin levels by regulating lignin-biosynthesis-related genes. Environ Exp Bot 169:103896
Ogita S, Nomura T, Kato Y, Uehara-Yamaguchi Y, Inoue K, Yoshida T, Sakurai T, Shinozaki K, Mochida K (2018) Transcriptional alterations during proliferation and lignification in Phyllostachys nigra cells. Sci Rep 8(1):1–11
Ogita S, Nomura T, Kishimoto T, Kato Y (2012) A novel xylogenic suspension culture model for exploring lignification in Phyllostachys bamboo. Plant Methods 8:40
Pathak M, Singh B, Sharma A, Agrawal P, Pasha SB, Das HR, Das RH (2006) Molecular cloning, expression, and cytokinin (6-benzylaminopurine) antagonist activity of peanut (Arachis hypogaea) lectin SL-I. Plant Mol Biol 62(4):529–545
Que F, Hou XL, Wang GL, Xu ZS, Tan GF, Li T, Wang YH, Khadr A, Xiong AS (2019) Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic Res 6(1):1–15
Roberts LW, Gahan PB, Aloni R (2012) Vascular differentiation and plant growth regulators. Springer Science & Business Media
Rogers LA, Campbell MM (2004) The genetic control of lignin deposition during plant growth and development. New Phytol 164(1):17–30
Sajjad Y, Jaskani MJ, Ashraf MY, Qasim M, Ahmad R (2014) Response of morphological and physiological growth attributes to foliar application of plant growth regulators in gladiolus ‘white prosperity’. Pak J Agric Sci 51(1):123–129
Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin–cytokinin interactions in the control of shoot branching. Plant Mol Biol 69:429–435
Simmons BA, Loqué D, Ralph J (2010) Advances in modifying lignin for enhanced biofuel production. Curr Opin Plant Biol 13:312–319
Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T (2004) Toward a systems approach to understanding plant cell walls. Science 306:2206–2211
Stiebeling B, Neumann KH (1987) Identification and concentration of endogenous cytokinins in carrots (Daucus carota L.) as influenced by development and a circadian rhythm. J Plant Physiol 127:111–121
Stiebeling B, Pauler B, Neumann KH (1987) The influence of 6-BA application on yield, phosphorus and nitrogen uptake and endogenous cytokinin concentrations in carrots (Daucus carota L.) grown in phosphorusor nitrogendepleted soil. J Plant Nutr Soil Sci 150:69–74
Svensson SB (1972) A comparative study of the changes in root growth, induced by coumarin, auxin, ethylene, kinetin and gibberellic acid. Physiol Plant 26:115–135
Tang Y, Liu F, Xing H, Mao K, Chen G, Guo Q, Chen J (2019) Correlation analysis of lignin accumulation and expression of key genes involved in lignin biosynthesis of ramie (Boehmeria nivea). Genes 10(5):389
Tian C, Jiang Q, Wang F, Wang GL, Xu ZS, Xiong AS (2015) Selection of suitable reference genes for qPCR normalization under abiotic stresses and hormone stimuli in carrot leaves. PLoS One:10(2)
Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153:895–905
Vazquez-Cooz I, Meyer R (2002) A differential staining method to identify lignified and unlignified tissues. Biotech Histochem 77:277–282
Wang GL, Que F, Xu ZS, Wang F, Xiong AS (2017) Exogenous gibberellin enhances secondary xylem development and lignification in carrot taproot. Protoplasma 254:839–848
Wang GL, Sun S, Xing GM, Wu XJ, Wang F, Xiong AS (2015) Morphological characteristics, anatomical structure, and gene expression: novel insights into cytokinin accumulation during carrot growth and development. PLoS One 10(7)
Wang J, Chen X, Gao Y, Zhang Y, Long S, Deng X, He D, Wang Y (2009) Expression of critical lignin metabolism genes in flax (Linum usitatissimum). Acta Agron Sin 35:1468–1473
Wang X, Ding J, Lin S, Liu D, Gu T, Wu H, Trigiano RN, McAvoy R, Huang J, Li Y (2020b) Evolution and roles of cytokinin genes in angiosperms 2: do ancient CKXs play housekeeping roles while non-ancient CKXs play regulatory roles? Hortic Res 7(1):29
Wang X, Lin S, Liu D, Gan L, McAvoy R, Ding J, Li Y (2020a) Evolution and roles of cytokinin genes in angiosperms 1: do ancient IPTs play housekeeping while non-ancient IPTs play regulatory roles? Hortic Res 7(1):28
Wang YH, Li T, Zhang RR, Khadr A, Tian YS, Xu ZS, Xiong AS (2020c) Transcript profiling of genes involved in carotenoid biosynthesis among three carrot cultivars with various taproot colors. Protoplasma 257(3):1–15
Wang YH, Wu XJ, Sun S, Xing GM, Wang GL, Que F, Khadr A, Feng K, Li T, Xu ZS, Xiong AS (2018) DcC4H and DcPER are important in dynamic changes of lignin content in carrot roots under elevated carbon dioxide stress. J Agric Food Chem 66(30):8209–8220
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4(3):162–176
Webster BD, Radin JW (1972) Growth and development of cultured radish roots. Am J Bot 59(7):744–751
Werner T, Schmülling T (2009) Cytokinin action in plant development. Curr Opin Plant Biol 12(5):527–538
Xu ZS, Tan HW, Wang F, Hou XL, Xiong AS (2014) CarrotDB: a genomic and transcriptomic database for carrot. Database 2014
Yasmeen A, Nouman W, Basra SMA, Wahid A, Hussain N, Afzal I (2014) Morphological and physiological response of tomato (Solanum lycopersicum L.) to natural and synthetic cytokinin sources: a comparative study. Acta Physiol 36:3147–3155
Zhao Q, Dixon RA (2011) Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci 16:227–233
Zhou L, Li S, Huang P, Lin S, Addo-Danso SD, Ma Z, Ding G (2018) Effects of leaf age and exogenous hormones on callus initiation, rooting formation, bud germination, and plantlet formation in Chinese fir leaf cuttings. Forests 9:478
Acknowledgments
The research was supported by National Natural Science Foundation of China (31872098), the Open Fund of the State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University (ZW201905) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: ASX, AK. Performed the experiments: AK, YHW, RRZ, and XRW. Analyzed the data: AK, YHW, RRZ, and ZSX. Contributed reagents/materials/analysis tools: ASX. Wrote the paper: AK. Revised the paper: ASX. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing financial interests
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khadr, A., Wang, YH., Zhang, RR. et al. Cytokinin (6-benzylaminopurine) elevates lignification and the expression of genes involved in lignin biosynthesis of carrot. Protoplasma 257, 1507–1517 (2020). https://doi.org/10.1007/s00709-020-01527-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01527-8