Abstract
Rice bacterial blight, caused by Xanthomonas oryzae pv. oryzae (Xoo), is a severe disease of rice plants. Upon pathogen infection, rice biosynthesizes phytoalexins, including diterpenoids such as momilactones, phytocassanes, and oryzalexins. However, information on headspace volatiles in response to Xoo infection is limited. We have examined headspace volatile terpenes, induced by the infection of Xoo, and investigated their biological roles in the rice plant. Monoterpenes α-thujene, α-pinene, sabinene, myrcene, α-terpene, and (S)-limonene and sesquiterpenes cyclosativene, α-copaene, and β-elemene were detected from 1-week-old Xoo-infected rice seedlings, by solid-phase microextraction–gas chromatography–mass spectrometry. All monoterpenes were constitutively released from rice seedlings before Xoo infection. However, (S)-limonene emission was further elicited after exposure of the seedlings to Xoo in coincidence with upregulation of limonene synthase gene (OsTPS20) transcripts. Only the stereospecific (S)-limonene [and not (R)-limonene or other monoterpenes] severely inhibited Xoo growth, as confirmed by disc diffusion and liquid culture assays. Rice seedlings showed suppressed pathogenic symptoms suggestive of resistance to Xoo infection after foliar treatment with (S)-limonene. Collectively, our findings suggest that (S)-limonene is a volatile phytoanticipin, which plays a significant role in suppressing Xoo growth in rice seedlings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xanthomonas oryzae pv. oryzae (Xoo) causes bacterial blight disease in rice plant, which severely affects rice production (Mew 1987). Xoo enters primarily through secretary tissues, such as hydathodes near the leaf tips, and proliferates within the internal tissues of infected plants. Usually, pathogenic symptoms appear at the tillering stage and a reduction in the yield of 10–20 % (as high as 50 % with severe infection) can occur. Occasionally, infection at seedling stage can cause severe pathogenic symptoms, called kresek, resulting in seedling death within a few weeks (Mew et al. 1993).
Plants have evolved mechanisms to produce a variety of secondary metabolites, including terpenoids, in interaction with environmental stimuli (Arimura et al. 2008; Bajpai et al. 2011; Loreto et al. 1998; Pare and Tumlinson 1999). Terpenes are largely classified into monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20), which are synthesized using geranyl diphosphate, farnesyl diphosphate, and geranylgeranyl diphosphate as substrates, respectively. Monoterpenes and diterpenes are mainly produced in the chloroplast via the 2-C-methyl-d-erythritol-4-phosphate pathway, while sesquiterpenes are synthesized in the cytoplasm through mevalonate-dependent pathway (Bohlmann et al. 1998; Chen et al. 2011; Lichtenthaler 1999; Tholl 2006). The amount and composition of terpenoids vary with plant species, and their production is related to environmental stimuli. For example, jasmonic acid induces the production of monoterpene linalool, which suppresses the growth of Xoo in rice in a concentration-dependent manner (Taniguchi et al. 2014a). Similarly, infection by the rice blast fungus Magnaporthe oryzae elevated the level of antifungal sesquiterpene β-elemene in rice; however, this terpene is not effective against Xoo (Taniguchi et al. 2014b). The fungus also increases the accumulation of diterpene phytoalexins in blast-resistant rice plants, but not in susceptible plants (Hasegawa et al. 2010). Rice plants biosynthesize non-volatile phytoalexins, such as a flavonoid sakuranetin (Kodama et al. 1992) and diterpenoids, including four groups of phytocassanes A–E, oryzalexins A–F, oryzalexin S, and momilactones A and B in response to biotic stresses (Cartwright et al. 1981; Kato et al. 1973, 1994; Koga et al. 1995). These phytoalexins are mainly produced against M. oryzae infection, but rice plants also accumulate atypical diterpenoids against bacterial blight Xoo, oryzalide-related compounds, which show anti-Xoo activity (Kono et al. 1991; Watanabe et al. 1990, 1992). These non-volatile compounds are constitutively produced and induced by Xoo infection and thus can be considered phytoanticipins. Recently, we discovered release of a bouquet of terpenes, mainly (S)-limonene, from rice exposed to various abiotic stresses, such as H2O2, UV-B, and γ-ray (Lee et al. 2015). Although (S)-limonene has been considered to play a role in rice seedlings as an antioxidant under oxidative environment, little is known of the biological (physiological) functions of this limonene.
The present study demonstrates profiling of headspace volatile terpenes released from the leaves of Xoo-infected rice. Biological functions of major Xoo-inducing volatiles, such as α-pinene, limonene, and α-copaene, were investigated by disc diffusion and liquid Xoo culture assays. Antibacterial limonene was specifically analyzed for its enantiomeric type, and only a stereospecific (S)-limonene showed highest anti-Xoo activity. Biological activity of (S)-limonene was further verified by in planta bioassay with its foliar pretreatment, followed by Xoo infection. The work provides fundamental understanding of rice–Xoo interactions, which can be helpful in developing a treatment method based on plant-derived natural pesticides to control bacterial blights.
Materials and methods
Plant materials and reagents
Rice plants (Oryza sativa L. spp. Japonica cv. Dongjin) were grown in half strength Murashige and Skoog (MS) solid media (10 plants/magenta box) in a magenta box [65 mm (L) × 200 mm (H) × 65 mm (W)] and maintained at a temperature of 25 °C with a light/dark cycle of 14:10 h. Authentic standards [i.e., α-pinene, (S)-limonene, and (R)-limonene] were purchased from Sigma-Aldrich, USA, and α-copaene was purchased from ACC Corporation (American Custom Chemicals Corporation, USA).
Analysis of headspace volatile compounds by gas chromatography–mass spectrometry
Leaves of 1-week-old rice seedlings were inoculated with X. oryzae pv. oryzae (Xoo) in a magenta box and transferred into a 20-mL glass vial (three seedlings/vial) after 3 days. After 1 day of incubation in the vial, headspace volatiles were collected for 60 min at 25 °C, using a 50:30 μm divinylbenzene (DVB)/carboxen (CAR)/polydimethylsiloxane (PDMS)-coated solid-phase microextraction (SPME) fiber (StableFlex 24Ga) (Supelco, USA) for gas chromatography–mass spectrometry (GC–MS) (Agilent GC system, 7890A; MSD, 5957C). The GC–MS analysis was performed with a HP-5MS capillary column (0.25 mm i.d. × 30 m, 0.25 μm film thickness) (Agilent Technologies, USA), maintaining a flow rate of helium as the carrier gas at 1 mL/min. The oven temperature was set to rise from 80 °C (3-min holding time) to 150 °C at a rate of 5 °C/min and a thermal gradient of 10 °C/min up to 250 °C and then 20 °C/min until 300 °C (3-min holding time). The conditions of mass spectrometry and chiral GC–MS were set according to Lee et al. (2015). Relative amount of volatiles was estimated in a percentile by comparing its peak area out of total peak area of all terpene volatiles detected. The enantiomeric identification of limonene was determined using an HP-Chiral-20B chiral capillary column (0.25 mm i.d. × 30 m with a 0.25-μm film) (Agilent Technologies, USA). Retention times and mass spectra of authentic (R)-limonene and (S)-limonene standards were compared with those of a volatile produced from the experimental examinations. GC program was set to a holding time of 3 min at 80 °C, followed by 1 °C/min gradient to 95 and 30 °C/min gradient up to 240 °C (3-min holding time).
Cultivation of Xoo
Xoo was cultured in nutrient broth (NB, 0.5 % peptone and 0.3 % beef extract) and a modified Wakimoto’s (WF-P) medium (2 % sucrose, 0.5 % Bacto Peptone, 0.05 % calcium nitrate, 0.082 % sodium phosphate, 0.005 % ferrous sulfate, 1.5 % agar) at 28 °C for 48 h (Karagnilla et al. 1973). For a disc diffusion assay, Xoo was cultured in NB until optical density at 595 nm (OD)595 = 1.0 and subsequently incubated on the WF-P solid medium.
Analysis of antibacterial activities of terpene hydrocarbons against Xoo (a liquid culture assay)
Xoo was cultivated in 10 ml NB for 48 h at 28 °C at a constant agitation speed of 180 rpm. The Xoo culture was diluted with NB at a ratio of 1:1 (v/v) (OD595 ≈ 1.0). Each terpene (in various concentrations) was then added into the culture and incubated at 28 °C at an agitation speed of 180 rpm. After 24 h, the optical density of the cultures, in the presence (or absence) of terpene hydrocarbons, was recorded using an Infinite™ M200 microplate reader (Tecan, Switzerland) to evaluate the inhibitory activities of the terpenes against Xoo.
Analysis of antibacterial activities of terpene hydrocarbons against Xoo (a disc diffusion assay)
Xoo was incubated on the solid WF-P medium at 28 °C for 48 h. A sterilized 8-mm paper disc (Advantec, Japan), saturated with α-pinene, (S)-limonene, (R)-limonene, and α-copaene in various concentrations, was placed in the middle of a medium plate from the beginning of incubation. The radius of inhibition zone around each disc was measured and recorded after 48 h at 28 °C. The experiments were run in triplicate.
In vivo leaf infection assay
Limonene solutions in various concentrations (i.e., 0.1, 1, 3, and 5 mM) were sprayed on the leaves of 1-week-old rice seedlings until saturation. After dryness, Xoo liquid culture in NB (OD595 ≈ 1.0) was subsequently inoculated on the leaves using the clipping inoculation method (Kauffman et al. 1973). After 1 week of incubation at 25 °C (14:10-h light/dark cycle), the level of in vivo antibacterial activity of limonene was evaluated by comparing the length of blight lesion with that of the control, which involved a spray of 0.01 % ethanol on rice leaves.
Real-time PCR and quantitative real-time PCR
The total RNA was extracted in accordance with the manufacturer’s instruction using an RNeasy Plant Mini Kit (Qiagen, USA), followed by complementary DNA synthesis using the M-MLV reverse transcriptase (Enzynomics, Republic of Korea). For reverse transcription-polymerase chain reaction (RT-PCR) with OsTPS20, 1-week-old seedlings grown in a magenta box were infected with Xoo and only the aerial plant parts were collected. OsACT (Os11g0163100) and OsUBQ (Os01g0328400) were used as internal control genes. A PCR reaction was performed as follows: 95 °C (3 min), 30 cycles of 95 °C (30 s), 55 °C (30 s), 72 °C (30 s), and 72 °C (10 min). For quantitative RT-PCR, 1-week-old seedlings were foliar sprayed with 5 mM of (S)-limonene (Fig. 4c) or were infected with Xoo on the leaves (Fig. 4b), harvesting at 24 h or 1 week after the treatments. Filter-sterilized 0.01 % ethanol (v/v) was sprayed as a control. The assay was performed using the SYBR premix reagent (Takara, Japan) with the Corbett Rotor-Gene 6000 (Corbett Life Science, Australia). The abundance of transcripts was calculated with 2−ΔΔCT method using the Rotor-Gene 6000 software pre-installed in the instrument (Livak and Schmittgen 2001). OsACT was used as an internal control. The primers used are listed in Table S2. The PCR reaction was carried out as follows: 95 °C (10 min), 40 cycles of 95 °C (10 s), 55 °C (15 s), and 72 °C (40 s), followed by 72 to 95 °C (1 °C/5 s).
Statistical analysis
Experimental data were analyzed using the Statistical Package for Social Sciences (SPSS)/Predictive Analytics Software (PASW) Statistics version 18 (Chicago, USA). Statistical significance was assessed using Duncan’s multiple range test at p < 0.01.
Results
Volatile terpenes from rice seedlings against Xoo infection
Various terpenes released from 1-week-old rice seedlings infected with Xoo are shown in Fig. 1. Rice seedlings without Xoo infection were treated as control. There were no significant differences between control and Xoo-infected rice seedlings in the species of the volatiles [i.e., monoterpenes: α-thujene, α-pinene, sabinene, phellandrene, limonene, and α-terpinolene; sesquiterpenes: cyclosativene, α-copaene, and (E)-caryophyllene], except for de novo production of β-elemene in infected seedlings (Fig. 1(A–D)). However, the infection treatment affected the amount of the volatiles, particularly limonene, which comprised ≈40 % of the total peak area of terpene volatiles (Fig. 1(E), Table S1). β-Elemene was the only terpene volatile to be de novo emitted from the Xoo-infected rice seedlings.
Volatile terpenes from rice seedlings analyzed using SPME–GC–MS [extracted ion chromatograph (m/z = 93.1)]. Monoterpenes from A control and C Xoo-infected rice seedlings, sesquiterpenes from B control and D Xoo-infected rice seedlings, and E relative amount of 10 representative volatile terpenes in control and Xoo-infected rice seedlings: 1 α-thujene, 2 α-pinene, 3 sabinene, 4 phellandrene, 5 limonene, 6 α-terpinolene, 7 cyclosativene, 8 α-copaene, 9 β-elemene, and 10 (E)-caryophyllene. Values are means ± SE (n = 3)
Chiral analysis for limonene emitted from the Xoo-infected rice seedlings
To determine the chirality of the limonene overproduced by Xoo infection, we collected the headspace volatiles from the Xoo-infected rice seedlings and analyzed using solid-phase microextraction (SPME)–chiral GC–MS. Interestingly, optical isomer of the limonene, which was (S)-limonene, was the same as that of the limonene produced by oxidative abiotic stresses (Fig. 2).
Screening of volatile terpenes for inhibitory effect on Xoo growth
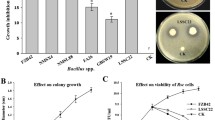
The inhibitory effect of various volatiles [i.e., α-pinene, (S)/(R)-limonene, and α-copaene] on Xoo growth is shown in Fig. 3a. β-Elemene was not included due to the absence of any antibacterial activity (Taniguchi et al. 2014b). α-Copaene did not affect the growth of Xoo in the liquid cultures at all concentrations, and only the higher concentrations (3 and 5 mM) of α-pinene and (R)-limonene significantly inhibited Xoo growth. Lower treatment concentrations of the two volatiles had no visible impact on Xoo growth (Fig. 3a), while (S)-limonene was highly effective in inhibiting Xoo growth at all treatment concentrations (Fig. 3a).
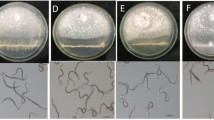
Biological activities of representative volatile terpenes against Xoo. a A liquid culture assay. b A disc diffusion assay of a α-pinene, b (S)-limonene, c (R)-limonene, and d α-copaene. c In vivo leaf infection assay. PC positive control (0.01 % ethanol spray only), NC negative control [Xoo infection without (S)-limonene pretreatment]. Images were photographed after detaching the leaves. Different letters above bars indicate significant difference among the treatments (p < 0.01, Duncan’s multiple range test). Values are means ± SE (n = 3)
Inhibition of Xoo growth in a disc diffusion assay
The antibacterial activity of these compounds was also confirmed by a disc diffusion assay (Fig. 3b). (S)-Limonene strongly suppressed Xoo in a concentration-dependent manner: 21.3 ± 1.2 mm zone of inhibition at 1 mM, 31.3 ± 1.5 mm zone of inhibition at 3 mM, and 41.3 ± 0.6 mm zone of inhibition at 5 mM (Fig. 3b). However, inhibitory effect of (R)-limonene, the opposite enantiomer of (S)-limonene, was almost half compared to that of (S)-limonene. The effect of α-pinene was similar to that of (R)-limonene, and α-copaene had little or no effect on Xoo inhibition.
Suppression of Xoo growth by (S)-limonene pretreatment
The strong inhibitory activity of (S)-limonene against Xoo as observed in vitro was verified in planta by comparing the length of blight lesions in intact rice leaves, sprayed with various concentrations of (S)-limonene (Fig. 3c). After 7 days of incubation, progressions of pathogenic symptoms from the tip to the base of the leaves were observed, where the leaves were not treated with (S)-limonene as a negative control (Fig. 3c). (S)-Limonene treatment largely inhibited the Xoo growth in a concentration-dependent manner as evident from the shorter length of lesions (10.7 ± 1.5 mm at 0.1 mM and 2.7 ± 1.2 mm at 1 mM) compared to the negative control leaves (12.0 ± 1.7 mm). No obvious pathogenic symptoms were observed beyond 3 mM of (S)-limonene treatment (Fig. 3c).
Transcript analyses of (S)-limonene synthase (OsTPS20) and pathogenesis-related genes
Xoo infection in rice seedlings caused a strong induction of OsTPS20 transcript, with 16-fold increase (Fig. 4a, b). We consider that the suppressive effect of (S)-limonene against Xoo might be derived from an innate defense system activated by (S)-limonene treatment. Representative 11 antibacterial pathogenesis-related (PR) genes [i.e., Os01g0124400 (trypsin inhibitor), Os01g0194300 (NPR1-like protein), Os01g0801500 (β-1,3-glucanase), Os02g0833900 (peroxidase), Os04g0350100 (protease inhibitor), Os04g0493400 (chitinase), Os06g0691200 (thaumatin-like protein), Os07g0129200 (PR1a protein), Os07g0677100 (peroxidase), Os11g0114900 (nonspecific lipid transfer protein), and Os11g0701800 (class III chitinase)] were considered (Taniguchi et al. 2014a), and their expressions were determined following (S)-limonene treatment. As there was no increase in the steady-state transcript levels with (S)-limonene treatment for all the genes, it is very likely that (S)-limonene interacts directly with Xoo.
Transcript levels of OsTPS20 and pathogenesis-related (PR) genes in response to Xoo infection. a Steady-state transcript level of OsTPS20; quantitative real-time PCRs for b OsTPS20 and c PR genes. a Os01g0124400 (trypsin inhibitor), b Os01g0194300 (NPR1-like protein), c Os01g0801500 (β-1,3-glucanase), d Os02g0833900 (peroxidase), e Os04g0350100 (protease inhibitor), f Os04g0493400 (chitinase), g Os06g0691200 (thaumatin-like protein), h Os07g0129200 (PR1a protein), i Os07g0677100 (peroxidase), j Os11g0114900 (nonspecific lipid transfer protein), and k Os11g0701800 (class III chitinase). OsACT and OsUBQ were used as internal controls. Values are means ± SE (n = 3)
Discussion
To delineate the functionality of the volatiles inducibly (or abundantly) released against Xoo-infected rice plants, we firstly examined the relative amount of volatiles from the rice plant and found that (S)-limonene was a major monoterpene to be induced and α-copaene was the most abundant sesquiterpene (Figs. 1 and 2). We have demonstrated that (S)-limonene was able to significantly suppress Xoo growth both in a liquid culture assay with 0.5 mM (S)-limonene (Fig. 3a) and in a disc diffusion assay with 1 mM (S)-limonene (Fig. 3b) in concentration-dependent manners. The anti-Xoo activity of (S)-limonene was further tested by spraying this limonene on leaves prior to infecting leaves with Xoo. Interestingly, suppression of pathogenic symptoms by 1 mM (S)-limonene treatment progressed in a concentration-dependent manner—no visible symptoms over 3 and 5 mM treatments (Fig. 3c). The results obtained provide new insights into the protective role of headspace volatiles against rice bacterial blight. Previously, non-volatile phenolic compounds, free radicals, or lignin-like polymers correlating with the activity of peroxidase have been implicated in plant defense against Xoo infection (Reimers et al. 1992), together with the presence of fibrillar materials, enveloping bacterial blights (Xanthomonas campestris pv. oryzae) in rice vessels of incompatible interactions (Horino and Kaku 1989).
The biological activities of terpenes appear to be specific not only to plant species and pathogen types but also to enantiomeric types and concentrations of terpenes. For instance, limonene in transgenic orange fruits, where the concentration of limonene was minimized by antisense expression of a limonene synthase, activated defense mechanisms against the fungus Penicillium digitatum (Pers.) Sacc. and Xanthomonas citri subsp. citri (Rodríguez et al. 2014). Conversely, high amounts of limonene in orange peels attracted more pests and pathogens, rather than repelling or inhibiting them (Rodríguez et al. 2011). Importantly, our study demonstrates that stereospecificity of the compound could be a key determinant of biological functions; only the specific enantiomer, (S)-limonene, and not (R)-limonene, retains a strong inhibitory activity against Xoo.
Rice constitutively releases (S)-limonene at low levels, but the level of this limonene is elevated during abiotic oxidative stresses (Lee et al. 2015) or biotic bacterial blight infection (in the present study), mainly biosynthesized by OsTPS20 (Lee et al. 2015). Rice bacterial blight infection usually occurs at the tillering stage, and the symptoms peak at the flowering stage. However, rice plants are most susceptible at the seedling stage (less than 21-day-old plants) and damage to plants from the disease at this stage of plant growth is more severe, with kresek symptoms observable (Mew et al. 1993). Therefore, it is critical to control rice bacterial blight at the early stage of plant development to ensure good yield of rice. The findings of our study point to the possibility that (S)-limonene can be used as an alternative environment-friendly chemical to manage the disease at the seedling stage. Furthermore, the development of rice breeding program for rice cultivars producing an appropriate amount of (S)-limonene will also be a valuable strategy.
Oxygenated monoterpenes have been considered as potential antibacterial agents and extensively screened against numerous bacterial strains (Kotan et al. 2007). Therefore, we determined the accumulation of carveol or carvone, which are oxygenated limonene derivatives. However, we did not observe any accumulation of these compounds under the experimental conditions of our work. In contrast, for direct suppression of Xoo by (S)-limonene as observed in our study, monoterpene alcohol linalool confers resistance indirectly to Xoo infection by inducing the expression of PR genes in rice (Taniguchi et al. 2014a). Because (S)-limonene treatment per se did not induce the expression of any of the PR genes (Fig. 4c), we assume that rice might have a dual-defense mechanism that controls Xoo growth, using (S)-limonene as a direct contact phytoanticipin and using linalool to activate innate defense systems. At least in rice, (S)-configuration of the compounds seems to be important for proper functioning of the mechanisms, as linalool was also documented to be (S)-enantiomer in rice (Yuan et al. 2008). Future functional analyses of many other volatiles (individually or in combination) may uncover further details on the interaction of rice with Xoo in respect of chemical defense. It would also be of interest to generate transgenic rice plant by overexpressing OsTPS20 to determine whether endogenously accumulated (S)-limonene induces the expression of defense-related genes or the vapor phase of the volatile could also inhibit bacterial blight.
References
Arimura G et al (2008) Herbivore-induced terpenoid emission in Medicago truncatula: concerted action of jasmonate, ethylene and calcium signaling. Planta 227:453–464
Bajpai VK, Kang S, Xu H, Lee S-G, Baek K-H, Kang SC (2011) Potential roles of essential oils on controlling plant pathogenic bacteria Xanthomonas species. Plant Pathol J 27:207–224
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci U S A 95:4126–4133
Cartwright DW, Langcake P, Pryce RJ, Leworthy DP, Ride JP (1981) Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 20:535–537
Chen F, Tholl D, Bohlmann J, Pichersky E (2011) The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J 66:212–229
Hasegawa M et al (2010) Phytoalexin accumulation in the interaction between rice and the blast fungus. Mol Plant Microbe Interact 23:1000–1011
Horino O, Kaku H (1989) Defense mechanisms of rice against bacterial blight caused by Xanthomonas campestris pv. oryzae. pp. 135–152. In: Bacterial blight of rice. IRRI, Los Banos, Philippines
Karagnilla A, Paris-Natural M, Ou SH (1973) A comparative study of culture media for Xanthomonas oryzae. Philipp Agric 57:141–152
Kato T et al (1973) Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett 14:3861–3864
Kato H, Kodama O, Akatsuka T (1994) Oryzalexin F, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry 36:299–301
Kauffman H, Reddy A, Hsieh S, Merca S (1973) Improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Disease Reporter
Kodama O, Miyakawa J, Akatsuka T, Kiyosawa S (1992) Sakuranetin, a flavanone phytoalexin from ultraviolet-irradiated rice leaves. Phytochemistry 31:3807–3809
Koga J, Shimura M, Oshima K, Ogawa N, Yamauchi T, Ogasawara N (1995) Phytocassanes A, B, C and D, novel diterpene phytoalexins from rice, Oryza sativa L. Tetrahedron 51:7907–7918
Kono Y et al (1991) Structures of oryzalides A and B, and oryzalic acid A, a group of novel antimicrobial diterpenes, isolated from healthy leaves of a bacterial leaf blight-resistant cultivar of rice plant. Agric Biol Chem 55:803–811
Kotan R, Kordali S, Cakir A (2007) Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z Naturforsch C 62:507–513
Lee G, Lee S, Chung M-S, Jeong Y, Chung B (2015) Rice terpene synthase 20 (OsTPS20) plays an important role in producing terpene volatiles in response to abiotic stresses. Protoplasma 252:997–1007
Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50:47–65
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Loreto F, Förster A, Dürr M, Csiky O, Seufert G (1998) On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ 21:101–107
Mew T (1987) Current status and future prospects of research on bacterial blight of rice. Annu Rev Phytopathol 25:359–382
Mew T, Alvarez A, Leach J, Swings J (1993) Focus on bacterial blight of rice. Plant Dis 77:5–12
Pare PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121:325–332
Reimers PJ, Guo A, Leach JE (1992) Increased activity of a cationic peroxidase associated with an incompatible interaction between Xanthomonas oryzae pv. oryzae and rice (Oryza sativa). Plant Physiol 99:1044–1050
Rodríguez A et al (2011) The monoterpene limonene in orange peels attracts pests and microorganisms. Plant Signal Behav 6:1820–1823
Rodríguez A et al (2014) Terpene down-regulation triggers defense responses in transgenic orange leading to resistance against fungal pathogens. Plant Physiol 164:321–339
Taniguchi S, Hosokawa-Shinonaga Y, Tamaoki D, Yamada S, Akimitsu K, Gomi K (2014a) Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ protein in rice. Plant Cell Environ 37:451–461
Taniguchi S et al (2014b) Isolation of jasmonate-induced sesquiterpene synthase of rice: product of which has an antifungal activity against Magnaporthe oryzae. J Plant Physiol 171:625–632
Tholl D (2006) Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol 9:297–304
Watanabe M et al (1990) Novel C19-kaurane type of diterpene (oryzalide A), a new antimicrobial compound isolated from healthy leaves of a bacterial leaf blight-resistant cultivar of rice plant. Agric Biol Chem 54:1103–1105
Watanabe M et al (1992) Structures of oryzalic acid B and three related compounds, a group of novel antibacterial diterpenes, isolated from leaves of a bacterial leaf blight-resistant cultivar of rice. Biosci Biotechnol Biochem 56:113–117
Yuan JS, Köllner TG, Wiggins G, Grant J, Degenhardt J, Chen F (2008) Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J 55:491–503
Acknowledgments
This work was supported by the Nuclear R&D Program of the Ministry of Science, ICT, and Future Planning (MSIP), Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Lee, G.W., Chung, MS., Kang, M. et al. Direct suppression of a rice bacterial blight (Xanthomonas oryzae pv. oryzae) by monoterpene (S)-limonene. Protoplasma 253, 683–690 (2016). https://doi.org/10.1007/s00709-015-0904-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0904-4