Abstract
Protium heptaphyllum is a Burseraceae species known by the production of aromatic resin with medicinal, economic, and ecological values. Information on the development, architecture, and lifetime of the secretory system are crucial to understand the resin production and contribute to a more sustainable tapping regime. We investigated the histology and ultrastructure of the secretory canals under a developmental point of view. Stem samples were analyzed under light and transmission electron microscopy by conventional and cytochemical methods. Secretory canals, originated from procambium and cambium, occurred immersed in the primary and secondary phloem. Mature canals have a secretory epithelium and a wide lumen where the exudate is accumulated. A sheath of parenchyma cells with meristematic features surrounds the epithelium. The canals originate by schizogenesis and develop by schyzolysigenesis. Canals active in secretion occurred since the shoot apex and near the cambium. In the dilation zone of the secondary phloem, secretory canals exhibit sclerified epithelial and sheath cells and are inactive in secretion. Secreting epithelial cells have subcellular apparatus consistent with oleoresin, polysaccharides, and enzymes secretion. Pectinase and cellulase were cytochemically detected in developing canals and are involved in cell wall changes associated to canal growth and release of exudate. In P. heptaphyllum, the secretory system has a complex structure resultant from longitudinal growth, lateral ramification, and fusion of the adjacent canals, in addition to intrusive growth of both epithelial and sheath cells. Although some anatomical results are already known, ultrastructural data represent the novelty of this work. Our findings can contribute to the establishment of more efficient and sustainable techniques for resin extraction in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the substances produced by plants, resin is considered the most versatile material since the Stone Age, going through the pre-industrial era, and reaching the present day (Langenheim 2003). Species of Burseraceae are known for the production of resin and essential oils used in religious ceremonies, medicine, and the production of cosmetics (Souza et al. 2016). The pharmacological properties of their secretions have been proven in the treatment against inflammations, pains, liver diseases, and malaria (Sussunaga 1996; Siani et al. 1999). The presence of canals secreting aromatic resin associated with the phloem is a constitutive characteristic of many Burseraceae species (Metcalfe and Chalk 1950).
Protium heptaphyllum (Aubl.) Marchand., popularly known as breu-branco (Lorenzi 2002), is a tree representative of Burseraceae widely distributed in different ecosystems in Brazil (Matos 1997; Citó et al. 2006; Daly and Fine 2011). The high diversity of terpenes in the aromatic resin of this species provides an effective defense against herbivores and pathogens (Bowers et al. 2001). The exudate from P. heptaphyllum stem is source of raw material to produce medicaments, cosmetics, hygiene products, perfumery, repellents, varnishes, incense, stains, and candles (Revilla 2001). The chemical composition and the medicinal properties of the aromatic resin produced by this species have been exhaustively studied (Lima et al. 2016 and references therein) and its cytotoxicity in breast cancer cells, antimicrobial activity, and its antimutagenicity in vivo have been proven (Lima et al. 2016).
In P. heptaphyllum, secretory canals are filled with a viscous and transparent material where phenolic compounds, alkaloids, essential oils, resin, and mucopolysaccharides was histochemically identified (Souza et al. 2016). Information on the development and architecture of the secretory system, structural variations, and lifetime of the secretory canals are lacking. These data are crucial to understand the resin production and contribute to a more sustainable tapping regime (Tolera et al. 2013).
In this study, we investigated the histology, ultrastructure, and cytochemistry of the secretory canals in P. heptaphyllum stem under a developmental point of view.
Materials and methods
Study site
This study was performed in a Restinga forest (coastal sand dune habitats) located in Itaputiua island (2° 25′ 56″ S; 44° 03′ 30″ W), Raposa city, Maranhão State, Brazil. Based on data from the National Institute of Meteorology (INMET), for the period 2007–2016, the climate in the region is characterized as Aw (tropical with a dry season) following Köeppen, with two distinctive seasons. The rainy season in the site is from January to June and the dry season is from July to December. The mean annual rainfall is 2.100 mm. The mean annual maximum and minimum temperatures are 28 and 18 °C, respectively. The study area is dominated by dystrophic acid sandy soil with high proportion of fine sand.
Plant material
Samples of shoot apex and mature stems were collected from ten adult trees of P. heptaphyllum with about 7 m height with a straight stem and diameter at breast height between 20 and 25 cm. Samples of stem in primary growth were obtained from 0.5 cm below the apex; samples of stem in secondary growth were obtained from the trunk at the breast height.
Vouchers were deposited in the herbarium “Irina Delanova de Gemtchujnicov” (BOTU) of the Department of Botany, Institute of Biosciences of Botucatu (IBB), São Paulo State University (UNESP).
Histology
For histological characterization and ontogenetic analysis, materials were fixed in FAA 50 (formalin/acetic acid/alcohol 50%) for 48 h (Johansen 1940). Samples of shoot apex (2 mm length) and young stems (5 mm length) were dehydrated in ethanol series and embedded in methacrylate resin (Gerrits 1991). Serial cross and longitudinal sections (5 μm thickness) were obtained using a rotary microtome and stained with Toluidine blue pH 4.7 (O’Brien et al. 1964). Stem portions in secondary growth (1.5 cm3) were sectioned (cross, tangential, and radial sections) using a sliding microtome and the sections (15 μm) were dyed in Safrablau (safranin and Astra blue) solution (Bukatsch 1972). Permanent slides were mounted with Entellan® and analyzed under an Olympus BX41 microscope coupled with a digital camera.
Histochemistry
The main classes of metabolite in the secretory canals were investigated in stems (primary and secondary growth). The sections of fresh material were obtained using Ranvier or sliding microtome and treated with the following: Sudan IV (Johansen 1940) and Sudan Black (Pearse 1980) for the detection of total lipids (Johansen 1940), Nadi reagent for terpenes (David and Carde 1964), ferric chloride solution (10%) for phenolic compounds (Johansen 1940), Ruthenium red solution (0.02%) for mucopolysaccharides (Chamberlain 1932),Wagner reagent for alkaloids (Furr and Mahlberg 1981), cupric acetate (10%) for resin (Johansen 1940), and Lugol reagent for starch and alkaloids (Johansen 1940). Control tests were performed according to the authors of the respective techniques. Observations and documentation were carried out using a light microscope (Olympus Cell B) equipped with a digital camera.
Transmission electron microscopy
To conventional analyses, portions of stems in different growth stages were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.3) for 24 h, post-fixed with 1% osmium tetroxide in the same buffer for 1 h, dehydrated through acetone series, and embedded in Araldite resin (Machado and Rodrigues 2004). Ultra-thin sections were post-stained with uranyl acetate and lead citrate (Reynolds 1963). The material was examined with Fei Tecnai transmission electron microscope (TEM) at 80 kV.
For ultracytochemical detection of pectinase, samples were incubated in 0.1 M sodium acetate buffer solution (pH 5.0) containing 0.5% pectin for 20 min at room temperature. For ultracytochemical detection of cellulase, the material was incubated in 0.05 M citrate buffer (pH 4.8) containing 0.02% carboxymethyl cellulose (CMC) for 10 min also at room temperature. Both treatments were transferred to Benedict reagent solution at 80 °C for 10 min and then washed in 0.1 M phosphate buffer. Then, the samples were processed according to conventional protocol described above. The control samples were processed without incubation in pectin and CMC (Bal 1974; Allen and Nessler 1984; Marinho and Teixeira 2016).
Results
Distribution, structure, and histochemistry of the secretory canals
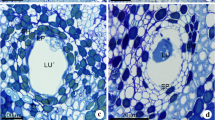
Mature secretory canals were observed immersed in the primary (Fig. 1a) and secondary (Fig. 1b) phloem of the stem. Structurally, in all the samples analyzed, the mature secretory canals were constituted by a uniseriate epithelium surrounding a wide lumen (Fig. 1c) where the secretion accumulates. These canals exhibited spherical lumen in cross section (Fig. 1a–d) and elongated lumen in longitudinal section (Fig. 1e). A discontinuous sheath constituted by one or more layers of tangentially elongated parenchyma cells with meristematic potential surrounded the canals (Fig. 1c, d).
Cross a–d, f–m and longitudinal sections e showing secretory canals in Protium heptaphyllum stem. a Secretory canals (arrows) in the primary phloem. b Secretory canals (arrows) in the secondary phloem. c mature canal with wide lumen (LU) and secretory epithelium (EP) surrounded by a parenchyma sheath (SH). d Secretory canal with trabeculate epithelium. Parenchyma sheath (SH) with meristematic features. e Secretory canal with tapered ends. f Secretory canal in the inner region of the secondary phloem with epithelial cells exhibiting only pecto-cellulosic walls. LU: lumen. g Secretory canal in the median region of the secondary phloem. The arrowhead indicates a sclerified cell in the epithelium. LU: lumen. h Secretory canal in the dilated zone of the secondary phloem constituted exclusively by sclerified epithelial cells. Observe lignified sheath cells. i–m Histochemical tests. i Lipids, j oleoresin, k mucopolysaccharides, l phenolic substances, and m alkaloids. Scale bars: a (150 μm); b, e (100 μm); d, f–h (50 μm); i–m (25 μm); and c (10 nm)
Epithelial cells showed different degrees of swelling and variable shape (Fig. 1c, d). Some epithelial cells were more elongated and protruded into the lumen, originating trabeculae (Fig. 1d). These cells could divide in the anticlinal and oblique planes (Fig. 1c).
In the primary phloem, these cells exhibited pecto-cellulosic walls (Fig. 1c, d). In the stem bark, the epithelial cells showed a progressive lignification of their walls as the canals distanced from the cambium. So, secretory canals in the conductive phloem (adjacent to the cambium) exhibited only epithelial cells with pecto-cellulosic walls (Fig. 1f); in the canals located in the median region of the secondary phloem (non-conductive region), some epithelial cells exhibited thicker and lignified walls (Fig. 1g); canals in the dilated zone of the secondary phloem were constituted exclusively by sclerified epithelial cells characterized by a narrow lumen and even thicker lignified walls with large, ramified pits, resembling a stone cell (Fig. 1h). In this last phloem region, lignified sheath cells were observed surrounding the canals (Fig. 1h).
Total lipids (Fig. 1i), oleoresin (Fig. 1j), mucopolysaccharides (Fig. 1k), and phenolic compounds (Fig. 1l) were histochemically identified in the epithelial cells and lumen of the secretory canals both in the primary and secondary phloem. Alkaloids (Fig. 1m) were detected only in the secretory canals present in the bark.
Ultrastructural characterization of epithelial cells
Secretory canals were active in secretion since their early developmental stages and presented lumen filled with exudate heterogeneous in aspect (Fig. 2a). Epithelial cells had dense cytoplasm, evident nucleus, and small vacuoles (Fig. 2b). The inner tangential cell wall, facing the lumen, was dense (Fig. 2a) and became loose in appearance (Fig. 2b, c). Anticlinal (Fig. 2a) and outer tangential (Fig. 2b) walls had plasmodesmata interconnecting the epithelial cells between themselves and with the sheath cells. The plasmalemma of the epithelial cells was sinuous, mainly in the cell face tangent to the lumen (Fig. 2b, c).
TEM images of the secretory canals in Protium heptaphyllum. a Secretory canal in early developmental stage with lumen (LU) filled with exudate heterogeneous in aspect. Observe plasmodestama (PD) connecting epithelial cells (EP). b Epithelial cell with dense cytoplasm, voluminous nucleus (NU), and small vacuole (VA). LU: lumen, MI: mitochondria. c Part of an epithelial cell showing mitochondria (MI), rough endoplasmic reticulum (RER), plastids (PL), oil drops (OL), and secretory vesicles (VE) filled with dense material. Dense material adhered to the surface of the tangential wall facing the lumen (LU). NU: nucleus, VA: vacuole. d Myelin-like figures (MF), mitochondria (MI), dictyosomes (DI) with numerous adjacent vesicles (VE), and oil drops (OL) in epithelial cell. e Epithelial cell with modified plastids (PL) with dense globules. MI: mitochondria, OL: oil drops. f Part of epithelial cell with smooth endoplasmic reticulum (SER) with dilated cisterns and hyperactive dictyosomes (DI). Vesicles (VE) in the peripheral cytoplasm. g Extensive rough endoplasmic reticulum (RER) and oil drops (OL) in epithelial cell. Scale bars: b–d, f, g (500 nm); a (2 nm); e (1 nm)
The cytoplasm of the epithelial cells was abundant with numerous polysomes, mitochondria (Fig. 2c–e), plastids (Fig. 2c–e), dictyosomes (Fig. 2d, f), extensive rough endoplasmic reticulum (Fig. 2g), and oil droplets spread in the cytoplasm (Fig. 2c–e, g). Plastids were rounded to ovoid in shape, devoid of thylakoids and had electron-dense stroma, and osmiophilic inclusions (Fig. 2c, e). The smooth endoplasmic reticulum presented dilated cisterns, mainly in the ends of their profiles (Fig. 2f). Myelin-like structures, consisted of several concentric membranes packed together in a vesicular compartment, were observed in the peripheral cytoplasm (Fig. 2d). Numerous vesicles were observed in epithelial cells, especially close to the plasma membrane (Fig. 2c–f). Clusters of electron-dense bodies were observed in the cytoplasm, mainly in the cell face adjacent to the lumen (Fig. 3a), in the periplasmic space (Fig. 3b), inside vacuole (Fig. 3a, c), and vesicles (Fig. 3a–c) and immersed in the cell wall facing the lumen (Fig. 2b, c; Fig. 3a–c). Images suggest that these substances crossed the plasma membrane and accumulated in the cell wall tangent to the lumen (Fig. 2b–e; Fig. 3a–c). Subsequently, this material was released into the lumen by a peeling process of cell wall (Fig. 3c).
TEM images of secretory canals in Protium heptaphyllum. a Cluster of electron-dense bodies in the cytoplasm and vacuole of epithelial cell. LU: lumen, VA: vacuole. b Electron-dense material accumulated in the periplasmic space (PS) of epithelial cell. LU: lumen. c Peeling of the tangential cell wall facing the lumen (LU). d Sheath cell (SH) with developed vacuoles (VA) and reduced cytoplasm with amyloplasts (AP). Tapered ends of the sheath cells encircled. EP: epithelial cell. e Detail of the sheath cell showing mitochondria (MI), dictyosomes (DI), rough endoplasmic reticulum (RER), and oil drops (OL).VA: vacuole, EP: epithelium. Scale bars: b, e (500 nm); d (2 nm); a, c (1 nm)
The sheath cells had more developed vacuome and reduced cytoplasm (Fig. 3d). Their plastids presented globular starch grains (Fig. 3d; insert). Mitochondria, rough endoplasmic reticulum, and oil droplets were observed in the cytoplasm (Fig. 3e). The sheath cells were tangentially elongated and acquired tapered ends that penetrated between the radial walls of the neighboring sheath cells, in a way that resembles apical intrusive growth (Fig. 3d).
Origin and development of the secretory canals
Secretory canals at different stages of development were observed side by side in the shoot apex and subjacent regions (Fig. 4a). In the bark, immature secretory canals were observed near the cambium (Fig. 4b); mature canals occurred in the conductive phloem (Fig. 4b), while senescent and non-functional canals occurred in the non-conductive phloem zone (Fig. 1b).
Origin and developmental features of secretory canals in Protium heptaphyllum. a, b, e–g Light microscopy. c, d TEM. a Longitudinal section through the shoot apex showing secretory canals (arrows) at different developmental stages. b Cross section of the stem showing immature secretory canal (arrow) near the cambium. c Precursor cells of the secretory canals. NU: nucleus, PL: plastids. d Cluster of derivative cells. Swollen middle lamella among them. MI: mitochondria, NU: nucleus. e–f Clustered derivative cells arranged around a small triangular intercellular space. g Epithelial cells (EP) arranged around a central lumen (LU). Scale bars: a (150 μm); b (50 μm); e–g (10 μm); c (2 nm); d (1 nm)
The secretory spaces were formed from procambial cells in the shoot apex and from cambial fusiform cells in the bark. The process of lumen formation and development of the secretory canals were similar in both shoot apex and bark. So, in this work, we illustrated the lumen formation and development only in the shoot apex.
Precursor cells of the secretory canals were pyramidal in shape (Fig. 4c) and divided in several planes forming a cluster with four to eight cells (Fig. 4d). The derivative cells (Fig. 4d) had irregular contour, thin walls, voluminous nucleus with nucleolus, abundant cytoplasm rich in mitochondria and plastids, and small vacuoles. The middle lamella among the clustered derivative cells became swollen (Fig. 4d) and a small triangular intercellular space appeared originating the lumen (Fig. 4e). This initial lumen expanded by the progressive dissolution of the middle lamella of the surrounding cells (Fig. 4f, g).
Mature secretory canals continued developing by elongation at their ends (Fig. 5a). The elongating canals were characterized by the irregular contour and diameter of the lumen (Fig. 5b). In longitudinal section, epithelial cells were tangentially elongated and exhibited tapered ends that penetrated between the radial walls of the neighboring epithelial cells (Fig. 5c), in a way that they resembled apical intrusive growth. In the both ends of an elongating canal, some epithelial cells exhibited swollen middle lamella with dissolution signals; this process culminated with the cell release into the lumen (Fig. 5d). Simultaneously, other epithelial cells in the canal ends exhibited signs of lyses such as irregular walls with loose appearance, reduced nucleus with irregular contour and dense lumps of chromatin (Fig. 5e), numerous vacuoles with membranous debris (Fig. 5f), large oil droplets (Fig. 5e, f) and mitochondria irregular in outline exhibiting electron lucent areas and disrupted cristae (Fig. 5e, f). The cell wall tangent to the lumen exhibited continuous peeling and discontinues areas forming hollows (Fig. 5e). Ultracytochemical tests revealed the presence of cellulase (Fig. 5g) and pectinase (Fig. 5h) in these cells walls. This process culminated with rupture of the epithelial cells (Fig. 5b, d).
Longitudinal sections of Protium heptaphyllum primary stem showing developmental aspects of secretory canals. a Light microscopy. b–h TEM. a Mature elongated canals with tapered ends and lumen with irregular width. b Elongating canal with irregular lumen (LU) and ruptured end. EP: epithelium, SH: sheath cell. c Epithelial cells (EP) with tapered ends between the radial walls of the neighboring epithelial cells. SH: sheath cell, LU: lumen. d End of an elongating canal with epithelial cells (EP) exhibiting swollen middle lamella with dissolution signals and ruptured cells. LU: lumen. e Epithelial cell showing irregular walls with loose appearance and discontinues areas forming hollows. Reduced nucleus (NU), vacuoles (VA), mitochondria (MI) with disrupted cristae, plastids (PL), and oil (OL) drop. LU: lumen. f Epithelial cell with nucleus (NU) with irregular contour, mitochondria (MI) with lysis signals of matrix, and vacuoles (VA) with membranous debris. PL: plastid. g–h Ultracytochemical tests for enzymes in the cell walls. g Cellulase. h Pectinase. Scale bars: a (150 μm); g, h (500 nm); b (10 nm); d (5 nm); c, e (2 nm); f (1 nm)
In the ends of the elongating secretory canals, the sheath cells divided periclinally and maintained their intrusive growth potential (Fig. 6a). Sheath cells substituted the ruptured epithelial cells, becoming part of the secretory epithelium.
Aspects of fusion and ramification of the secretory canals in Protium heptaphyllum primary stem. a, c TEM. b, d–k Light microscopy. a Part of a secretory canal showing epithelial (EP) and sheath cells (SH) dividing periclinally. LU: lumen. b Longitudinal rows of small cells (encircled) adjacent to an enlarging canal. c Small-rowed cells adjacent to the secretory epithelium (EP) showing irregular walls, swollen middle lamellae, and dense and abundant cytoplasm. NU: nucleus. d Formation of a lateral ramification of secretory canal. E. Secretory canal with evident lateral ramification. f Adjacent canals separated by parenchyma cells. g Fused secretory canals. h–k Cross sections showing fusion of adjacent canals. Note: dissolution of the secretory epithelium in the contact region between canals. Scale bars: a, c (2 nm); b (10 μm); h–k (25 μm); d–g (100 μm)
Secretory canals ramified among the neighboring cells. Serial sections showed small cells arranged in longitudinal rows, adjacent to enlarging secretory canals (Fig. 6b). These small cells, which were immersed in parenchyma tissue, exhibited meristematic features, such as thin and irregular walls, voluminous nucleus, and dense and abundant cytoplasm (Fig. 6b, c). The middle lamellae among these cells showed dissolution signals (Fig. 6c). As the middle lamellae dissolved, these cells became part of the secretory epithelium and gave rise to a branch of the canals (Fig. 6d, e). The presence of pectinase was ultracytochemically detected in the middle lamellae region of these cells.
In longitudinal (Fig. 6f, g) and cross (Fig. 6h–k) sections, some secretory canals were observed very close to each other, separated only by few parenchyma cell layers (Fig. 6f), sharing the same parenchyma sheath (Fig. 6h) or with the secretory epithelium in direct contact (Fig. 6i). The middle lamellae among these parenchyma cells were dissolved, allowing the junction of adjacent canals in a similar way to observe in the ramification canals. In the regions of contact between the neighboring canals, epithelial cells exhibited signals of lyses and dissolution of the middle lamellae (Fig. 6i, j). This process culminated with the total fusion of the adjacent canals (Fig. 6k, g). Pectinase and cellulase were ultracytochemically marked in the cell walls in these regions.
Discussion
Looking at different regions of the stem in P. heptaphyllum and using light and electron microscopy methods, we got insights on the origin and development of the secretory canals, ramification of canals by incorporation of neighboring cells, and fusion between canals, in addition to evidence of intrusive growth. Although some anatomical and histochemical results are already known for some Protium species (Souza et al. 2016), ultrastructural data represent the novelty of this work. Our findings lead us to understand the complexity of the tridimensional secretory system in this Burseraceae species.
The histochemical characterization of the secretory canals in P. heptaphyllum corroborates the data obtained by Souza et al. (2016). The subcellular apparatus of the epithelium of mature secretory canals in P. heptaphyllum is consistent with the production of polysaccharides and proteins, in addition to the lipophilic compounds. In fact, these substances were histochemically detected in these glands. The occurrence of dictyosomes and abundant vesicles in these cells indicates the production of hydrophilic substances, as polysaccharides (Fahn 1979; Evert 2006). The abundance of oil drops in the cytoplasm, presence of plastids without thylakoids, and smooth endoplasmic reticulum with dilated cisterns are consistent to the synthesis of lipophilic substances (Fahn 1979; Evert 2006) and are commonly reported in cells secreting monoterpenes (Cheniclet and Carde 1985; Turner et al. 1999; Rodrigues et al. 2011a; Machado et al. 2017).
In the present study, the placement of the myelin-like figures (multilamellar bodies) in the peripheral cytoplasm of epithelial cells, in the interface with plasmalemma, suggests their role in the secretion of lipids and other molecules to the outside of the protoplast, as described to animal cells (Paquet et al. 2013). The origin of myelin-like figures in these cells is obscure and can be associated to autophagic process and degeneration of mitochondria and plastids, among others (van Doorn and Papini 2016).
The abundance of polysomes and profiles of rough endoplasmic reticulum in the epithelial cells of P. heptaphyllum are consistent to the synthesis of lytic enzymes required to the growth of the secretory canals (Marinho and Teixeira 2016) and the cell wall changes involved in the exudate release (Machado and Carmello-Guerreiro 2001; Rodrigues et al. 2011b; Machado et al. 2017).
Regarding the exudate release toward the lumen, our images suggest the occurrence of different mechanisms in P. heptaphyllum. The observation of oil drops and osmiophilic bodies in the cytoplasm of the epithelial cells, mainly in the cell side facing the lumen, in the periplasmic space and immersed in the cell wall matrix suggests lipid compounds can cross the cell wall and reach the lumen (Nair et al. 1981). These features are typical of eccrine process of secretion release (Evert 2006; Rodrigues and Machado 2012) which is favored by the loose arrangement of the cellulose microfibrils of the cell wall facing the lumen. In addition, the abundance of vesicles in the outer cytoplasm and the irregular contour of the plasmalemma of the epithelial cells indicate that a fraction of the secretion, mainly the hydrophilic compounds, can be released toward the lumen by granulocrine mechanism (Fahn 1979; Evert 2006). In P. heptaphyllum, it is evident that the release of the secretion to the lumen is associated to the peeling of the outer layers of the tangential wall of the epithelial cells facing the lumen (Rodrigues et al. 2011a, b; Machado et al. 2017). Still, in this species, the epithelial cells in the ends of the growing secretory canals can be broken, and their protoplast content is released to the lumen, characterizing holocrine mechanism of secretion (Machado and Carmello-Guerreiro 2001).
The secretory canals in P. heptaphyllum were originated from the procambium in the shoot apex and from cambium in the stem bark. Despite their different meristematic origin, these canals are similar in structure and development, including the occurrence of longitudinal growth, lateral ramification, and fusion. In both cases, the lumen is initiated by the separation of the cluster cells by the dissolution of the middle lamellae, characterizing a schizogenous process (Fahn 1979; Evert 2006). However, the canals with well-established lumen continue developing. Such growth of the secretory canals occurs by a combination between cell separation and rupture, characterizing a schizolysigenous process of growth (Fahn 1979; Evert 2006; Rodrigues et al. 2011b).
In this paper, the occurrence of cell wall peeling, vacuoles with membranous debris, nucleus with irregular contour and dense lumps of chromatin, and mitochondria with cristolysis signals in epithelial cells are apoptotic signals consistent with programmed cell death (PCD) (Danon et al. 2000; Brighigna et al. 2006) during the growth of the secretory canals. In fact, the occurrence of PCD has been demonstrated during the development of the oil secretory spaces (Rodrigues et al. 2011a; Liu et al. 2012). Vacuoles containing membranous inclusions, probably sequestered portions of cytoplasm, can be interpreted as autophagic structures (autophagosomes) and indicate the occurrence of macroautophagic process (Papini et al. 2014) during the development of the secretory system in P. heptaphyllum.
The occurrence of an anastomosed secretory network has already been reported in other Burseraceae species (Mc Nair 1918; Tolera et al. 2013). By comparing different developmental stages of stem in light and electron microscopy, we were able to access and understand the complexity of the resin secretory system in P. heptaphyllum. In this species, the secretory system has a complex structure resulting from longitudinal growth, lateral ramification, and fusion of the adjacent canals, in addition to intrusive growth of both epithelial and sheath cells. Occurrence of intrusive growth of secretory cells has been described to secretory cavities of Metrodorea nigra (Machado et al. 2017) and in laticifers of Tabernaemontana catharinensis (Canaveze and Machado 2015). Epithelial and sheath cells with tapered ends penetrating the swollen middle lamella among adjacent cells are consistent with intrusive growth (Siedlecka et al. 2008; Machado et al. 2017) that was here confirmed by serial anatomical section analysis. This observation is of particular importance if we consider the spatial adjustment of the secretory canals occurred during their longitudinal growth, lateral ramification, and fusion. In addition, the maintenance of the secretory active in the mature canals through the stem development is ensured by the replacement of the damaged epithelial cells by the parenchyma sheath that surrounds the secretory epithelium, as reported for different plant species (Bosabalidis and Tsekos 1982a, b; Rodrigues et al. 2011a, b; Machado et al. 2017). Differently from the reported to other species, the sheath cells in older stem regions of P. heptaphyllum can become sclerified and lose their meristematic capacity. Considering that both epithelial and parenchyma sheath cells in the older canals can also lignify, the lifetime of these glands is determinate in P. heptaphyllum.
The observation of rows of cells with meristematic features immersed in the parenchyma tissue adjacent to mature canals is a novelty in the development of the secretory canals in Burseraceae. This characteristic suggests the existence of an induction action of secretory canals on the neighboring cells as discussed for laticifers (Canaveze and Machado 2015); however, the action-inducting mechanism and the inductive properties of secretory cells are still poorly investigated. Anastomosis of secretory, as observed here in P. heptaphyllum, implies in a direct communication between the two adjacent canals (Bosshard and Hug 1980) with biological and yield implications. In biological terms, this anastomosed secretory system facilitates the transport of resin to long distances and toward different directions ensuring a more effective protection against herbivores (Tolera et al. 2013). Regarding the resin yield, this tridimensional network of the resin secretory system contributes to a more efficient tapping of resin (Tolera et al. 2013).
Our findings demonstrated that in the trunk of P. heptaphyllum, secretory canals active in secretion occurred deeper in secondary phloem; on the other hand, canals inactive in secretion occurred more superficially in the bark. The functional status of the secretory canals was attributed based on the features of the epithelial cell walls. So, epithelial cells with thin pecto-cellulosic walls and abundant cytoplasm characterize canals active in secretion, while epithelial cells with thicker and lignified walls, devoid of protoplast, characterize canals inactive in secretion. Sclerified cell walls can act in the protection against the rupture of the canals and the reflux of the storage secretion in the lumen of secretory ducts. The presence of exudate in the lumen of inactive canals coupled with our observation of the sclerified epithelial cells indicates that, even inactive in secretion, these canals remain important sites of resin storage. So, superficial wounds maybe will be enough to the overflow of resin. These findings coupled to the occurrence of an anastomosed and tridimensional resin secretory system in P. heptaphyllum can contribute to the establishment of more efficient and sustainable techniques for resin extraction.
References
Allen RD, Nessler CL (1984) Cytochemical localization of pectinase activity in laticifers of Nerium oleander L. Protoplasma 119(1–2):74–78. https://doi.org/10.1007/BF01287819
Bal AK (1974) Cellulase. In: Hayat MA (ed) Electron microscopy of enzymes v3. Van Nostrand Reinhold, New York
Bosabalidis A, Tsekos I (1982a) Ultrastructural studies on the secretory cavities of Citrus deliciosa. Early stages of the gland cell differentiation. Protoplasma 112(1–2):55–62. https://doi.org/10.1007/BF01280215
Bosabalidis A, Tsekos I (1982b) Glandular scale development and essential oil secretion in Origanum dictamnus L. Planta 156(6):496–504. https://doi.org/10.1007/BF00392771
Bosshard HH, Hug UE (1980) The anastomoses of the resin canal system in Picea abies (L.) Karst., Larix decidua Mill. and Pinus sylvestris L. Holz Roh Werkst 38(9):325–328. https://doi.org/10.1007/BF02611082
Bowers WS, Evans PH, Venable DL, Becerra JX (2001) Interactions between chemical and mechanical defenses in the plant genus Bursera and their implications for herbivores. Am Zool 41:865–876
Brighigna L, Milocani E, Papini A, Vesprini JL (2006) Programmed cell death in the nucellus of Tillandsia (Bromeliaceae). Caryologia 59(4):334–339. https://doi.org/10.1080/00087114.2006.10797935
Bukatsch F (1972) Bemerkungen zur Doppelfárbung Astrablau-Safranin. Mikrokosmos 61:255
Canaveze Y, Machado SR (2015) Leaf colleters in Tabernaemontana catharinensis (Apocynaceae, Rauvolfioideae): structure, ontogenesis, and cellular secretion. Botany 93(5):287–296. https://doi.org/10.1139/cjb-2014-0229
Chamberlain CJ (1932) Methods in plant histology. The University of Chicago, Chicago
Cheniclet C, Carde JP (1985) Presence of leucoplasts in secretory cells and of monoterpenes in the essential oil: a correlative study. Isr J Bot 34:219–238
Citó AGL, Costa FB, Lopes JAD, Oliveira VMM, Chaves MH (2006) Identificação dos constituintes voláteis de frutos e folhas de Protium heptaphyllum Aubl (March). Revista Brasileira de Plantas Medicinais 8:4–7
Daly DC, Fine WA (2011) A new amazonian section of Protium (Burseraceae) including both edaphic specialist and generalista taxa. Studies in Neotropical Burseraceae XVI. Sistematic Bot 36(4):939–949. https://doi.org/10.1600/036364411X604958
Danon A, Delorme V, Mailhac N, Gallois P (2000) Plant programmed cell death: a common way to die. Plant Physiol Biochem 38(9):647–655
David R, Carde JP (1964) Coloration différentielle dês inclusions lipidique et ter-peniques des pseudophylles du pine maritime aumoyen du reactif Nadi Paris. CR Acad Sci Paris 257:1338–1340
Evert RF (2006) Esau’s plant anatomy. In: meristems, cells and tissues of the plant body their structure, function and development, 3rd edn. John Wiley and Sons, New Jersey. https://doi.org/10.1002/0470047380
Fahn A (1979) Secretory tissues in plants. Academic Press, London
Furr M, Mahlberg PG (1981) Histochemical analyses of lacticifers and glandular trichomes in Cannabis sativa. J Nat Prod 44(2):153–159. https://doi.org/10.1021/np50014a002
Gerrits PO (1991) The application of glycol methacrylate in histotechnology: some fundamental principles. Departament of Anatomy and Embryology State University of Gröningen, Gröningen
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Langenheim JH (2003) Plant resins: chemistry, evolution, ecology and ethnobotany. Timber Press, Portland
Lima TAAC, Ribeiro JELS, Marques MOM, Facanali R, Lima MP (2016) Estimulo para produção de resina em Protium hebetatum Daly e avaliação dos constituintes químicos voláteis. Scientia Amazonia 5:21–24
Liu P, Liang S, Yao N, Wu H (2012) Programmed cell death of secretory cavity cells in fruits of Citrus grandis cv. Tomentosa is associated with activation of caspase 3-like protease. Trees Struct Funct 26(6):1821–1835. https://doi.org/10.1007/s00468-012-0752-1
Lorenzi H (2002) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas do Brasil. Instituto Plantarum, Brasil
Machado SR, Carmello-Guerreiro SM (2001) Estrutura e desenvolvimento de canais secretores em frutos de Schinus terebinthifolius Raddi (Anacardiaceae). Acta Bot Bras 15(2):189–195. https://doi.org/10.1590/S0102-33062001000200005
Machado SR, Rodrigues TM (2004) Anatomia e ultra-estrutura do pulvino primário de Pterodon pubescens Benth. (Fabaceae-Faboideae). Rev Bras Bot 27(1):135–147. https://doi.org/10.1590/S0100-84042004000100015
Machado SR, Canaveze Y, Rodrigues TM (2017) Structure and functioning of oil cavities in the shoot apex of Metrodorea nigra A. St.-Hil. (Rutaceae). Protoplasma 254(4):1661–1674. https://doi.org/10.1007/s00709-016-1056-x
Marinho C, Teixeira S (2016) Cytochemical localization of pectinases and cellulases in developing laticifers of Maclura tinctoria and Ficus Montana (Moraceae). European Microscopy Congress: Proceedings
Matos FJA (1997) O Formulário Fitoterápico do professor Dias da Rocha, 2nd edn. UFC, Fortaleza
Mc Nair JB (1918) Secretory canals of Rhus diversiloba. Bot Gaz 65(3):268–273. https://doi.org/10.1086/332233
Metcalfe CR, Chalk L (1950) Anatomy of the dicotyledons leaves, stem and wood in relation to taxonomy with notes on economy uses. Clarendon press, Oxford
Nair GM, Patel KR, Subrahmanyam SV, Shah JJ (1981) Secretion of resin across the wall of the epithelial cell in the gum-resin canal of Comiphora mukul Engl. Ann Bot 47(3):419–421. https://doi.org/10.1093/oxfordjournals.aob.a086035
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue. Protoplasma 59(2):368–373. https://doi.org/10.1007/BF01248568
Papini A, Mosti S, van Doorn WG (2014) Classical macroautophagy in Lobivia rauschii (Cactaceae) and possible plastidial autophagy in Tillandsia albida (Bromeliaceae) tapetum cells. Protoplasma 251(3):719–725. https://doi.org/10.1007/s00709-013-0567-y
Paquet VE, Lessire R, Domergue F, Fouillen L, Filion G, Sedighi A, Charette SJ (2013) Lipid composition of multilamellar bodies secreted by dictyostelium discoideum reveals their amoebal origin. Eukaryot Cell 12 (10):1326–1334
Pearse AGE (1980) Histochemistry theoretical and applied, vol II, 4th edn. Longman Group Limited, London
Revilla J (2001) Plantas da Amazônia: oportunidades econômicas e sustentáveis. SEBRAE-AM/INSPA, Manaus
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17(1):208–212. https://doi.org/10.1083/jcb.17.1.208
Rodrigues TM, Machado SR (2012) Oil glands in Pterodon pubescens Benth. (Leguminosae-Papilionoideae): distribution, structure, and secretion mechanisms. Int J Plant Sci 173(9):984–992. https://doi.org/10.1086/667609
Rodrigues TM, Santos DC, Machado SR (2011a) The role of the parenchyma sheath and PCD during the development of oil cavities in Pterodon pubescens (Leguminosae-Papilionoideae). Comptes Rendus Biologies 334(7):535–543. https://doi.org/10.1016/j.crvi.2011.04.005
Rodrigues TM, Teixeira SP, Machado SR (2011b) The oleoresin secretory system in seedlings and adult plants of copaíba (Copaifera langsdorffii Desf., Leguminosae–Caesalpinioideae). Flora - Morphol Distribution Funct Ecol Plants 206(6):585–594. https://doi.org/10.1016/j.flora.2010.10.002
Siani AC, Ramos MFS, Menezes-de-Lima O, Soares ROA, Rosas EC, Susunaga GS, Guimarães AC, Zoghbi MGB, Henriques MGMO (1999) Evaluation of anti-inflamatory-related activity of essential oils from the leaves and resin of species of Protium. J Ethopharmacol 66(1):57–69. https://doi.org/10.1016/S0378-8741(98)00148-2
Siedlecka A, Wiklund S, Péronne MA, Micheli F, Lesniewska J, Sethson I, Edlund U, Richard L, Sundberg B, Mellerowicz EJ (2008) Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol 146(2):554–565. https://doi.org/10.1104/pp.107.111963
Souza LR, Trindade FG, Oliveira RA, Costa LCB, Gomes VM, Cunha M (2016) Histochemical characterization of secretory ducts and essential oil analysis of Protium species (Burseraceae). J Essent Oil Res 28(2):166–171. https://doi.org/10.1080/10412905.2015.1092478
Sussunaga GS (1996) Estudo químico e biológico da resina produzida pela espécie Protium heptaphyllum March. (Burseraceae). Dissertation, University of Amazonas
Tolera M, Menger D, Sass-Klaassen U, Sterck FJ, Copini P, Bongers F (2013) Resin secretory structures of Boswellia papyrifera and applications for franckincense yield. Ann Bot 111(1):61–68. https://doi.org/10.1093/aob/mcs236
Turner GW, Gershenzon J, Nielson EE, Froehlich JE, Croteau RB (1999) Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol 120(3):879–886. https://doi.org/10.1104/pp.120.3.879
van Doorn WG, Papini A (2016) Plastid degeneration in Tillandsia albida (Bromeliaceae) and Lobivia rauschii (Cactaceae) provides evidence about the origin and destiny of multilamellar bodies in plants. Phytomorphology 66:103–112
Acknowledgments
We thank Dr. Douglas C. B. Daly for the botanical identification, and the technical team of the Electron Microscopy Center, IBB UNESP, for assistance in processing the materials. FH Palermo (CNPq/Master) and J Nicolai (CNPq/PIBIC) received scholarship from Conselho Nacional de Desenvolvimento e Pesquisa, CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling Editor: Peter Nick
Electronic supplementary material
ESM 1
(GIF 801 kb)
Rights and permissions
About this article
Cite this article
Palermo, F.H., Rodrigues, M.I.d.A., de Nicolai, J. et al. Resin secretory canals in Protium heptaphyllum (Aubl.) Marchand. (Burseraceae): a tridimensional branched and anastomosed system. Protoplasma 255, 899–910 (2018). https://doi.org/10.1007/s00709-017-1197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1197-6