Abstract
By a detailed ontogenetic study of Polemonium caeruleum pollen, tracing each stage of development at high TEM resolution, we aim to understand the establishment of the pollen wall and to unravel the mechanisms underlying sporoderm development. The main steps of exine ontogeny in Polemonium caeruleum, observed in the microspore periplasmic space, are spherical units, gradually transforming into columns, then to rod-like units (procolumellae), the appearance of the initial tectum, growth of columellae in height and tectum in thickness and initial sporopollenin accumulation on them, the appearance of the endexine lamellae and of dark-contrasted particles on the tectum, the appearance of a sponge-like layer and of the intine in aperture sites, the appearance of the foot layer on the base of the sponge-like layer and of spinules on the tectum, and massive sporopollenin accumulation. This sequence of developmental events fits well to the sequence of self-assembling micellar mesophases. This gives (together with earlier findings and experimental exine simulations) strong evidence that genome and self-assembly probably share control of exine formation. It is highly probable that self-assembly is an intrinsic instrument of evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pollen wall is the most complex wall found in plants. To understand regulation of pollen wall development, a detailed ontogenetic study, capturing each stage of development at a high level of resolution, is needed. The tetrad period, understood as crucial for pattern determination in the pollen wall by some authors (Dickinson 1970; Heslop-Harrison 1972; Blackmore and Barnes 1987; Gabarayeva 2000, 2014; Vinckier and Smets 2005; Taylor and Osborn 2006; Taylor et al. 2013, 2015), is most often underestimated (or represented by one stage) by many investigators, whereas almost all the main characters of the future exine are defined in this period. Consequently, observations presented here focus on the tetrad period of development.
Our next point of special interest concerns mechanisms, underlying sporoderm construction processes, which are not under direct genetic control. The pioneering idea of the universal importance of self-organization for pattern formation in nature by Thompson (1961) was then picked up by several biologists (Heslop-Harrison 1972; Gerasimova-Navashina 1973; Sheldon and Dickinson 1983; Dickinson and Sheldon 1986; van Uffelen 1991; Hemsley et al. 1992b, 2003; Collinson et al. 1993; Gabarayeva 1993, 2000, 2014; Southworth and Jernstedt 1995; Hemsley and Griffiths 2000) and discussed in reviews (Wellman 2004; Blackmore et al. 2007; Ariizumi and Toriyama 2011). The essence of our hypothesis (Gabarayeva and Hemsley 2006; Hemsley and Gabarayeva 2007) on the role of self-assembly processes in spore and pollen wall development is that the sequence of sporoderm developmental events represents the sequence of self-assembling micellar mesophases, initiated by genomically given parameters of surfactant glycoproteins and sporopollenin (SP) precursors and monomers (phenyl-propanoids and fatty acids) at increasing concentrations. Correspondingly, our goal in this study is to determine whether our hypothesis, which proved to be applicable to a wide diversity of other plants (about 30 species from remote taxa, viz. from Cyatheaceae (leptosporangiate fern), Cycadaceae, gymnosperms, basal and advanced angiosperms—see our papers in References), also applies in Polemonium.
Another point in question for discussion, based on observations of developmental events in Polemonium and other species in the course of exine development, is the localization of SP precursor synthesis. Is this synthesis localized exclusively to the tapetum, or does it also proceed in microspores? Pro and contra reasons for this are discussed as one of the fundamental questions.
Polemonium caeruleum L. is a hardy perennial herbaceous flowering plant and produces cup-shaped, lavender-colored, or white flowers. The plant usually reaches a height from 45 to 60 cm. The plant is native to damp grasslands, woodlands, meadows, and rocky areas in temperate areas of Europe and Asia. The plant flowers in June–July during 15–20 days.
We have chosen this species for our study because its pollen wall has not been ontogenetically studied and because it was easy to collect many flower-buds at different stages of development. We believe that self-assembly is a kind of universal mechanism, taking part in spore and pollen development. In essence, any species is suitable for our studies, if only a plant is rich in flower-buds/sporangium.
Pollen grains of Polemonium caeruleum are spherical, pantoaperturate, with ornamented aperture membrane; the structure of the pollen wall is semitectate-columellate (Halbritter and Svojtka 2005). No ontogenetic studies on pollen wall of Polemonium have been previously undertaken.
Material and methods
Flower-buds of Polemonium caeruleum (L.) were obtained from the Garden of the Komarov Botanical Institute, St. Petersburg for 3 years, 80–100 buds every year. Stamens were fixed for 24 h at 20 °C in 3% glutaraldehyde in 0.1 M cacodylate buffer pH 7.4 with 2.5% sucrose and lanthanum nitrate (1%) for better preservation of the plasma membrane glycocalyx. After post-fixation in 2% osmium tetroxide for 4 h at 20 °C followed by acetone dehydration, the samples were embedded in a mixture of Epon and Araldite. Ultrathin sections were contrasted with 1% aquatic solution of uranyl acetate and 0.2% lead citrate. Sections were examined with a Hitachi H-600 transmission electron microscope (TEM). For scanning (SEM) observations, mature pollen grains were mounted on stubs, coated with gold/palladium and examined with Jeol JSM-6390.
Results
Early tetrad stage
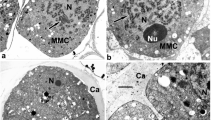
After the completion of meiosis, four early microspores of each tetrad, surrounded by the callose special cell wall, are united in planar (Fig. 1a) or tetrahedral tetrads. Remnants of the cytomictic channels which united the late spore mother cells are still evident (Fig. 1a, arrows). A large nucleus with a nucleolus occupies a considerable part of the microspore volume (Fig. 1b).
Early post-meiotic tetrads of microspores in Polemonium caeruleum. a A planar tetrad with four microspores, surrounded by callose (Ca). Adjacent tetrads are still connected with each other (arrows) via cytomictic channels. b A microspore of a tetrad. Large nucleus (N) with nucleolus, plastids (P), vacuoles (V), cisternae of ER, and dictyosomes (D) are seen in the cytoplasm. Bars at 1 μm
When first signs of the microspore surface coat—the glycocalyx—appear in the periplasmic space between the callose layer and the plasma membrane, the latter has an even profile (Fig. 2a). Its contour becomes uneven sometime later (Fig. 2b) and then takes a sinusoidal profile (Fig. 2c). In parallel, the glycocalyx layer becomes gradually thicker (Fig. 2b, c). Some plastids are in tight contact with the plasma membrane, others have a cup-like form (Fig. 2c). Then, the structure of the glycocalyx changes: Spherical units with diameter appr. 16 nm appear near the plasma membrane (Fig. 2d and magnified fragment as inset, arrows) which in the outer portion of the glycocalyx are arranged into rod-like units—columns of spherical units (Fig. 2d, arrowheads).
Young tetrad stage in Polemonium caeruleum. a A thin layer of the glycocalyx (G) appears on the plasma membrane (PM). Plenty of ribosomes (R) are seen in the cytoplasm; ER cisterna is parallel to the plasma membrane. b Some later step. The plasma membrane is slightly wavy, discontinuous glycocalyx becomes thicker (arrowheads). c The plasma membrane acquires sinusoidal outline. The glycocalyx appears more distinct. Cisternae of RER persist their orientation. d and inset Tangential section reveals tightly packed spherical units (arrows) of the glycocalyx, which are rearranged into rod-like structures on the surface (arrowheads). For details at higher magnification, see inset. CP cup-like plastid, Mi mitochondrion, P plastid. Bars in a–d at 0.25 μm. Inset in d at 0.1 μm
Middle tetrad stage
Initial columellae appear on the plasma membrane (Fig. 3a, arrowheads). The substructure of this procolumella, pointed by the left arrowhead, is shown in the inset: a tuft of rod-like units, each unit consists of columns of spherical subunits of appr. 16 nm. Many rough endoplasmic reticulum (RER) cisternae are seen in the microspore cytoplasm (Fig. 3a, b, arrows). Inside the plasma membrane invaginations, the glycocalyx appears as a network with roundish meshes (Fig. 3b). Slightly later, the procolumellae acquire more complex substructure (Fig. 3c): They appear as a tuft of rod-like units with diameter appr. 60 nm, tied by another subunit like a sheaf (left arrowhead and left inset, tiny white arrowheads), or have a wigwam-like form (the right arrowhead and the right inset). It is well discernible in Fig. 3c and insets that rod-like units consist of columns of spherical units. RER cisternae are often outstretched to the plasma membrane under the developing columellae. A very thin protectum (Fig. 3c, arrows) is a newcomer at this stage.
Middle tetrad stage in Polemonium caeruleum. a and inset The initial procolumellae appear in the glycocalyx (G) on pinnacles of the plasma membrane (arrowheads). The procolumella in inset reveals the substructure as several columns of spherical units (white arrowhead). RER cisternae are parallel to the plasma membrane (arrows). b Deep invaginations of the plasma membrane appear, filled with the glycocalyx (G). Note RER cisternae, outstretched to the plasma membrane (arrows). c Well-discernible procolumellae, consisting of rod-like units (arrowheads; see them also in insets), surrounded by another subunit (tiny white arrowheads), support a thin initial protectum (arrows). Ca callose, MC microspore cytoplasm. Bars in a–c at 0.25 μm. Insets in a at 0.1 μm. Both insets in c at 0.1 μm
Late tetrad stage
The tetrahedral tetrad is shown in Fig. 4a. The cytoplasm of two adjacent tetrads is full of organelles (Fig. 4b), and the developing sporoderm is noticeable (Fig. 4b, arrowhead). Higher magnification reveals the primexine with a well discernible tectum, its thickness is appr. 0.07 μ (Fig. 4c, d, arrowheads) and short columellae. Many dictyosomes and plastids (some are cup-like) are seen in the microspore cytoplasm (Fig. 4c). RER cisternae are in direct contact with the nuclear envelope (Fig. 4e, arrows).
Late tetrad stage in Polemonium caeruleum. a A survey of a tetrahedral tetrad. b Two adjacent tetrads, their cytoplasm contains many small vacuoles (V), lipid globules (LG), and cup-like plastids (CP). c The primexine is well discernible with procolumellae and protectum (arrowheads). d Proximal sides of the adjacent tetrad microspores. The protectum (arrowheads) is well developed. e A border of the microspore nucleus. Note contacts (arrows) of ER cisternae with the nuclear envelope (NE). AL anther loculus, Ca callose, N nucleus. Bars at 1 μm
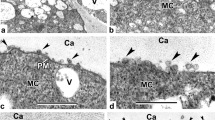
The contacts of RER cisternae and plastids with the plasma membrane attract special attention (Fig. 5a–d). Many images of RER cisternae, extended to and contacting with the plasma membrane, are observed (Fig. 5a, c, d, arrows); some cisternae open directly to the periplasmic space (Fig. 5b, arrowhead). Plastids are often seen in the close vicinity to the plasma membrane (Fig. 5e–g). Plastids produce clusters of tiny osmiophilic vesicles (Fig. 5e–g, arrowheads) which evidently are directed to the plasma membrane.
Late tetrad stage in Polemonium caeruleum. a Contacts of ER cisternae (arrows) with the plasma membrane. b A fragment of ER cisternae, directly contacting the plasma membrane and opening to the periplasmic space (arrowhead). c, d Cisternae of ER in contact with the plasma membrane, some in sites of developing procolumellae. Autolytic vacuoles (AV) (c) and endocytotic channel (EC in d). e–g Plastids (P) in close vicinity (arrowheads) to the plasma membrane. Ca callose, G glycocalyx, GV Golgi vesicles, MC microspore cytoplasm, PEx primexine, PG plastoglobule, PT protectum. Bars at 0.25 μm
The aperture sites lack glycocalyx, and correspondingly, primexine is not developed (Fig. 6a, b). The cytoplasm of the tapetal cells is overloaded with RER cisternae (Fig. 6c).
Late tetrad stage in Polemonium caeruleum. Aperture sites (a, b) and tapetum (c). a, b At the sites of the future apertures the layer of the glycocalyx is scanty (a) or absent (b) and primexine is absent. c The cytoplasm of the tapetal cells (Ta) is full of RER cisternae. AL anther loculus, Ca callose, CP cup-like plastid, G glycocalyx, MC microspore cytoplasm, Mi mitochondrion, Pex primexine. Bars at 0.25 μm
Stage of disintegrating tetrad
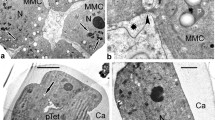
The callose envelope appears discontinuous: It is partly dissolved (Fig. 7a) and partly remains in remnants (Fig. 7b). Because the tectum in Polemonium caeruleum is discontinuous and represented by short striae, oriented in different directions, it appears on sections differently, depending on the plane of section: as widenings of tops of columellae (Fig. 7a, b) or as rather long fragments of typical tectum (Fig. 7c). A new layer, which is a forerunner of the first endexine lamella (Fig. 7a, arrows), occurs inward to the glycocalyx (arrowheads). Double-membrane organelles, containing needle-like precipitations, attract attention (Fig. 7a, c, stars). Similar crystals were observed in analogous organelles of Passiflora racemosa at young tetrad stages (Fig. 7a, corner insets). Large lipoid globules are seen beyond the plasma membrane, inside its invaginations (Fig. 7a). Large plastids with dark stroma and osmiophilic inclusions are plentiful in the microspore cytoplasm (Fig. 7a, b).
Stage of disintegrating tetrad (a, b) and young free microspore stage (c) in Polemonium caeruleum. a Initial discontinuous endexine layer (arrows) appears between the dark contrasted plasma membrane (PM) and the glycocalyx (arrowheads). Large lipid globule (L) in the periplasmic space. Some double-membrane organelles (stars) contain osmiophilic needle-like crystals, the left one is in contact with the plasma membrane. Similar crystals in plastids of Passiflora racemosa at young tetrad stages (corner insets in a). b and inset The initial fragments of the endexine as tripartite layer with central white line (arrows). c The ectexine consists of a thick tectum and thin columellae with spiral-like shield (tiny arrowheads). The endexine is represented by primordial white-lined lamella (arrowheads). Note dark-contrasted lipid droplets on the plasma membrane, in the peripheral cytoplasm (white asterisks) and on the plastid surface (P). Double membrane organelles with needle-like crystals (stars). CaR callose remnants, D dictyosome, G glycocalyx, MC microspore cytoplasm, N nucleus. Bars in a–c at 0.25 μm. Insets in a, b at 0.1 μm
Slightly later well-discernible fragments of the tripartite endexine lamella with typical central white line occur (Fig. 7b and inset, arrows).
Young free microspore stage
The columellae become higher and the tectum more prominent: Its thickness has increased up to 0.12 μ (Fig. 7c). Note that young columellae still preserve their substructure: Careful observation reveals a kind of spiral shield around columellae, initiated at the middle tetrad stage (Fig. 3c). The white-lined primordial endexine lamella appears continuous (arrowheads). The plasma membrane is overloaded with lipid globules (Fig. 7c, asterisks); such globules are seen also in the cortical cytoplasm and as inclusions in plastids. The participation of plastids in synthesis and release of lipid substance beyond the plasma membrane seems evident (Fig. 8a–d). Plastids, situated in close vicinity to the plasma membrane, pinch off lipid globules (Fig. 8a, asterisks) which are also observed on the plasma membrane. Other plastids are seen in direct contact with the plasma membrane (Fig. 8b–d). Note that double-membrane organelles with needle-like crystals—most probably another generation of plastids or mitochondria—persist in the cortical cytoplasm (Fig. 8a–d, stars). Similar organelles were found in the microspore cortical cytoplasm of Chamaedorea microspadix (Fig. 8e, star), where the plasma membrane was also loaded with lipids.
Young free microspore stage in Polemonium caeruleum (a–d) and late tetrad microspore in Chamaedorea microspadix (e). a Plastids (P) evidently synthesize some lipid substance, pinching them off as globules (asterisks) and governing them to the plasma membrane, the latter is heavily loaded with lipid substance. Note double-membrane organelles with needle-like crystals (stars). b–d Close contacts of plastids with the plasma membrane which looks unusually thick because of heavy accumulation of lipids (asterisks). Double-membrane organelles (stars) with needle-like crystals accompany plastids, some of them are in contact with the plasma membrane. The endexine in a–d shown by arrowheads. e Similar double-membrane organelles (star) with needle-like crystals in Chamaedorea microspadix late tetrad microspores (Fig. 6c from Gabarayeva and Grigorjeva 2010, Grana 49: 91–114). C columella, GR glycocalyx remnants, MC microspore cytoplasm, T tectum. Bars at 0.5 μm
Middle free microspore stage
The endexine is considerably thicker: It consists of several lamellae, visible by their central white lines (Fig. 9a, arrowhead). Dark-contrasted particles appear on the tectum surface (Fig. 9a, white arrows). A slightly oblique section shows that such particles cover the whole tectum surface (Fig. 9b, black arrows). Many plastids, some of them are in contact with RER cisternae (Fig. 9, black arrowhead), persist in the cortical cytoplasm, sliding probably along the bundles of microtubules towards the plasma membrane (Fig. 9b, white arrowheads). Golgi stacks are very active, and many Golgi vesicles are accumulated under the plasma membrane.
Middle free microspore stages in Polemonium caeruleum. a The endexine is more developed and contains several lamellae with white lines (arrowhead). Small roundish osmiophilic particles—so-called sporopollenin-acceptor particles (SAPs)—appear on the surface of tectal elements (white arrows). A bundle of microtubules (black arrow) contacts the plasma membrane. b An oblique section through the microspore surface reveals the tectum surface with SAPs (arrows) and the surface of the endexine lamella (End). RER cisterna is in contact with plastid (black arrowhead). Note microtubules (white arrowheads), some are connected with plastids. C columella, D dictyosome, GR glycocalyx remnants, GV Golgi vesicles, MC microspore cytoplasm, PM plasma membrane, T tectum. Bars at 0.25 μm
In the aperture sites, the endexine lamellae are separated from each other and fragmented (Fig. 10a, arrowheads). In the thick zone of the aperture, glycocalyx clusters of dark-contrasted particles (Fig. 10a, arrows), similar to those on the tectum (Fig. 10b, arrows), appear. The tectum is as thick as 0.17 μ. Similar tectal particles in developing microspores of Borago officinalis are shown in Fig. 10c (arrows).
Middle free microspore stages in Polemonium caeruleum. a An aperture site (A) of a microspore is filled with the glycocalyx (G). The endexine lamellae (End) divergent and are partly fragmented (arrowheads). Clusters of SAPs appear in the outer part of the glycocalyx (arrows). b The ectexine is represented by tectum and columellae. Note SAPs on the tectum surface (black arrows). The endexine is shown by white arrows. c similar clusters of SAPs on tops of columellae in Borago officinalis free microspore (arrows). d Tapetum is fragmented, its remnants (TaR) include plastids (P) and many lipid globules: large (LG) and small dark-contrasted (arrows), the latter on their way to developing exine (Ex). AL anther loculus, C columella, G glycocalyx, MC microspore cytoplasm, PM plasma membrane, T tectum. Bars in a–c at 0.25 μm and in d at 0.5 μm
The tapetum has lost its integrity and appears as fragments, mainly as plastids and lipid globules of different size and osmiophility. Small high-osmiophilic droplets are evidently on their way to the developing exine (Fig. 10d, arrows).
Late free microspore stage
Two new layers appear at the beginning of this stage: a dark-contrasted, sponge-like layer on the surface of the endexine (Fig. 11a–d), and the channeled intine at aperture sites (Fig. 11a, b). Later on, this sponge-like layer is transformed into a foot layer with thickness of 0.13 μ (Fig. 11e, f). The tectum thickness has reached 0.35 μ, and the maximal thickness of the wavy endexine is 0.15 μ. Semi-mature exine of total thickness about 1 μ consists of ectexine and a comparatively thin endexine (Fig. 12a–c). The ectexine (Fig. 12a, double-headed arrow) includes a discontinuous tectum of appr. 0.35 μ in thickness, supported by columellae, and a foot layer of the same thickness. The whole ectexine is penetrated by microchannels (Fig. 12a–c). The lamellated endexine is discernible by its white lines (Fig. 12a–c) and compressed in result of the increasing of the cytoplasmic volume: Its thickness has decreased from 0.15 μ (Fig. 11e) to 0.08 μ. In the aperture sites (Fig. 12b), the endexine is seen in fragments. Spinules appear on the tectum (Fig. 12b, arrowheads).
Late free microspore stage in Polemonium caeruleum. a, b Two new layers—osmiophilic sponge-like layer (SL) and channeled intine (Int) at aperture sites appear. c, d Interaperture regions with sponge layer. e, f Later developmental step. Rather thick foot layer (FL), penetrated by microchannels, appears on the base of sponge layer. The lamellated endexine is discernible by their white lines (restricted by white arrows). SAPs on the tectum surface (black arrows). A aperture site, C columella, End endexine, MC microspore cytoplasm, P plastid, PM plasma membrane, T tectum. Bars at 0.25 μm
Late free microspore stage in Polemonium caeruleum. a An interaperture zone of the exine. The ectexine (double-headed arrows) consists of a very thick tectum and foot layer, penetrated by microchannels, and of thin columellae. The endexine is comparatively thin and consists of several lamellae, discernible by their white lines. b A fragment of the exine with an aperture site (A) in the middle. Note that the foot layer and the endexine are completely absent in the aperture. Spinules occur on the tectum on the base of SAPs. c Note that the foot layer, developed on the base of sponge layer, is ultimately very thick. White-lined endexine shown by arrowheads. Columellae are thin (arrows). End endexine, FL foot layer, MC microspore cytoplasm, T tectum. Bars at 0.25 μm
Mature pollen grains contain two sperm nuclei, and the cytoplasm of vegetative cell is overloaded with starch grains and lipid globules (Fig. 13a). The endexine is not evident. A SEM image of a pollen grain shows multiple apertures (Fig. 13b, arrowheads). Higher magnification shows tiny spinules on the tectal striae (Fig. 13c, arrows).
Mature pollen grains in Polemonium caeruleum. a Two sperm nuclei (SN) in a pollen grain after the completion of mitosis. The cytoplasm of the vegetative cell (VC) with starch grains (SG) and lipid globules (LG). Note that endexine is indiscernible in mature pollen grain. b SEM image of a pollen grain with irregular striato-reticulate sculpture (rugulate) and multiple apertures (arrowheads). c Pollen grain surface. Note tiny spinules on the tectum (arrows) which were developed on the base of SAPs. Apertures (asterisks). A aperture, Ex exine, Int intine. Bars in a at 2 μm, in b at 10 μm, and in c at 1 μm
Discussion
Sequence of exine construction
The main steps of sporoderm ontogeny in Polemonium caeruleum are as follows: (1) spherical units of the glycocalyx appear on the plasma membrane (Fig. 14a); (2) spherical units gradually transform into columns on the surface of the glycocalyx (Fig. 14a), then to tufts or wigwam-like groups of rod-like units at sites of the future columellae (Fig. 14b). Simultaneously, the initial tectum appears; (3) groups of these rod-like (cylindrical) units start to extend, with a corresponding growth of columellae in height. The tectum increases in thickness (Fig. 14c); (4) the first endexine lamella appears simultaneously with massive overloading of the plasma membrane with lipids (Fig. 14d) and with multiple contacts of lipid-producing microspores’ plastids with the plasma membrane (Fig. 8); (5) accumulation of SP monomers in the periplasmic space, the multiplication of the endexine lamellae, and the appearance of dark-contrasted particles (SAPs) on the tectum (Fig. 14e); (6) further SP monomers accumulation in the periplasmic space, the appearance of a sponge-like layer and of the channeled intine in aperture sites (Fig. 14f); and (7) the appearance of the foot layer on the base of sponge-like layer and of spinules on the tectum on the base of SAPs (Fig. 14g); massive SP accumulation completes the maturation of the exine.
Semi-scheme showing the main developmental stages in Polemonium caeruleum (left column) and micellar interpretation of the processes (right column). a-c Tetrad stages. a Fragment of Fig. 2d. Spherical units (arrows) of the glycocalyx are accumulated on the plasma membrane at early tetrad stage: These units are the first micellar mesophase (a′) of the self-assembling sequence—spherical micelles. A transition to the next mesophase—columns of spherical micelles (a″)—is seen on the glycocalyx surface (arrowheads). b Fragment of Fig. 3c. Initial columellae are arranged from several columns of spherical micelles (right arrowhead), which self-arrange into cylindrical micelles, collected into a tuft (left arrowhead in b, b′). Initial tectum layer (arrow). c Fragment of Fig. 4d. Tightly packed cylindrical micelles—the next, hexagonal mesophase—form the layer of the glycocalyx, where procolumellae stand out (arrows) as tufts of cylindrical micelles (c′). Sporopollenin precursors are accumulated on the primexine. d Fragment of Fig. 7c. At the foot of columellae, the first endexine lamella with central white line appears (arrow), based on the next mesophase—laminate micelles (d′) with typical gap between bilayers. e Fragment of Fig. 9b. The endexine consists of several white-lined lamellae (arrow), based on laminate micelles with their typical gaps (e′). SAPs appear on the tectum surface: the sites of future spinules, accumulating sporopollenin (SP). f Fragment of Fig. 11a. New sponge-like layer (asterisk) is the base of the foot layer. It represents sponge mesophase (f′)—a bicontinual structure with unequal subvolumes of immiscible liquids. g Semi-mature ectexine includes a thick discontinuous tectum, columellae, and very thick foot layer (asterisk), interrupted at aperture sites. The foot layer appears on the base of sponge mesophase (f, asterisk). Lamellate endexine is seen by their central white lines. Spinules (arrowheads) on the tectum appear on the base of SAPs (g′). Microchannels pierce the whole ectexine. They appear in semi-liquid ectexine on the base of bicontinuous double-labyrinth structure, where oil and water are mixed, stabilized by surfactants at the interfaces (g″ a fragment from P. Ball, A soft and sticky world. 1994)
Exine construction, regarded as a sequence of micellar self-assembly
The supposition that the microspore glycocalyx, this framework for the future exine appearing in the tetrad period, is a self-assembling colloidal system (Gabarayeva 1990, 1993) was based on histochemical data showing that the microspore glycocalyx consisted mainly of glycoproteins, with the addition of lipopolysaccharides (Rowley 1971, 1973, 1975; Pettitt and Jermy 1974; Rowley and Dahl 1977; Pettitt 1979). Many glycoproteins and lipopolysaccharides are known to be surface-active substances (surfactants): Their molecules are diphilic. This means that at some definite concentration (so-called critical micelle concentration—Fridrichsberg 1995), these molecules are capable of self-association, leading to the appearance of micelles.
Micelles, these supra-molecular aggregates, appear in colloidal solutions of surfactants in definite sequence, as their concentration increases. The sequence of the main mesophases (pseudophases) is as follows: spherical micelles, transitive “columns of coins,” cylindrical micelles, and layer of close-packed cylindrical micelles (hexagonal mesophase). Further increase in the concentration and/or addition of lipid substances leads to the appearance of laminate micelles, formed by parallel packing of bimolecular layers, separated by a stratum of water. The latter are seen with TEM as typical “central white lines” of endexine lamellae.
The sequence of sporoderm developmental events represents, in essence, the unfolding sequence of self-assembling micellar mesophases, initiated by genomically given physico-chemical parameters and induced by surfactant glycoproteins at increasing concentrations.
At the right vertical column of Fig. 14, a sequence of micellar mesophases is shown which corresponds to the main developmental events in the course of exine ontogeny in Polemonium caeruleum. If we trace this sequence from a′ to e′, it is clear that the sequence includes the main set of micellar mesophases. If one now regards the sequence of the main developmental steps at the left vertical column and compares it with the corresponding mesophases on the right, the conformity between structural units in the micrographs and schemes appears evident. The first glycocalyx units, spherical ones (Fig. 14a, arrows), observed at the early tetrad stage, are most probably spherical micelles (Fig. 14a′). The latter, as the concentration of glycoproteins, delivered by Golgi vesicles into the periplasmic space increases, re-arrange first into transitional mesophase—columns of spherical micelles (Fig. 14a, arrowheads and a″), then to cylindrical micelles (Fig. 14b, arrowheads and b′). Simultaneously, a thin protectum appears (Fig. 14b, arrow). Singular cylindrical micelles grow in number and self-assemble into tufts of parallel, close-packed ones, forming the next mesophase—hexagonal (Fig. 14c′): These are the base for future columellae (Fig. 14c, arrows). Meanwhile, SP precursors and monomers accumulate on the protectum.
When the tetrads disintegrate, tufts of cylindrical micelles grow up in height: Columellae appear taller (Fig. 14d). The concentration of lipophilic SP precursors in the periplasmic space increases, hence the next micellar mesophase—laminate one, with typical gaps filled with supporting liquid (Fig. 14d′) and seen with TEM as white lines. The first lamella of the endexine is formed on the base of such a laminate micelle after SP accumulation (Fig. 14d, arrow). Then, the number of laminate micelles (Fig. 14e′) and—correspondingly—of the endexine lamellae increases (Fig. 14e, arrow).
Then, a thick sponge-like layer appears (Fig. 14f, asterisk)—the so-called sponge mesophase (Fig. 14f′). This is most probably a highly concentrated colloid emulsion which is formed of two immiscible liquids (of hydrophobic SP precursors and water-based medium). Sponge mesophases are also regarded as bicontinuous structures with uneven subvolumes. This sponge-like layer is the base of the belated foot layer (Fig. 14g, asterisk)—an unusual case when the endexine appears earlier than the foot layer.
The last ontogenetic step of the exine in Polemonium is connected with the appearance of microchannels, piercing the foot layer, columellae, and tectum (Figs. 14g and 12 for higher magnification). How could it be that microchannels appear inside a solid ectexine? The fact is that semi-mature microspore exines before anther dihescence remain semi-liquid (Rowley and Rowley 1998) and ultimate SP polymerization proceeds after the contact of pollen grains with air. When an additional amount of SP hydrophobic precursors are added from the tapetum, the balance in semi-liquid exine mixture changes, and a bicontinuous double-labyrinth structure may occur (Fig. 14g″). The oil and water phases may form a continuous interpenetrating network (Ball 1994).
Spinules (Fig. 14g, arrowheads) on the tectum appear on the base of SAPs after SP accumulation (g′).
Comparison with ontogenetic sequences in other species
Ours and other authors’ ontogenetic studies of a number of species have shown that in spite of great differences in the ultimate exine structure and sculpture of mature pollen grains, there is much in common between their developmental sequences. A surprising uniformity is observed in the chain of events in the course of exine development, with some variations. The reason for this, in our opinion, is that self-assembly interferes in genomically controlled process and is an intrinsic phenomenon which assists the exine ontogeny. We have studied the exine development in about 30 species from remote taxa (ferns, cycads, Gnetopsida, gymnosperms, basal and advanced angiosperms—e.g. Gabarayeva 1991, 1995, 1996, 2014; Zavada and Gabarayeva 1991; Gabarayeva and Rowley 1994; Gabarayeva and Grigorjeva 2002, 2004, 2010, 2011, 2012, 2014, 2016; Gabarayeva et al. 1998, 2003b, 2009a, b, 2010a, b, 2011a, b, 2013a, b, 2014, 2016; Grigorjeva and Gabarayeva 2015), and in all of them, the constructive units (or “building blocks”) are the same and appear in the same sequence. All these building blocks fit well to the sequence of self-assembling micellar mesophases. In some species, reiteration of the sequence, partial or total, is observed—another feature of the self-assembling systems. Some other, more complex self-assembling structures as nematic liquid-crystals and bicontinuous phases also occur.
Rowley, who examined substructure of developing exines in tens of species during tens of years, has shown (1990) that the universal unit of exines is rod-like and called it a tuft, because this unit consisted of several rod-like subunits, twined round by another subunits like a sheaf; Rowley called them correspondingly core subunits and binder subunit. We suggested (Gabarayeva and Hemsley 2006; Hemsley and Gabarayeva 2007; Gabarayeva et al. 2016) that the tuft of Rowley corresponds most probably to a group (a tuft) of cylindrical micelles, braided with another one or by a filament (long cylindrical micelle). Images in Figs. 3c and inset and 7c (follow tiny arrowheads) confirm Rowley’s ideas.
Laminate micelles with their gaps between bilayers are evidently not only the base for all species with lamellate endexines, but they occur also in orbicule-like formations in Magnolia sieboldii (Gabarayeva and Grigorjeva 2012), as tripartite lamellae lying free in sporangium of Alsophila cetosa (Gabarayeva et al. 2011b), in anther loculus in Plantago major (Gabarayeva et al. 2017), in primexine (Dickinson 1976), and as radial lines in Hedycarya arborea exine (Sampson 1977, 2000). Earlier Scott (1994) suggested self-assembly for tripartite lamellae.
Sponge-like layers were also observed in Passiflora racemosa during the formation of the second endexine layer (Gabarayeva et al. 2013a, b, Fig. 4). In both Polemonium and Passiflora, SP gradually impregnates sponge-like layers, turning their friable structure to an unbroken one.
The concept of SP-acceptor particles (SAPs) was introduced by Rowley and coauthors (Skvarla and Rowley 1987; Rowley and Claugher 1991). They showed that in many species, early SP accumulation in the middle tetrad stage was connected with some osmiophilic particles (SAPs). Particles of similar size and electron density were also observed during exine formation in Asimina (Waha 1987), Nuphar (Rowley et al. 1995), Borago (Gabarayeva et al. 1998; Rowley et al. 1999), Stangeria (Gabarayeva and Grigorjeva 2002), Encephalartos (Gabarayeva and Grigorjeva 2004), and Symphytum (Gabarayeva et al. 2011b). SAPs probably include enzyme molecules for SP polymerization (Gabarayeva and Hemsley 2006). The proteinaceous nature of these particles was suggested based on results of oxidative experiments on a variety of species (Rowley et al. 2001; Gabarayeva et al. 2003a, b). SAPs, observed in Polemonium exine development, determine the sites of the future spinules. In Borago and Symphytum, they are sites of gemmae appearance. Receptive sites of the primexine (a boundary layer) were also described in Compositae and other species using oxidation by potassium permanganate (Blackmore and Barnes 1987; Blackmore and Claugher 1987). SAPs determine SP accumulation at specific sites. It is probable that in the Arabidopsis male-sterile mutant efd (Hu et al. 2014), where such “specific sites” of SP accumulation are disturbed, SAPs are lost. Therefore, the role of the genome is not restricted to the initial stage but applies also to later stages, determining specific positions of SP accumulation.
The involvement of self-assembly processes in exine development is supported by (1) morphological-ontogenetic data and (2) in vitro experimental simulations of spore-like and exine-like patterns (Hemsley et al. 1996a, 1998, 2000; Griffiths and Hemsley 2001; Hemsley et al. 2003; Moore et al. 2009; Gabarayeva and Grigorjeva 2013, 2016). Simulations, obtained in vitro conditions of colloidal mixtures, when the influence of genome was absent, represented a number of patterns, mimicking structure of spores and separate layers of microspore exines—ectexine, endexine, sponge-like layers, spine-like, and gemma-like structures—at different development stages.
The evidence suggests that genome and self-assembly share control of exine formation.
Self-assembly as an instrument of evolution
Self-assembly is a physico-chemical phenomenon, operating in colloidal systems. Because the cytoplasm and spore/microspore periplasmic space are colloidal systems, self-assembly a priori should function there (self-assembly of microtubules, microfilaments, plasma membrane, etc.). Mandelbrot (1982) suggested that nature need not overload genetic code with detail, much is predetermined by self-assembly. The genetic code need not describe the complexity of the ultimate structure, but only the initial conditions (Regier and Hatzopoulos 1988). The role of self-organization in living nature was discussed by Kurakin (2005), in an interdisciplinary review of Benítez (2013). Lintilhac (2014) confirms that physical forces play a prominent role in development.
The intrinsic feature of self-assembly is its nonlinear regularities. Self-assembly mechanisms are governed by attractors—a limited number of possible end states (Kauffman 1993). Hemsley has shown (1998) that a sudden, spasmodic character of self-assembly may result in well-known phenomena: appearance of similar patterns in genetically remote species and sharply different patterns in genetically close species. Slight deviation in genetic control (of substance concentrations, etc.) may cause an abrupt or no change in exine development. Indeed, a switch from mainly radially oriented ectexine constructive elements (columellae, alveolae, etc.) to tangentially oriented endexine lamellae is reminiscent of a phase transition in physics when gradual changes in concentration or in other parameters produce abrupt macroscopic changes between discrete stable states (Ingber 2003).
Why has evolution saved such a “wilful” instrument in its toolbox? Hemsley and Griffiths suggested (2000) that the incorporation of self-assembly mechanisms in development is advantageous to organisms in some respects. First, such systems are energy-saving in comparison with those where for ultimate structure the encoding in genome and then decoding via enzymes are needed. The energy needed for aggregation of two colloidal particles in a colloidal system is less than the energy required for their dissociation, so such a system will tend to form aggregates. Therefore, if an organism synthesizes a colloidal dispersion (e.g. in the microspore periplasmic space), the formation of colloidal crystals would be a regular thing. Second, self-assembly systems can switch from the production of one pattern to another with only minor modification of the initial conditions. The latter is supported by simulation experiments (see above). This confirms the opinion (Hemsley 1998) that evolutionary change (in the form of speciation) could be relatively rapid and that the deviation from the primary type of development may be multiplied up many times as the consequence of nonlinear character of self-assembly.
Role of plastids and RER in exine development
It was shown (Piffanelli et al. 1998; Murphy and Vance 1999) and currently generally accepted by numerous studies on Arabidopsis and rice mutants that SP monomers are synthesized in the tapetum and are delivered into the developing microspore walls after tetrad disintegration (e.g. Ariizumi et al. 2004; Ariizumi and Toriyama 2011; Dobritsa et al. 2011; Liu and Fan 2013; Lallemand et al. 2013; Lou et al. 2014; Quilichini et al. 2014; Shi et al. 2015). It was shown that in Arabidopsis tapetal plastids, de novo synthesized fatty acids undergo consecutive modifications in ER or in plastids themselves (Dobritsa et al. 2009; Chen et al. 2011). A number of genes proteins form a sporopollenin metabolon in the ER to effectively produce lipidic and phenolic precursors for sporopollenin biosynthesis (Lallemand et al. 2013).
Though it has been shown that the synthesis of the sporopollenin and tryphine precursors (fatty acids and phenylpropanois) was controlled by the sporophytic genes expressed in tapetal cells (Li and Zhang 2010), some authors hold the opinion that the source of the SP monomer during the tetrad period can be the microspore itself (Heslop-Harrison and Dickinson 1969; Echlin 1971; Heslop-Harrison 1976; Dickinson 1976; Dickinson and Potter 1976; Audran 1981; Gabarayeva 1991, 1993, 1996; Gabarayeva and Grigorjeva 2002; Gabarayeva and Hemsley 2006; Hemsley and Gabarayeva 2007). An interesting remark appeared in the review of Wallace and coauthors (2011, p. 10): “The observation that many of the genes … are predominately expressed sporophytically is somewhat at odds with the fact that exine development mostly occurs at the surface of individual microspores after meiosis”. These authors have concluded that wall development is controlled by both the diploid tapetum and haploid microspores, and identified microspores as an important additional site of SP biosynthesis. Tapetum is the major tissue for synthesizing sporopollenin precursors, as evidenced by expression analysis (Shi et al. 2015), but additional issue could be microspores themselves, even if low in quantity. Indeed, it is strange enough that the tetrad period is persistently ignored or neglected in the bulk of studies on mutants (a rare exclusion see in Fig. 1 of Shi et al. 2015) apparently because SP accumulation in the tetrad period is scanty in comparison with that in the post-tetrad one. But, an advantage in quantity far from always is the main point in comparison with the advantage in quality. The oxidation degradation experiments of a number of authors (Southworth 1974, 1986; Rowley and Prijianto 1977; Rowley and Dahl 1977; Blackmore and Claugher 1987; Rowley and Claugher 1991; Gabarayeva et al. 2003a, b) have unambiguously shown that the so-called receptor-dependent SP, which accumulates in small amount exclusively in the tetrad period, is very resistant to severe degradation—unlike massive amounts of receptor-independent, tapetum-derived SP, accumulating in the post-tetrad period. If the process of complex exine development is imagined in architect-engineering terms, all the main work—preparation of blueprints (genome-determined concentrations of substances in the periplasmic space), house basement and installation of the scaffold (the appearance of the glycocalyx by self-assembly), marking (the appearance of SAPs, under genome control), erection of walls, and installation of overlapping (the appearance of the primexine, with all but fragile characters of the mature ectexine)—all these key operations proceed in the tetrad period, whereas dress work or decorating (massive accumulation of tapetum-originated SP on the primexine) is an auxillary, cumulative process, a matter of post-tetrad time.
Another doubt is cast upon exclusively tapetal origin of SP precursors because of the problem with penetration of fatty acids and phenylpropanoids through the thick callose envelope to the periplasmic space in the tetrad period. Recent investigations indicate that lipid transfer protein (LTP) proteins, may be responsible for the transport of sporopollenin precursors through the plasma membrane (Zhang and Li 2014), not through callose.
Our data on Polemonium show that plastids (Fig. 5e–g) and RER (Fig. 5a–d) of the microspores apparently participate in synthesis of some lipoid substances which are delivered to the microspore plasma membrane and transferred (probably by active monomolecular transport—Markham et al. 2000a, b) into the periplasmic space: Both are observed in direct contact with the plasma membrane at the late tetrad stage and slightly later (Figs. 7 and 8), and plastids are often in close contacts with RER cisternae (Fig. 9b). These tight contacts bring about heavy lipid accumulations on the plasma membrane. Lipid transfer proteins (LTPs) may be also involved in the process (Li-Beisson et al. 2010). A generation of small plastids (or mitochondria—these double-membrane organelles is difficult to specify) bears needle-like crystals, like in developing microspores in Passiflora racemosa (Fig. 7a, corner insets) and in Chamaedorea microspadix (Fig. 8a, corner inset). The explanation for this could be that these organelles synthesize fatty acids, which are known to be turned on certain conditions to lead soaps, and the latter have the form of needle-like crystals (Geyer 1973). The evidence that long-chain fatty acid synthesis is delegated to the plastids has been suggested by Roughan and Slack (1982) and confirmed later: In Arabidopsis, de novo fatty acid synthesis is localized in plastids (Li-Beisson et al. 2010; Ariizumi and Toriyama 2011).
The main groups of lipoid SP precursors and monomers are aliphatic units—fatty acids (de Leeuw et al. 2006) and phenyl-propanoids—and aromatic units, especially cinnamic acid derivations (Gubatz et al. 1986; Herminghaus et al. 1988; Gubatz and Wiermann 1992, 1993; Wiermann and Gubatz 1992; Gubatz et al. 1993; Hemsley et al. 1992a, 1993, 1996b; Wilmesmeier and Wiermann 1995, 1997; Niester-Nyveld et al. 1997; Van Bergen et al. 2004; Grienenberger et al. 2010; Legrand 2010; Quilichini et al. 2015).
It is known that enzymes of phenyl-propanoid metabolism are located on ER membranes in Arabidopsis thaliana (Burbulis and Winkel-Shirley 1999) and Nicotiana tabacum (Achnine et al. 2004). It has been also shown that plastids take part in the synthesis of different phenolics, including p-coumaric acid (Goodwin and Mercer 1983; Mousdale and Coggins 1985; Zaprometov 1993; Muravnik and Shavarda 2011). These data, together with our morphological evidence, add some weight to the supposition that plastids of microspores, in tandem with RER cisternae, synthesize some small but very important part of SP precursors/ monomers, accumulated on the primexine in the tetrad period.
Tapetum
The tapetum during the pollen wall development in Polemonium caeruleum is surprisingly not impressive: Its ultrastructural features do not change significantly from the early tetrad period up to middle free microspore stage and are shown in Fig. 6c. In free microspores, the tapetum is already disintegrated, supplying actually completed exine with additional SP precursors. No orbicules or triphyne are evident.
Conclusions
-
1.
The sequence of the exine developmental events in Polemonium caeruleum, traced in detail, has shown that it fits well to the sequence of self-assembling micellar mesophases. The same phenomenon was observed in all other species under our (and other authors’) studies. These observations, together with experimental mimicking of exine in vitro in colloidal solutions (Gabarayeva and Grigorjeva 2013, 2016), provide strong evidence for the important role of self-assembly in exine development.
-
2.
The evidence suggests that genome and self-assembly probably share control of exine formation.
-
3.
It is highly probable that self-assembly is an intrinsic instrument of evolution. Being an energy-favorable physico-chemical phenomenon operating in colloidal systems, self-assembly plays a prominent role in living nature, particularly in sporoderm development. One of advantages, conferred by self-assembly, is a high potential of variability, inherent in non-linear systems.
-
4.
The data obtained allow to conclude that microspore plastids, as well as tapetal ones, participate in the synthesis of lipid substances—probably fatty acids and phenyl-propanoids (sporopollenin monomers) for developing exine. Close contacts of two types of plastids and RER cisternae with the microspore plasma membrane suggest transfer of these substances to the periplasmic space.
Abbreviations
- ER:
-
Endoplasmic reticulum
- RER:
-
Rough endoplasmic reticulum
- SAPs:
-
SP-acceptor particles
- SEM:
-
Scanning electron microscope
- TEM:
-
Transmission electron microscope
- SP:
-
Sporopollenin
References
Achnine L, Blancaflor EB, Rasmussen S, Dixon RA (2004) Colocalization of l-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channelling in phenylpropanoid biosynthesis. Plant Cell 16:3098–3109
Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Ann Rev Plant Biol 62:437–460
Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39:170–181. doi:10.1111/j.1365-313X.2004.02118.x
Audran J-C (1981) Pollen and tapetum development in Ceratozamia mexicana (Cycadaceae): sporal origin of the exinic sporopollenin in cycads. Rev Palaeobot Palynol 33:315–346
Ball P (1994) Designing the molecular world. Princeton University Press, Princeton
Benítez M (2013) An interdisciplinary view on dynamic models for plant genetics and morphogenesis: scope, examples and emerging research avenues. Front Plant Sci 4:7. doi:10.3389/fpls.2013.00007
Blackmore S, Barnes SH (1987) Pollen wall morphogenesis in Tragopogon porrifolius L. (Compositae: Lactuceae) and its taxonomic significance. Rev Palaeobot Palynol 52:233–246
Blackmore S, Claugher D (1987) Observations on the substructural organization of the exine in Fagus sylvatica L. (Fagaceae) and Scorzonera hispanica L. (Compositae: Lactuceae). Rev Palaeobot Palynol 5:175–184
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498
Burbulis IE, Winkel-Shirley B (1999) Interactions among enzymes of the Arabidopsis flavonoid biosynthetic pathway. Proc Nat Acad Sci USA 96:12929–12934
Chen W et al (2011) Male Sterile2 encodes a plastid-localized fatty acyl carrier protein reductase required for pollen exine development in Arabidopsis. Plant Physiol 157:842–853
Collinson ME, Hemsley AR, Taylor WA (1993) Sporopollenin exhibiting colloidal organization in spore walls. Grana Suppl 1:31–39
de Leeuw JW, Versteegh GJM, van Bergen PF (2006) Biomacromolecules of algae and plants and their fossil analogues. Plant Ecol 182:209–233
Dickinson HG (1970) Ultrastructural aspects of primexine formation in the microspore tetrad of Lilium longiflorum. Cytobiologie 1:437–449
Dickinson HG (1976) Common factors in exine deposition. In: Ferguson IK, Muller J (eds) The evolutionary significance of the exine. Academic Press, London, pp 67–89
Dickinson HG, Potter U (1976) The development of patterning in the alveolar sexine of Cosmos bipinnatus. New Phytol 76:543–550
Dickinson HG, Sheldon JM (1986) The generation of patterning at the plasma membrane of the young microspore of Lilium. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Linn Soc Symp Ser, no. 12. Academic Press, London, pp 1–18
Dobritsa AA et al (2009) CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol 151:574–589
Dobritsa AA, Geanconteri A, Shrestha J et al (2011) A large-scale genetic screen in Arabidopsis to identify genes involved in pollen Exine production. Pl Physiol (Lancaster) 157:947–970. doi:10.1104/pp.111.179523
Echlin P (1971) The role of the tapetum during microsporogenesis of angiosperms. In: Heslop-Harrison J (ed) Pollen development and physiology. Butterworths, London, pp 41–61
Fridrichsberg DA (1995) Colloidal chemistry. Chemistry, St.-Petersburg
Gabarayeva NI (1990) Hypothetical ways of exine structure determination. Botanicheski Zhurnal 75:1353–1362 (in Russian, with English abstract)
Gabarayeva NI (1991) Patterns of development in primitive angiosperm pollen. In: Blackmore S, Barnes SH (eds) Pollen and spores. Clarendon Press, Oxford, pp 257–268
Gabarayeva NI (1993) Hypothetical ways of exine pattern determination. Grana 33(Suppl 2):54–59
Gabarayeva NI (1995) Pollen wall and tapetum development in Anaxagorea brevipes (Annonaceae): sporoderm substructure, cytoskeleton, sporopollenin precursor particles, and the endexine problem. Rev Palaeobot Palynol 85:123–152
Gabarayeva NI (1996) Sporoderm development in Liriodendron chinense (Magnoliaceae): a probable role of the endoplasmic reticulum. Nordic J Bot 16:1–17
Gabarayeva NI (2000) Principles and recurrent themes in sporoderm development. In: Harley MM, Morton CM, Blackmore S (eds) Pollen and spores: morphology and biology. Royal Botanic Gardens, Kew, pp 1–17
Gabarayeva NI (2014) Role of genetic control and self-assembly in gametophyte sporoderm ontogeny: hypotheses and experiment. Russian J Develop Biol 45:177–195
Gabarayeva NI, Grigorjeva VV (2002) Exine development in Stangeria eriopus (Stangeriaceae): ultrastructure and substructure, SP accumulation, the equivocal character of the aperture, and stereology of microspore organelles. Rev Palaeob Palynol 122:185–218
Gabarayeva NI, Grigorjeva VV (2004) Exine development in Encephalartos altensteinii (Cycadaceae): ultrastructure, substructure and the modes of SP accumulation. Rev Palaeobot Palynol 132:175–193
Gabarayeva NI, Grigorjeva VV (2010) Sporoderm ontogeny in Chamaedorea microspadix (Arecaceae). Self-assembly as the underlying cause of development. Grana 49:91–114
Gabarayeva NI, Grigorjeva VV (2011) Sporoderm development in Swida alba (Cornaceae), interpreted as a self-assembling colloidal system. Grana 50:81–101
Gabarayeva NI, Grigorjeva VV (2012) Sporoderm development and substructure in Magnolia sieboldii and other Magnoliaceae: an interpretation. Grana 51:119–147
Gabarayeva NI, Grigorjeva VV (2013) Experimental modelling of exine-like structures. Grana 52:241–257. doi:10.1080/00173134.2013.818165
Gabarayeva NI, Grigorjeva VV (2014) Sporoderm and tapetum development in Eupomatia laurina (Eupomatiaceae). An interpretation Protoplasma 251:1321–1345
Gabarayeva N, Grigorjeva V (2016) Simulation of exine patterns by self-assembly. Pl Syst Evol 302:1135–1156. doi:10.1007/s00606-016-1322-6
Gabarayeva NI, Hemsley AR (2006) Merging concepts: the role of self-assembly in the development of pollen wall structure. Rev Palaeobot Palynol 138:121–139
Gabarayeva NI, Rowley JR (1994) Exine development in Nymphaea Colorata (Nymphaeaceae). Nord J Bot 14:671–691
Gabarayeva NI, Rowley JR, Skvarla JJ (1998) Exine development in Borago (Boraginaceae). 1. Microspore tetrad period. Taiwania 43:203–214
Gabarayeva NI, Blackmore S, Rowley JR (2003a) Observations on the experimental destruction and on substructural organization of the pollen wall of some selected gymnosperms and angiosperms. Rev Palaeobot Palynol 124:203–226
Gabarayeva NI, Grigorjeva VV, Rowley JR (2003b) Sporoderm ontogeny in Cabomba aquatica (Cabombaceae). Rev Palaeobot Palynol 127:147–173
Gabarayeva N, Grigorjeva V, Rowley JR, Hemsley AR (2009a) Sporoderm development in Trevesia burckii (Araliaceae). I. Tetrad period: further evidence for the participation of self-assembly processes. Rev Palaeobot Palynol 156:211–232
Gabarayeva N, Grigorjeva V, Rowley JR, Hemsley AR (2009b) Sporoderm development in Trevesia burckii (Araliaceae). II. Post-tetrad period: further evidence for participating of self-assembly processes. Rev Palaeob Palynol 156:233–247
Gabarayeva NI, Grigorjeva VV, Rowley JR (2010a) A new look at sporoderm ontogeny in Persea americana. Micelles and the hidden side of development. Ann Bot 105:939–955
Gabarayeva NI, Grigorjeva VV, Rowley JR (2010b) Sporoderm development in Acer tataricum (Aceraceae). An interpretation. Protoplasma 247:65–81
Gabarayeva NI, Grigorjeva VV, Polevova SV (2011a) Exine and tapetum development in Symphytum officinale (Boraginaceae). Exine substructure and its interpretation. Plant Syst Evol 296:101–120
Gabarayeva NI, Grigorjeva VV, Marquez G (2011b) Ultrastructure and development during meiosis and the tetrad period of sporogenesis in the leptosporangiate fern Alsophila setosa (Cyatheaceae) compared with corresponding stages in Psilotum nudum (Psilotaceae). Grana 50:235–262
Gabarayeva NI, Grigorjeva V, Kosenko Y (2013a) I. Primexine development in Passiflora racemosa Brot. Overlooked aspects of development. Plant Syst Evol 299:1013–1035
Gabarayeva N, Grigorjeva V, Kosenko Y (2013b) II. Exine development in Passiflora racemosa Brot.: post-tetrad period. Overlooked aspects of development. Plant Syst Evol 299:1037–1055
Gabarayeva N, Grigorjeva V, Polevova S (2014) Sporoderm and tapetum ontogeny in Juniperus communis (Cupressaceae). Connective structures between tapetum and microspores. Rev Palaeobot Palynol 206:23–44
Gabarayeva NI, Grigorjeva VV, Blackmore S (2016) Pollen wall substructure and development in Tanacetum vulgare (Compositae: Anthemideae): revisiting hypotheses on pattern formation in complex cell walls. Int J Plant Sci 177:347–370. doi:10.1086/684946
Gabarayeva N, Grigorjeva V, Polevova S, Hemsley AR (2017) Pollen wall and tapetum development in Plantago major (Plantaginaceae): assisting self-assembly. Grana 56:81–111. doi:10.1080/ 00173134.2016.1159729
Gerasimova-Navashina EN (1973) Physico-chemical nature of primexine formation of angiosperm pollen grains. In: Kovarski A (ed) Embryology of angiosperms. Ştiinţǎ, Kishinev, pp 57–70
Geyer G (1973) Ultrahistochemie. Veb Gustav Fischer Verlag, Jena
Goodwin TW, Mercer EI (1983) Introduction to plant biochemistry. Pergamon, Oxford
Grienenberger E, Kim SS, Lallemand B, Geoffroy P, Heintz D, Souza CA, Heitz T, Douglas CJ, Legrand M (2010) Analysis of tetraketide α-pyrone reductase function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Pl Cell 22:4067–4083. doi:10.1105/tpc.110.080036
Griffiths PC, Hemsley AR (2001) Rasberries and muffins—mimicking biological pattern formation. Colloids and Surfaces B Biointerfaces 25:163–170
Grigorjeva V, Gabarayeva N (2015) The development of sporoderm, tapetum and Ubisch bodies in Dianthus deltoides (Caryophyllaceae): self-assembly in action. Rev Palaeobot Palynol 219:1–27
Gubatz S, Wiermann R (1992) Studies on sporopollenin biosynthesis in Tulipa anthers. 3. Incorporation of specifically labeled C-14 phenylalanine in comparison to other precursors. Bot Acta 105:407–413
Gubatz S, Wiermann R (1993) Studies on sporopollenin biosynthesis in Cucurbita maxima. 1. The substantial labeling of sporopollenin from Cucurbita maxima after application of [C-14] phenylalanine. J Biosci 48:10–15
Gubatz S, Herminghaus S, Meurer B, Strack D, Wiermann R (1986) The location of hydroxycinnamic acid amides in the exine of Corylus pollen. Pollen Spores 28:347–354
Gubatz S, Rittscher M, Meuter A, Nagler A, Wiermann R (1993) Tracer experiments on sporopollenin biosynthesis. Grana Suppl 1:12–17
Halbritter H, Svojtka M (2005) Polemonium caeruleum. In: PalDat (2005–06-01)—a palynological database
Hemsley AR (1998) Nonlinear variation in simulated complex pattern development. J Theor Biol 192:73–79
Hemsley AR, Gabarayeva NI (2007) Exine development: the importance of looking through a colloid chemistry “window”. Plant Syst Evol 263:25–49
Hemsley AR, Griffiths PC (2000) Architecture in the microcosm: biocolloids, self-assembly and pattern formation. Phil Trans R Soc Lond A 358:547–564
Hemsley AR, Chaloner WG, Scott AC, Groombridge CJ (1992a) Carbon-13 solid-state nuclear magnetic resonance of sporopollenins from modern and fossil plants. Ann Bot (Oxford) 69:545–549
Hemsley AR, Collinson ME, Brain APR (1992b) Colloidal crystal-like structure of sporopollenin in the megaspore walls of recent Selaginella and similar fossil spores. Bot J Linn Soc 108:307–320
Hemsley AR, Barrie PJ, Chaloner WG, Scott AC (1993) The composition of sporopollenin: its contribution to living and fossil spore systematics. Grana Suppl 1:2–11
Hemsley AR, Jenkins PD, Collinson ME, Vincent B (1996a) Experimental modelling of exine self-assembly. Bot J Linn Soc 121:177–187
Hemsley AR, Scott AC, Barrie PJ, Chaloner WG (1996b) Studies of fossil and modern spore wall biomacromolecules using 13C solid state NMR. Ann Bot (Oxford) 78:83–94
Hemsley AR, Vincent B, Collinson ME, Griffiths PC (1998) Simulated self-assembly of spore exines. Ann Bot (Oxford) 82:105–109
Hemsley AR, Collinson ME, Vicent B, Griffiths PC, Jenkins PD (2000) Self-assembly of colloidal units in exine development. In: Harley MM, Morton CM, Blackmore S (eds) Pollenand spores: morphology and biology. Whitstable Printers Ltd, Whitstable, pp 31–44
Hemsley AR, Griffiths PC, Mathias R, Moore SEM (2003) A model for the role of surfactants in the assembly of exine sculpture. Grana 42:38–42
Herminghaus S, Gubatz S, Arendt S, Wiermann R (1988) The occurrence of phenols as degradation products of natural sporopollenin—a comparison with “synthetic sporopollenin”. Z Naturf 43c:491–500
Heslop-Harrison J (1972) Pattern in plant cell wall: morphogenesis in miniature. Proc Royal Inst Great Brit 45:335–351
Heslop-Harrison J (1976) The adaptive significance of the exine. In: Ferguson IK, Muller J (eds) The evolutionary significance of the exine. Academic Press, London, pp 27–37
Heslop-Harrison J, Dickinson HG (1969) Time relationships of sporopollenin synthesis associated with tapetum and microspores in Lilium. Planta 84:199–214
Hu J, Wang Z, Zhang L, Sun MX (2014) The Arabidopsis Exine formation defect (EFD) gene is required for primexine patterning and is critical for pollen fertility. New Phytol 203:140–154
Ingber DE (2003) Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci 116:1397–1408
Kauffman SA (1993) The origins of order. Univ. Press, Oxford
Kurakin A (2005) Self-organization versus watchmaker: stochastic dynamics of cellular organization. Biol Chem 386:247–254
Lallemand B, Erhardt M, Heitz T, Legrand M (2013) Sporopollenin biosynthetic enzymes interact and constitute a metabolon localized to the endoplasmic reticulum of tapetum cells. Plant Physiol 162:616–625
Legrand M (2010) Analysis of of tetraketide α-pyrone reductase function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Pl Cell 22:4067–4083
Li H, Zhang D (2010) Biosynthesis of anther cuticle and pollen exine in rice. Plant Signal Behav 5:1121–1123
Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, DeBono A, Durrett TP, Franke RB, Graham IA, Katayama K, Kelly AA, Larson T, Markham JE, Miquel M, Molina I, Nishida I, Rowland O, Samuels L, Schmid KM, Wada H, Welti R, Xu C, Zallot R, Ohlrogge J (2010) Acyl-Lipid Metabolism. The Arabidopsis Book 8:e0133. doi:10.1199/tab.0133
Lintilhac PM (2014) The problem of morphogenesis: unscripted biophysical control systems in plants. Protoplasma 251:25–36. doi:10.1007/s00709-013-0522-y
Liu L, Fan X-D (2013) Tapetum: regulation and role in SP biosynthesis in Arabidopsis. Plant Mol Biol 83:165–175
Lou Y, Xu XF, Zhu J, Gu JN, Blackmore S, Yang ZN (2014) The tapetal AHL family protein TEK determines nexine formation in the pollen wall. Nature Plants. doi:10.1038/ncomms4855
Mandelbrot BB (1982) The fractal geometry of nature. W. H. Freeman and co., San Francisco
Markham KR, Gould KS, Winefield CS, Mitchell KA, Bloor SJ, Boase MR (2000a) Anthocyanic vacuolar inclusions–their nature and significance in flower colouration. Phytochemistry 55:327–336
Markham KR, Ryan KG, Gould KS, Rickards GK (2000b) Cell wall sited flavonoids in Lisianthus flower petals. Phytochemistry 54:681–687
Moore SEM, Gabarayeva N, Hemsley AR (2009) Morphological, developmental and ultrastructural comparison of Osmunda regalis L. spores with spore mimics. Rev Paleobot Palynol 156:177–184
Mousdale DM, Coggins JR (1985) Subcellular localization of the common shikimate-pathway enzymes in Pisum sativum L. Planta 163:231–249
Muravnik LE, Shavarda AL (2011) Pericarp peltate trichomes in Pterocarya rhoifolia: histochemistry, ultrastructure, and chemical composition. Int J Plant Sci 172(2):159–172
Murphy DJ, Vance J (1999) Mechanisms of lipid-bodies formation. TIBS 24:109–115
Niester-Nyveld C, Haubrich A, Kampendonk H et al. (1997) Immunocytochemical localization of phenolic compounds in pollen walls using antibodies against p-coumaric acid coupled to bovine serum albumin. Protoplasma 197: 148-159
Pettitt JM (1979) Ultrastructure and cytochemistry of spore wall morphogenesis. In: Dyer AF (ed) The experimental biology of ferns. Academic Press, London – New York – San Francisco, pp 211–252
Pettitt JM, Jermy AC (1974) The surface coats on spores. Biol J Linn Soc 6:245–257
Piffanelli P, Ross JHE, Murphy DJ (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod 11:65–80
Quilichini TD, Douglas CJ, Samuels AL (2014) New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann Bot, mcu042. doi:10.1093/aob/mcu042
Quilichini TD, Grienenberger E, Douglas CJ (2015) The biosynthesis, composition and assembly of the outer pollen wall: a tough case to crack. Phytochemistry 113:170–182
Regier JC, Hatzopoulos AK (1988) Evolution in steps: the role of regulatory alterations in the diversification of the moth chorion morphogenetic pathway. In: Varner JE (ed) Self-assembling architecture. Alan R. Liss, New York, pp 179–202
Roughan PG, Slack CR (1982) Cellular organization of glycerolipid metabolism. Ann Rev Plant Physiol 33:97–132
Rowley JR (1971) Implications on the nature of sporopollenin based upon pollen development. In: Brooks J, Grant PR, Muir M, van Gijzel P, Shaw G (eds) Sporopollenin. Academic Press, London, pp 174–219
Rowley JR (1973) Formation of pollen exine bacules and microchannels on a glycocalyx. Grana 13:129–138
Rowley JR (1975) Lipopolysaccharide embedded within the exine of pollen grains. In: Bailey GW (ed), 33rd Ann Proc Electron Microscopy Soc Amer Las Vegas, Nevada, pp 572–573
Rowley JR (1990) The fundamental structure of the pollen exine. Plant Syst Evol Suppl 5:13–29
Rowley JR, Dahl AO (1977) Pollen development in Artemisia vulgaris with special reference to glycocalyx material. Pollen Spores 19:169–284
Rowley JR, Claugher D (1991) Receptor-independent sporopollenin. Botanica Acta 104:316–323
Rowley JR, Rowley JS (1998) Stain reversal in pollen exines. In: Narendra MD (ed) Current concepts in pollen-spore and biopollination research. Research Periodicals and Book Publishing House, India – USA – UK, pp 223–232
Rowley JR, Prijianto B (1977) Selective destruction of the exine of pollen grains. Geophytology 7:1–23
Rowley JR, Flynn JJ, Takahashi M (1995) Atomic force microscope information on pollen exine substructure in Nuphar. Bot Acta 108:300–308
Rowley JR, Skvarla JJ, Gabarayeva NI (1999) Exine development in Borago (Boraginaceae). 2. Free microspore stages. Taiwania 44:212–229
Rowley JR, Gabarayeva NI, Skvarla JJ, El-Ghazaly G (2001) The effect of 4-methylmorpholine N-oxide monohydrate (MMNO.H2O) on pollen and spore exines. Taiwania 46:246–273
Sampson FB (1977) Pollen tetrads of Hedycarya arborea J. R. Et G. Forst. (Monimiaceae). Grana 16:61–73
Sampson FB (2000) Pollen diversity in some modern magnoliids. Int J Plant Sci 161:193–210
Scott RJ (1994) Pollen exine—the sporopollenin enigma and the physics of pattern. In: Scott RJ, Stead MA (eds) Society for Experimental Biology seminar Ser 55: molecular and cellular aspects of plant reproduction. Cambridge Univ Press, Cambridge, pp 49–81
Sheldon JM, Dickinson HG (1983) Determination of patterning in the pollen wall of Lilium henryi. J Cell Sci 63:191–208
Shi J, Cui M, Yang L, Kim Y-J, Zhang D (2015) Genetic and biochemical mechanisms of pollen wall development. Trends Pl Sci 20:741–753
Skvarla JJ, Rowley JR (1987) Ontogeny of pollen in Poinciana (Leguminosae). I. Development of exine template. Rev Palaeobot Palynol 50:239–311
Southworth D (1974) Solubility of pollen exines. Am J Bot 61:36–44
Southworth D (1986) Substructural organization of pollen exines. In: Blackmore S, Ferguson IK (eds) Pollen spores: form and function. Acad. Press, London, pp 61–69
Southworth D, Jernstedt JA (1995) Pollen exine development precedes microtubule rearrangement in Vigna unguiculata (Fabaceae): a model for pollen wall patterning. Protoplasma 187:79–87
Taylor ML, Osborn JM (2006) Pollen ontogeny in Brasenia (Cabombaceae, Nymphaeales). Amer J Bot 93:344–356
Taylor ML, Hudson PJ, Rigg JM, Strandquist JN, Green JS, Thiemann TC, Osborn JM (2013) Pollen ontogeny in Victoria (Nymphaeales). Int J Plant Sci 174:1259–1276
Taylor M, Cooper RL, Schneider EL, Osborn JM (2015) Pollen structure and development in Nymphaeales: insights into character evolution in an ancient angiosperm lineage. Am J Bot 102:1–18. doi:10.3732/ajb.1500249
Thompson DW (1961) On growth and form. Abridged edition. Cambridge university Press, Cambridge
van Bergen PF, Blokker P, Collinson ME, Sinninghe Damsté JS, de Leeuw JW (2004) Structural biomacromolecules in plants: what can be learnt from the fossil record? In: Hemsley AR, Poole I (eds) The evolution of plant physiology. Academic Press, Amsterdam, pp 134–154
van Uffelen GA (1991) The control of spore wall formation. In: Blackmore S, Barnes SH (eds) Pollen and spores: patterns of diversification. Clarendon Press, Oxford, pp 89–102
Vinckier S, Smets E (2005) A histological study of microsporogenesis in Tarenna gracilipes (Rubiaceae). Grana 44:30–44
Waha M (1987) Sporodcrm development of pollen tetrads in Asimina triloba (Annonaceae). Pollen Spores 29:31–44
Wallace S, Fleming A, Wellman CH, Beerling DJ (2011) Evolutionary development of the plant spore and pollen wall. AoB PLANTS 2011:plr027. doi:10.1093/aobpla/plr027
Wellman CH (2004) Origin, function and development of the spore wall in early land plants. In: Hemsley AR, Poole I (eds) The evolution of plant physiology. Royal Botanic Gardens, Kew, pp 43–63
Wiermann R, Gubatz S (1992) Pollen wall and sporopollenin. Int Rev Cytol 140:35–72
Wilmesmeier S, Wiermann R (1995) Influence of EPTC (S-ethyl-dipropyl-thiocarbamate) on the composition of surface waxes and sporopollenin structure in Zea mays. J Pl Physiol 146:22–28
Wilmesmeier S, Wiermann R (1997) Immunocytochemical localization of phenolic compounds in pollen walls using antibodies against p-coumaric acid coupled to bovine serum albumin. Protoplasma 197:148–159
Zaprometov MN (1993) Phenolic compounds: distribution, metabolism and functions in plants. Nauka, Moskow
Zavada MS, Gabarayeva NI (1991) Comparative pollen wall development of Welwitschia mirabilis and selected primitive angiosperms. Bull Torrey Bot Club 118:292–302
Zhang D, Li H (2014) Exine export in pollen. In: Geisler M. et al., eds. Plant ABC Transporters. Springer, IPS, pp 49–62
Acknowledgements
This work was partly supported by grant no. 17-04-00517 of Russian Foundation for Basic Research for Nina Gabarayeva (chemicals) and partly provided in the framework of institutional research project of the Komarov Botanical Institute of the Russian Academy of Sciences (using equipment of The Core Facility Center “Cell and Molecular Technologies in Plant Science”). The authors wish to thank Dr. F. Bruce Sampson for some comments and suggested improvements to the English. Our special thanks to our anonymous reviewers.
Author information
Authors and Affiliations
Contributions
Conceptualization: N.G.; Methodology: N.G., V. G.; Investigation: N.G., V.G.; Righting original draft, review and editing: N.G.; Funding acquisition: N.G.; Resources: V.G.; Supervision: N.G.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Handling Editor: Benedikt Kost
Rights and permissions
About this article
Cite this article
Grigorjeva, V.V., Gabarayeva, N. Pollen wall ontogeny in Polemonium caeruleum (Polemoniaceae) and suggested underlying mechanisms of development. Protoplasma 255, 109–128 (2018). https://doi.org/10.1007/s00709-017-1121-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1121-0