Abstract
A significant amount of work has been expended to identify the elusive components of plasmodesmata (PD) to help understand their structure, as well as how proteins are targeted to them. This review focuses on the role that lipid membranes may play in defining PD both structurally and as subcellular targeting addresses. Parallels are drawn to findings in other areas of research which focus on the lateral segregation of membrane domains and the generation of three-dimensional organellar shapes from flat lipid bilayers. We conclude that consideration of the protein–lipid interactions in cell biological studies of PD components and PD-targeted proteins may yield new insights into some of the many open questions regarding these unique structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasmodesmata (PD) are membrane-rich structures that are characterised by a close association of two cellular membrane systems: the plasma membrane and the endoplasmic reticulum (ER), which within PD forms a narrow rod called the desmotubule (DT; Maule 2008). However, the role that the lipid environment within these membranes plays in plasmodesmal structure and function, as well as protein targeting to PD has so far received little attention. This despite a majority of identified PD proteins being either integrally or peripherally membrane-associated, or utilising the secretory route through the endomembrane system for delivery to PD (Table 1).
The study of membranes and their associated proteins has been slow partly because of the problems inherent in investigating the hydrophobic lipid phase with laboratory techniques that have mostly been developed to study proteins in aqueous media (Mouritsen 2010; von Heijne 2006). But although our understanding of protein–lipid interactions in biological membranes is far from complete, accumulating evidence shows that membrane proteins preferentially associate with specific membrane lipids and that the biophysical properties of lipid environments facilitate specific protein–protein interactions and protein intracellular targeting, structure and function (Dowhan and Bogdanov 2009; Hancock 2006; Hurley and Meyer 2001; Jacobson et al. 2007; Janmey and Kinnunen 2006; Kusumi and Suzuki 2005; Sprong et al. 2001; Stahelin 2009). In other words, ‘proteins and lipids make up indissociable parts of an intricate sorting machinery’ (Sprong et al. 2001). Protein–lipid interactions are also responsible for the generation of membrane curvature and thus the shape of organelles (Voeltz and Prinz 2007). Therefore, the membrane domains characteristic for PD, i.e. their hydrophobic protein–protein and protein–lipid interactions, can be viewed as defining them both structurally and as subcellular targeting addresses. Consequently, it might for instance be more useful to ask what defines the membrane domain that a plant viral movement protein (MP) associates with rather than to search for a specific ‘receptor’, i.e. a unique protein–protein interaction. This review summarises what is known about the membranes in plasmodesmata and places these data into the context of recent research on protein–lipid interactions and membrane shaping in other areas of cell biology, as well as highlighting some experimental approaches that may be applicable to the study of PD structure and function. The properties of the plasma membrane at PD and the putative role of lipid rafts are discussed first, then the structure and function of the desmotubule and its role in virus movement, followed by a consideration of the establishment of desmotubular ER-plasma membrane adhesion sites during PD biogenesis.
Lipid rafts and the PD plasma membrane
Continuity and lateral heterogeneity of the plasma membrane in PD
Electron microscopy (EM) has shown that the plasma membrane, cytosol, and ER are all intercellularly connected through PD, but this does not necessarily mean that these compartments continue homogenously across the cell boundary. What evidence is there regarding the similarity or difference of the plasma membrane at PD to the surrounding plasmalemma? Little is known about the constituents of PD and such data are mostly indirect, but they do indicate that the plasma membrane within the plasmodesmal pore differs from its surroundings. In electron micrographs, globular proteins can often be seen to cover the surface of the PD plasma membrane (Blackman and Overall 2001; Botha et al. 1993; Botha and Cross 2000; Ding et al. 1992; Ehlers and Kollmann 2001; Overall and Blackman 1996; Robards 1968a) and immuno-gold localisation of plasma membrane H+-ATPase detected the protein in pit fields, but it was excluded from the inside of PD (Fleurat-Lessard et al. 1995). These data show that the plasma membrane lining the pore is distinct morphologically and in its protein composition, but there are also functional differences.
In photobleaching studies in soybean root cell suspension culture with a fluorescently labelled sphingolipid that partitions predominantly to the outer leaflet of the plasma membrane, the plasma membrane within PD formed a barrier for lipid diffusion (Grabski et al. 1993). On the other hand, a thioredoxin (Trx h9) was recently identified that can move intercellularly in Arabidopsis roots. Trafficking probably occurred in a plasma membrane-associated manner as it required peripheral association with the cytoplasmic face of the plasma membrane via putative myristoylation and palmitoylation motifs (Meng et al. 2010). Also, sucrose transmembrane transporters localised in the plasma membrane of phloem sieve elements are probably delivered through PD from the companion cells (Kühn et al. 1997; Lalonde et al. 2003), although intercellular transport may be ER associated, with secretion to the plasma membrane taking place in the sieve element (Oparka and Turgeon 1999; Wright and Oparka 2006).
Protein targeting to the PD plasma membrane
Recently, an integral membrane protein localising to the plasma membrane within PD was identified (plasmodesmata-located protein 1 = PDLP1; Thomas et al. 2008). Intriguingly, the single transmembrane domain (TMD) of PDLP1 is sufficient to target a fused fluorescent protein to PD via the Brefeldin A-sensitive secretory pathway. PD targeting of the isolated TMD shows that lipophilic protein interactions or co-sorting into membrane microdomains specify the PD plasma membrane as a subcellular targeting address. However, whilst secretory delivery may occur close to PD (Rinne et al. 2001), the actual channel is probably too narrow for vesicle docking and fusion events, and no indications of endo- or exocytosis within PD have ever been observed in EM. Therefore, the PDLP1 TMD must reach its final localisation through lateral diffusion and anchoring in the PD-specific part of the plasma membrane, indicating that it does have unique features (Lucas et al. 2009).
One mechanism for sorting of single-pass transmembrane proteins to the plasma membrane is based on the length of their TMD. As the membranes along the secretory pathway from the ER to the plasma membrane increase in thickness, ER-resident single-pass transmembrane proteins tend to have shorter, and plasma membrane-resident proteins longer TMD (Brandizzi et al. 2002; Mitra et al. 2004; Ronchi et al. 2008). In agreement with this, C-terminal shortening of the 21 amino acid PDLP1 TMD to 18 residues resulted in retention in the ER (Thomas et al. 2008). However, analysis of mutants individually substituting residues 19–21 with alanine (maintaining nonpolarity) identified the penultimate valine 20 as essential for PD targeting. Therefore, specific protein–lipid, or hydrophobic protein–protein interactions within membranes are necessary for targeting, rather than TMD length per se. Bioinformatic analysis of the conserved TMDs of PDLPs from a range of plant species has identified several highly conserved residues including Val20 as well as Ala2 and Gly6 (K. Amari and C. Ritzenthaler, unpublished data). These three residues all face the same side of the helical wheel of the TMD. Additionally, Ala2 and Gly6 form a putative ‘smXXXsm’ motif (where sm designates a small residue, either Gly, Ala or Ser; Lemmon et al. 1992) which facilitates TMD interhelix interactions by permitting extensive van der Waals contacts (Senes et al. 2004). The ‘smXXXsm’ motifs function mostly in homo-oligomerization, but can also be involved in hetero-dimerization (Ciczora et al. 2007). PD-localised, full-length PDLP1 shows homotypic interactions in fluorescence resonance energy transfer experiments (K. Amari and C. Ritzenthaler, unpublished data), but it is not yet clear if the TMD, and specifically Val20 is necessary for the interaction, and if oligomerisation is required for PD targeting. In the Val20Ala mutant, the TMD was retained in the ER, i.e. TMD delivery to the plasma membrane was affected, rather than sorting within the plasma membrane. If mutants could be identified in which the TMD is still transported to the plasma membrane but does not localise to PD, these would enable to also dissect the lateral sorting to PD.
Lipid raft formation and function
The most important concept dealing with lateral heterogeneity of the plasma membrane is that of ‘lipid rafts’ (Bagatolli et al. 2010; Bhat and Panstruga 2005; Hancock 2006; Jacobson et al. 2007; Kusumi and Suzuki 2005; Lichtenberg et al. 2005; Lingwood and Simons 2010; Mouritsen 2010; Pike 2009). Biological membranes contain a variety of different lipids. According to the raft hypothesis, these do not form a homogenous mixture. Rather, saturated phospholipids—mostly sphingolipids—segregate into domains which adopt a more tightly packed, ‘liquid-ordered’ (Lo) phase. By contrast, the bulk membrane, consisting mostly of unsaturated glycero-phospholipids with acyl chains kinked at cis-double bonds, is in a less tightly packed ‘liquid-disordered’ (Ld) state. Importantly, the Lo phase is not a gel (=‘solid-ordered’, So) phase, but an intermediate phase that depends on the presence of sterols and is thought to share the lipid acyl chain order of the So state while only reducing lateral diffusion rates by two- to threefold compared with Ld (Hancock 2006; Mouritsen 2010; van Meer et al. 2008). Membrane phase transition is temperature-dependent, with lower temperatures favouring raft formation. The classic lipid raft model viewed these Lo phases as stable, macroscopic domains into which specific proteins would segregate. However, more recently, rafts are thought to be highly dynamic entities (see below).
The standard biochemical method for identifying raft-associated proteins has been the isolation of detergent resistant membrane (DRM) fractions (Lichtenberg et al. 2005). Treatment of biological membranes with the mild, non-ionic detergent Triton X-100 at 4°C solubilises the unsaturated phospholipids, but not the sphingolipid- and sterol-enriched DRM fractions which remain floating on sucrose density gradients. Proteins associating with DRMs were viewed as raft-associated and a relatively good agreement between studies has resulted in a high level of confidence in the approach. DRM preparations include ~10% of bulk plasma membrane proteins (Mongrand et al. 2004) and consistently contain certain classes of proteins (Bhat and Panstruga 2005; Borner et al. 2005; Kierszniowska et al. 2009; Mongrand et al. 2004; Pike 2009). These include glycosyl-phosphatidyl inositol (GPI)-anchored proteins, which are secreted proteins that remain associated with the outer leaflet of the plasma membrane through their lipid anchor.
However, the concept of pre-existing, stable lipid rafts in the plasma membranes of living cells has provoked much debate because of the instability (half life < 1 ms) and small size (10–20 nm) of Lo domains forming in in vitro systems under physiological conditions and the resulting difficulty to study them in vivo. As a result, recent work on lipid rafts has focussed more on imaging techniques that allow the quantitation of biomolecular interactions and diffusion rates and the tracking of sub-resolution fluorescent particles. This led to a refined raft concept (Bagatolli et al. 2010; Hancock 2006; Jacobson et al. 2007; Kusumi and Suzuki 2005; Lichtenberg et al. 2005; Lingwood and Simons 2010; Pike 2009) which views them as “small (10–200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalise cellular processes” (Pike 2006).
According to this model (Fig. 1a), spontaneously forming Lo domains remain small and short-lived unless they are stabilised. Stabilisation is mediated by the saturated lipid anchors and transmembrane domains of proteins, both of which favour interactions with specific membrane lipids. Membrane proteins thus distribute along a continuous spectrum of raft-stabilising effects which is reflected in their DRM association. Transmembrane domains initially segregate into the Lo–Ld phase boundary layer, acting as surfactants that reduce line tension (Hancock 2006). Additional stabilisation can then be achieved through interactions between raft proteins and the resulting size increase of the combined captured Lo phases, which further reduces line tension per area unit. Furthermore, the increased lifetime of the stabilised raft allows for longer diffusion distances and increased chances of encountering more stabilising proteins. Under these circumstances, rafts of ~200 nm diameter and with life times >1 s can form.
In biological membranes, which are nearly saturated with proteins and strongly compartmentalised for lateral diffusion (see below), complete Lo–Ld phase separation of the entire membrane could theoretically occur. It is thought that continuous endocytosis is the main factor preventing this (Hancock 2006; Hanzal-Bayer and Hancock 2007; Helms and Zurzolo 2004, Parton and Richards 2003). Continuous spontaneous formation, stabilisation and dissociation results in a great heterogeneity of rafts, rather than a uniform compartment gathering DRM proteins (Hancock 2006; Jacobson et al. 2007; Kusumi and Suzuki 2005; Lichtenberg et al. 2005; Lingwood and Simons 2010; Pike 2009). Various biological functions have been attributed to lipid rafts (Bhat and Panstruga 2005; Hancock 2006; Hanzal-Bayer and Hancock 2007; Helms and Zurzolo 2004; Pike 2009) and it now appears that most of these (including the function as signalling ‘platforms’ in various pathways) are associated with small, short-lived rafts. In contrast, larger, more stable domains are limited to specialised functions such as endocytosis, caveolae and T-cell synapses (Hancock 2006).
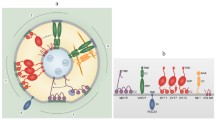
The lipid raft model applied to the plasma membrane in the plasmodesmal channel. a Small (~10–20 nm) liquid-ordered (Lo) domains can form spontaneously in the plasma membrane. Their lifetime remains in the sub-millisecond range (blue) unless they are stabilised by proteins (hexagons). Association of a protein with, initially, the phase boundary between liquid-ordered and liquid-disordered (Ld) membrane areas increases the lifetime of the raft (green) and thereby also the distance it can travel in the membrane and the chance of encountering other proteins. Protein-protein interactions can then further stabilise the raft (yellow), enclosing the proteins in the Lo phase. Rafts containing several interacting proteins can be stabilised for several seconds (red) and reach sizes of 100–200 nm. In the plasmodesmal plasma membrane (right side), a high density of raft proteins which are anchored to the cell wall and underlying cytoskeleton, including callose-anchored GPI-proteins providing fixed seeds for spontaneous raft formation (brown hexagons), increases the probability that raft lipids dissociating from the Lo phase will encounter another raft immediately und leads to an overall enrichment (‘trapping’) of rafts in the PD channel (modified after Hancock 2006). b The plant-specific protein remorin forms 70–80 nm clusters on the cytosolic face of the plasma membrane in a sterol-dependent manner. (from Raffaele et al. 2009). c Purified remorin oligomerises into tubules with a diameter of 4–8 nm in vitro (from Bariola et al. 2004)
Accumulating evidence for the existence of lipid rafts in PD
Could lipid rafts play a role in distinguishing the PD plasma membrane from the surrounding plasmalemma? Recently, it was shown that remorin, a member of a plant-specific protein family, is associated with the cytosolic face of the plasma membrane and clusters on detergent-resistant membrane patches ca. 75 nm across, consistent with a raft association (Fig. 1b; Raffaele et al. 2009). Sterol depletion of plasma membrane preparations abolished clustering, but not membrane association of remorin. In vivo localisation of remorin by fluorescence microscopy showed that the protein labelled punctae on the plasma membrane, but was also enriched within PD (Raffaele et al. 2009) indicating the presence of lipid rafts within the channel. In agreement with this, two of the PD-localised proteins identified so far are GPI-anchored. One of these is a β-1,3-glucanase probably involved in breakdown of the cell wall polysaccharide callose which contributes to regulating PD channel constriction (Roberts and Oparka 2003; Epel 2009), the other a protein that binds callose but without any apparent enzymatic activity, called PD callose-binding protein1 (PDCB1, Simpson et al. 2009). In mature plant cells, callose is found predominantly in the neck region around the plasmodesmal orifice and it was suggested that PDCB1 could function in anchoring the plasma membrane to the cell wall (Simpson et al. 2009). Applying the lipid raft model in this context allows us to expand on this interpretation (Fig. 1a): The GPI anchor of PDCB1 is expected to capture Lo domains. Since the protein is locally enriched and positionally fixed at PD through callose binding, this increases the chances of rafts becoming trapped and stabilised in the vicinity, leading to their merging and further stabilisation. Under these circumstances, rafts of ~200 nm diameter can form, which have the same surface area as a PD channel with a diameter of 50 and a length of 200 nm. Furthermore, endocytosis—a main factor-limiting raft size—probably does not occur in the pore. Thus, it is possible that the entire plasma membrane surface of PD could consist of trapped Lo domains, constituting not a stable structure but a dynamic local enrichment zone.
Lipid rafts and PD targeting and function
If a large percentage of the plasma membrane within PD consists of Lo domains, they could play a role in localisation of transmembrane proteins to PD. However, the heterogeneity of lipid rafts (Pike 2006) implies that not all raft-associated proteins would also localise to PD. For instance, the plasma membrane H+-ATPase is often found in DRM preparations (Bhat and Panstruga 2005; Borner et al. 2005), but excluded from PD (Fleurat-Lessard et al. 1995) and PD targeting probably also requires specific protein–protein interactions. For PDLP proteins, there is currently no evidence for or against a raft-association and it might be interesting to test their fractionation into DRMs and identify the affinity of the conserved TMD to different membrane lipids in vitro. If PDLPs do not associate with DRMs, they could still be raft-localised through hydrophobic protein–protein interactions of the TMD with Lo-stabilising proteins. Sphingolipids and sterols are synthesised in the endomembrane system and Lo domains already begin to segregate on the inside of trans-Golgi and exocytic vesicles (Hanzal-Bayer and Hancock 2007; Helms and Zurzolo 2004; Laloi et al. 2007; van Meer and Sprong 2004). Therefore, it may not be possible to identify mutations in the PDLP1 TMD that separate delivery to the plasma membrane from lateral sorting to PD because targeting would have already been disrupted within the secretory pathway. Instead, the effects of sterol- and sphingolipid-synthesis mutants (e.g. Diener et al. 2000; Gechev et al. 2004, 2008; Wang et al. 2008) and the sphingolipid biosynthesis inhibitor fenpropimorph (Laloi et al. 2007) on PDLP1 TMD targeting could be tested. Also, identifying PDLP TMD-interacting proteins within the endomembrane system may lead to identification of factors involved in the secretory targeting route to PD.
Interestingly, all eight Arabidopsis PDLP proteins have been found to interact with the tubule-forming movement proteins of grapevine fanleaf and cauliflower mosaic viruses in vivo as well as to co-localise with the cytoplasmically exposed base of the tubules. Inhibition of the secretory pathway, which interferes with PDLP localisation, also disrupted tubule formation and simultaneous knockout of three PDLP isoforms reduced tubule formation by 46% and delayed virus infection. Thus, PDLP proteins appear to act as receptors for these MPs (Amari et al. 2010). As the cytoplasmic C-terminus of the PDLPs is very variable, the conserved TMD is the most likely site of contact, again indicating that protein–protein interactions within membranes are important for PD targeting.
Lipid rafts are also viewed as signalling hubs and often contain receptor-like kinases (Bhat and Panstruga 2005, Kierszniowska et al. 2009; Pike 2009; Shahollari et al. 2004). Consistent with a view of PD as important gatekeepers in plant intercellular communication (Lucas et al. 2009; Maule 2008; Roberts and Oparka 2003) and the presence of lipid rafts in the channel, the receptor tyrosine kinase CRINKLY4 is enriched in the PD of specific cell–cell interfaces (Tian et al. 2007). Moreover, the proteomic screen that yielded PDLP1 and PDCB1 has identified a number of additional transmembrane proteins as targeted to PDs, including some receptor-like kinases (C. Faulkner, A.J. Maule, personal communication; see review in this issue). Therefore, it appears that in addition to a role in defining PD structurally and as subcellular targeting addresses, lipid rafts could also be important for their functions in cell–cell communication.
A potential scaffolding role for remorin at PD
Remorin associates with the plasma membrane despite being strongly hydrophilic, it homo-oligomerises, and the purified protein forms filaments in vitro and possibly in vivo (Fig. 1c; Bariola et al. 2004). This behaviour resembles caveolins, the key structural components of caveolae, which are large, invaginated raft domains that function in endocytosis, plasma membrane-cytoskeleton anchoring and cellular signalling (Hansen and Nichols 2010). Caveolae are absent in plants, but the biochemical similarities and raft-association of remorins indicate that they could function as scaffolding proteins (Lefebvre et al. 2010), stabilising lipid rafts on their cytoplasmic face and mediating protein interactions. The plasma membrane and ER (desmotubule) in PD are connected through protein ‘spokes’ attached to both membranes (Overall and Blackman 1996; Botha et al. 1993; Ding et al. 1992; Ehlers and Kollmann 2001; Overall et al. 1982), which have been interpreted as class VIII myosins (Overall and Blackman 1996; but see detailed discussion below). One function of lipid rafts is the anchoring of the plasma membrane to the underlying cytoskeleton (Bhat and Panstruga 2005; Kusumi and Suzuki 2005; Pike 2009; Simpson-Holley et al. 2002). The occurrence of a raft-associated putative scaffolding protein within PD makes it tempting to speculate that remorin could be directly or indirectly involved in anchoring the spokes on the plasma membrane side.
Remorin interacts with the triple gene block (TGB)1 movement protein of potato virus X and influences viral cell-to-cell transport: overexpression reduces, whilst knock-down enhances movement (Raffaele et al. 2009). The authors hypothesised that remorin binding might titrate TGB1-containing ribonucleoprotein complexes out of the pool available for cell-to-cell transport. But another interpretation is that conversely TGB1 binding to remorin could block interaction surfaces involved in stabilising plasmodesmata. In a fully turgid plant cell, the cell wall counterbalances the osmotic pressure of the protoplast. Although the inherent stability of the wall accounts for most of this counterforce, turgor pressure could presumably lead to a slight dilation of the pore if the spokes were untethered and this could underlie the gating capability (Howard et al. 2004) of TGB1. If TGB1-mediated PD dilation depended on quantitative occupation of remorin interaction surfaces, then overexpression of remorin would require more, and knock-down less TGB1 to achieve the same degree of PD gating, explaining the dosage effect on virus movement. Answering these open questions will require analysis of the mechanism by which the highly hydrophilic remorin protein binds to the plasma membrane, testing of its ability to stabilise Lo domains in vitro and identification of its cytosolic binding partners.
The diffusion barrier in the plasmodesmal plasma membrane
An enrichment of lipid rafts in the plasma membrane at the neck of and within PD could contribute to protein PD targeting and the structural integrity of the pore. But because the Lo phase reduces lateral diffusion of membrane lipids only by two- to threefold, additional factors must play a role in the ability of the PD plasma membrane to act as a barrier to lateral diffusion. A possible explanation is provided by a lateral compartmentation mechanism acting within the plasma membrane termed the ‘picket fence’ model (Auth and Gov 2009; Jacobson et al. 2007; Kusumi et al. 2005; Kusumi and Suzuki 2005). According to this model, a high density of TM proteins (‘pickets’) that are anchored to the underlying cytoskeleton (‘fence’) and/or the extracellular matrix (i.e. the cell wall in plants) divide the plasma membrane into small compartments a few tens or hundreds of nanometers across. These compartments restrict mobility of non-anchored membrane components because lateral escape of lipids and proteins requires ‘hop diffusion’ across the barriers. Within PD, the inner leaflet of the plasma membrane contains a high density of embedded globular proteins linked to the DT through the protein spokes (Overall and Blackman 1996; Botha et al. 1993; Ding et al. 1992; Ehlers and Kollmann 2001; Overall et al. 1982) as well as cell wall-anchored membrane proteins (PDCB1 and probably others). In some electron micrographs, proteinaceous rings or spokes can also be observed enclosing the apoplastic face of the plasma membrane or linking it to the cell wall (Badelt et al. 1994; Overall and Blackman 1996; Cook et al. 1997). These structural features probably contribute to the restricted lateral diffusion within the plasmodesmal plasma membrane.
Membrane curvature and the desmotubule
The structure of the desmotubule
The unique nature of the desmotubular ER within PD is apparent from electron micrographs, where the DT appears as a tubule approximately 15–20 nm in diameter (Fig. 2a) which usually contains a dark, proteinaceous ‘central rod’ in the lumen (Overall and Blackman 1996; Botha and Cross 2000; Botha et al. 1993; Ding et al. 1992; Ehlers and Kollmann 2001; Hepler 1982), and a ring of helically arranged globular proteins on the outside (Overall and Blackman 1996; Botha et al. 1993; Ding et al. 1992; Ehlers and Kollmann 2001; Hepler 1982; Overall et al. 1982; Robards 1968a; Robinson-Beers and Evert 1991). The latter are linked to proteins on the plasma membrane via spoke-like extensions (Botha and Cross 2000; Botha et al. 1993; Ding et al. 1992; Ehlers and Kollmann 2001; Robards 1968a; Robinson-Beers and Evert 1991; Schulz 1995). Subtracting the globular proteins, the diameter of the DT membrane tubule is between <10 and 15 nm, which may well make it the most strongly constricted lipid bilayer known in nature. Early electron microscopists even doubted whether such a structure could contain a membrane at all and the name ‘desmotubule’ thus derives from a structural comparison with microtubules (Robards 1968a, b). Today, it is clear that the DT is physically continuous with the cortical ER on both sides of the pore (Hawes et al. 1981; Hepler 1982; Oparka et al. 1994) and transport studies with endomembrane dyes (Grabski et al. 1993; Martens et al. 2006), transmembrane and lumenal green fluorescent protein (GFP) fusion proteins (Guenoune-Gelbart et al. 2008; Oparka et al. 1999), as well as peptides and dextrans microinjected into the ER lumen (Cantrill et al. 1999) have all shown that both the membrane and lumenal space continue uninterrupted across the cell boundary. This poses the question ‘How and why is this energetically unfavourable membrane curvature maintained?’.
Constriction of the desmotubule. a Staining of the ER lumen in maize root meristem cells by zinc iodide/osmium tetroxide. The extreme constriction of the desmotubule compared with the cortical ER is apparent. Bar = 250 nm (Image courtesy of C. Hawes, Oxford Brookes University). Narrow membrane tubules like this can be formed by a number of mechanisms: a greater number of cylindrical lipid molecules (e.g. phosphatidylcholine) in the outer leaflet (b), which may be achieved through the action of a ‘flippase’ lipid transporter (Holthuis and Levine 2005). Preferential sorting or generation of lipids with large head groups (i.e. phosphoinositides or lysophospholipids, which are missing one acyl chain due to the action of a phospholipase A) in the outer, and of cone-shaped lipids (i.e. diacylglycerol or phosphatidylethanolamine) in the inner monolayer (c), again possibly assisted by the activity of specific ‘flippases’. Insertion of protein amphipathic helix ‘wedges’ into the outer leaflet (d) or of kinked helices (‘re-entrant loops’) that can penetrate into the bilayer to different extends (e) increases the area of the cytoplasmic monolayer, leading to positive curvature. Proteins can assemble into scaffolds on the outside (cytoplasmic face, f) or inside (endomembrane lumen, g) of the membrane and bend it by oligomerising into a rigid cage structure (f) or having a curved membrane contact surface (g), or both. Scaffolds can be bound to the membrane through transmembrane domains, insertion of re-entrant loops or amphipathic helices, lipid anchors (black in f), polar interactions with lipid head groups, or any combination of these. Note that often several curvature-inducing mechanisms work cooperatively
Generation and stabilisation of membrane curvature
A homogenous, unconstricted lipid bilayer in an aqueous medium would normally form a symmetrical sphere. Tubules, sheets (flattened spheres) and other membrane shapes encountered in cells require the generation of additional positive (away from the cytoplasm) or negative (towards the cytoplasm) curvature. Asymmetries in the specific lipids comprising the monolayer leaflets can induce curvature, but the stable maintenance of membrane shapes requires the interaction with proteins (Fig. 2b–g; Shibata et al. 2009; van Meer and Sprong 2004; Voeltz and Prinz 2007). In recent years, an ever-increasing number of proteins have been found to influence membrane curvature. Some of the common mechanisms are illustrated in Fig. 2, but a full overview is beyond the scope of this review (see instead Bauer and Pelkmans 2006; Cory and Cullen 2007; Drin and Antonny 2010; Farsad and De Camilli 2003; Frost et al. 2009; Itoh and Takenawa 2009; Praefcke and McMahon 2004; Voeltz and Prinz 2007). Generally, narrower constriction of membranes requires tighter, more rigid protein–protein interactions. Hu et al. (2008) have calculated that a non-bulging (straight) tubule with a diameter of 17.4 nm requires constricting protein rings no more than 2 nm apart. A structure as narrow as the DT therefore needs to be maintained by a protein scaffold along its entire length. In agreement with this, Tilney et al. (1991) found that the DT in fern gametophytes was resistant to extraction with the mild detergent Triton X-100, but not to protease. By contrast, boiling of wall preparations in Triton X-100, but also in urea, CHAPS (3-[(3-cholamidopropyl) dimethyl-ammonio] 1-propane-sulphonate) or the strong ionic detergent SDS (sodium dodecyl sulphate) extracted proteins from PD and led to a disappearance of all internal substructure (Turner et al. 1994).
Are reticulons and DP1/Yop1 proteins sufficient to shape the desmotubule?
The identities of proteins constricting the DT are unknown, but two protein families that maintain tubule curvature in the cortical ER, from which the DT is derived, have recently been identified (Hu et al. 2008; Voeltz et al. 2006). These are the reticulons and DP1/Yop1 (deleted in polyposis 1/YIP (Ypt-interacting protein) one partner 1) family proteins. Proteins in both families contain two unusually long (~30–35 residues) hydrophobic helices that form re-entrant loops (Hu et al. 2008; Sparkes et al. 2010; Voeltz et al. 2006), inducing membrane curvature by the molecular wedge principle (Fig. 2e). Arabidopsis has 21 reticulon homologs (Nziengui et al. 2007; Sparkes et al. 2010), significantly more than yeast or animals, possibly reflecting the diversity of distinct functional domains of the plant ER (Sparkes et al. 2009a; Staehelin 1997). Only some of the plant reticulons have so far been characterised (Nziengui et al. 2007; Sparkes et al. 2010; Tolley et al. 2010) and it is unclear if any are present in the DT or if some isoforms might even be PD-specific. The reticulons have strong oligomerisation tendencies which allow them to act as scaffolds that can stabilise as well as induce membrane curvature (Shibata et al. 2008, 2009). They could probably induce sufficient curvature to generate the extreme constriction of the DT as yeast Yop1 and Rtn1 were able to deform a lipid bilayer into 15–17 nm diameter tubules in vitro (Hu et al. 2008). Moreover, over-expression of the plant reticulon RTNLB13 constricted cortical ER tubules so much that lumenal GFP was excluded in vivo (Tolley et al. 2010), resembling behaviour of the DT in many tissues (Crawford and Zambryski 2000; Martens et al. 2006; Oparka et al. 1999 and see below).
However, a number of observations make it unlikely that these are the only proteins contributing to DT structure. The cortical ER connecting to the DT is also constricted, although to a lesser extent (Fig. 2a). Either a higher density of reticulons must be maintained in the DT or it needs to contain unique reticulons/Yop1 family proteins that must be prevented from escaping laterally into the surrounding endomembranes. It is likely that there are lateral diffusion barriers at the ends of the DT and Overall and Blackman (1996) have proposed that the calcium-dependent contractile protein centrin (Blackman et al. 1999) may play a role in regulating lateral connectivity of PD to the cytoplasm. However, centrin itself is not membrane-associated and would need interacting partners on the DT. The spokes connecting the DT to the plasma membrane may also contribute to maintaining membrane-constricting proteins within PD, e.g. through interactions with reticulon cytoplasmic domains (Sparkes et al. 2010).
The central rod components within the DT lumen appear to have thin connections to the globular proteins on the cytoplasmic face of the DT, implying transmembrane connections (Robards 1968a; Ding et al. 1992). Reticulons/Yop1s have no significant lumenal domains and are thus unlikely to play any part in making up the central rod (Sparkes et al. 2010; Voeltz et al. 2006). In animal cells, the CLIMP-63 protein is an ER transmembrane protein that forms lumenal scaffolds. Oligomerisation of the lumenal domain is required to target CLIMP-63 to the tubular cortical ER (Klopfenstein et al. 2001), but scaffold formation probably does not contribute to tubule constriction. The cytoplasmic domain of CLIMP-63 attaches the animal ER to the microtubule cytoskeleton, and this attachment is necessary to maintain a cortical ER network (Vedrenne and Hauri 2006). Plants have no direct homologs of CLIMP-63 but can be expected to possess similar proteins for actin-anchoring of the ER, possibly also within PD. CLIMP-63 protein may therefore give an indication of the properties and domain structure required for components of the central rod. Lastly, it can also be expected that the DT membrane has a lipid composition that differs from the cortical ER because differences in membrane curvature lead to differential lipid sorting, an effect that is more pronounced in the presence of proteins (Sorre et al. 2009). Lipid-specific immuno-electron microscopy might be able to identify major DT lipids in situ (see Downes et al. 2005).
Association of the cytoskeleton with the desmotubule
Apart from its strong constriction and rigid scaffolding, the DT also differs from the surrounding ER in its association with the cytoskeleton. The plant cortical ER is closely linked with actin microfilaments, and ER movement and remodelling depends on the action of myosin motors (Boevink et al. 1998; Sparkes et al. 2009a, c). There are two classes of higher plant myosins, VIII and XI (Sparkes 2010), of which myosin XI-K is the isoform most important for ER motility (Sparkes et al. 2009c; Sparkes 2010). Myosin XI motors are similar to animal myosin V. Both classes consist of a homodimer of heavy chains that interact via a coiled coil domain and additionally bind several light chain cofactors (Vale 2003). Each heavy chain has an N-terminal motor domain of approximately 16.5 × 6.5 × 4 nm (Rayment et al. 1993), followed by a neck region containing six light chain-binding IQ (isoleucine–glutamine) motifs, the coiled-coil region and a globular tail domain that binds membrane cargo (Avisar et al. 2009; Li and Nebenführ 2007, 2008; Sparkes 2010; Vale 2003). The neck region is more than 20 nm long, and the entire molecule ~50 nm (Sakamoto et al. 2003). Even in a folded, inhibited state myosin V is ~30 nm long and ~20 nm wide (Li et al. 2006; Liu et al. 2006). Accordingly, EM studies show parallel strands of ER and actin mostly separated by 10–100 nm gaps with only punctate closer contacts (Hepler 1982; Lichtscheidl et al. 1990; Staehelin 1997). By comparison, PD are only about 50 nm in diameter, with the cytoplasmic sleeve between the plasma membrane and DT around ~10 nm in the neck region. It is obvious that the DT cannot have a similar cytoskeletal scaffold as the cortical ER. Nevertheless, both actin (White et al. 1994; Blackman and Overall 1998) and myosin (Blackman and Overall 1998; Radford and White 1998; Reichelt et al. 1999) have been immuno-localised along the PD pore and appear to play a role in regulating PD transport (Avisar et al. 2008; Baluska et al. 2001; Ding et al. 1996; Radford and White 1998; Su et al. 2010; White et al. 1994).
Overall and Blackman (1996) have proposed that the globular proteins encircling the DT in a helical arrangement are actin molecules and the spokes are myosin. Single actin fibres have a diameter of ~7 nm (Holmes et al. 1990), which is larger than the globular proteins surrounding the DT (2.5–4.5 nm; Botha et al. 1993; Ding et al. 1992; Overall et al. 1982), but they constitute a double helix of two strings of monomers. If the globular proteins of the DT coat are indeed actin they would have to be bound directly to or even embedded within the membrane and perhaps arranged in an unusual conformation. Alternatively, the effects of aldehyde fixation and/or osmium tetroxide and tannic acid staining may have led to inaccurate size estimates, as there are also disagreements over interpreting electron-dense or electron-lucid material as proteinaceous (Blackman and Overall 2001; Ding et al. 1992; Overall and Blackman 1996). Antibodies specific to the animal actin-stabilising protein tropomyosin have recently been found to label PD (Faulkner et al. 2009) even though no direct tropomyosin homologs have been found in higher plants. The specificity must be due to an actin-binding protein because the antibodies also co-localised with microfilaments. Perhaps the detected protein plays a role in the unusual membrane-attachment of actin within PD.
The PD-localised myosins belong to class VIII (Reichelt et al. 1999) which are smaller than type XI with only three to four IQ motifs, a shorter coiled coil domain and a characteristic C-terminus lacking a globular tail domain (Volkmann et al. 2003; Avisar et al. 2009; Sparkes 2010). However, as myosin motor domains are highly conserved and based on primary sequence comparison, it is unlikely that they are significantly shorter than ~20 nm. Thus, it remains unclear how myosin VIII fits into the PD structure as seen in electron microscopy. Its C-terminal domain contains clusters of basic residues which might bind to phospholipid headgroups (Knight and Kendrick-Jones 1993; Volkmann et al. 2003). If the head domains bind actin on the DT side, they might form part of the globular DT surface. The C-terminal tails might then bind the plasma membrane, with only the neck and coiled coil regions appearing as spokes. Whilst the nature of the cytoskeletal association of the desmotubular ER remains a matter of speculation, it is apparent that it differs significantly from that of the cortical ER.
Macromolecular trafficking through the desmotubule
Since the generation of the DT requires such extensive and unique remodelling of the cortical ER, it constitutes one of the defining features of PD, distinguishing them from other sites of plasma membrane adhesion or membrane tubulation and begs the question, ‘What is its function?’ The most likely answer is that it limits intercellular transport of lumenal and membrane contents of the ER and provides mechanical stability to PD. In budding yeast cells, a ring of the protein septin constricts the ER and thereby slows the diffusion of membrane proteins between mother and daughter cells (Luedeke et al. 2005). In plants, both endomembrane markers (Grabski et al. 1993; Martens et al. 2006) and non-protein ER-lumenal tracers up to 10 kDa (Cantrill et al. 1999) move cell-to-cell whereas GFP fusions targeted to the lumen show only very limited movement (Crawford and Zambryski 2000; Guenoune-Gelbart et al. 2008; Martens et al. 2006; Oparka et al. 1999). By contrast, GFP-fusions localised within the ER membrane moved intercellularly in Nicotiana benthamiana (Guenoune-Gelbart et al. 2008). This latter result does not seem to support a function of DT constriction in limiting cell-to-cell trafficking in the endomembrane system. However, it has to be kept in mind that plants may be able to modify DT constriction and different tissues and experimental systems may vary considerably. In particular, N. benthamiana is known to facilitate more extensive epidermal protein trafficking than closely related species (Howard et al. 2004; Krishnamurty et al. 2002).
In some species, the DT can be unconstricted in the centre of PD (Overall and Blackman 1996; Ding et al. 1992; Ehlers and Kollmann 2001; Glockmann and Kollmann 1996; Robinson-Beers and Evert 1991), or lack a central rod and appear slightly widened (Waigmann et al. 1997). In cytoplasmically sealed PD, it can even be either electron lucid (i.e. depleted in proteins) or completely absent (Botha and Cross 2000) whereas in other instances, PD may be dilated without any change in DT diameter (Schulz 1995). Ehlers and Kollmann (2001) have pointed out that in gating studies based on transient expression of fluorescent fusion proteins in epidermal cells, trafficking is usually observed only laterally within the epidermis, and not into the mesophyll. Ideally, trafficking studies should be combined with ultrastructural analysis of the same tissue and cell boundary. Unfortunately, this may be hard to implement because EM studies often do not achieve transverse views of PD with sufficient resolution and clarity to resolve DT substructure and small changes in diameter or a central rod may not be apparent depending on tissue preservation, fixation and staining procedures.
A role of the desmotubule in stabilising PD
Removal of the DT from PD near cutting surfaces in fern gametophytes by proteinase incubation resulted in pore dilation and random outward bulging of the plasma membrane in the central area (Tilney et al. 1991). The authors concluded that the function of the DT was to maintain the constriction of the PD channel. This is also supported by the fact that the DT is often not constricted in the dilated central cavities (Overall and Blackman 1996; Ding et al. 1992; Ehlers and Kollmann 2001; Glockmann and Kollmann 1996; Robinson-Beers and Evert 1991) but is always constricted in the narrow neck regions which are viewed as the areas where the size exclusion limit and macromolecular trafficking are regulated (Blackman and Overall 2001, Overall and Blackman 1996; Oparka 2004). Opening of PD does not, however, seem to require a removal of DT constriction (Moore et al. 1992; Schulz 1995). A possible explanation for this is that rigidity of the DT rather than its constriction per se is required for maintenance of PD structure. If the spokes are maintaining the constriction of the cytoplasmic sleeve, they need to be firmly attached to both the DT and the plasma membrane. A loosening of the central rod and DT surface may then lead to increased motility or detachment of the anchoring sites on the DT, whilst only resulting in a moderate, hard to detect increase of DT diameter due to the remaining presence of reticulons (Fig. 3).
Model of possible gating mechanisms employed by viral movement proteins. a In the non-gated state, constriction of the desmotubule is maintained by a rigid protein scaffold. This scaffold probably has elements within the DT lumen (the central rod) and some electron micrographs appear to show links from the central rod to the spokes in the cytoplasmic sleeve, implying transmembrane domains. According to previous PD models (Overall and Blackman 1996) and immunolocalisations, actin is shown associated with the DT, but only monomers are small enough to match the globular proteins observed by EM. The spokes are shown as myosin VIII (only monomers depicted) neck domains. Whether reticulons are present in or excluded from the DT membrane is unclear. Remorin is shown decorating the cytoplasmic face of lipid rafts which are ‘trapped’ by PDCB1. Because rafts are also viewed as anchoring points for the cytoskeleton, myosin VIII is shown contacting the remorin-covered rafts, not necessarily implying a direct protein-protein interaction. b Two mechanisms contributing to gating: movement proteins inserting into the ER membrane, in this case PMTV TGB2 and 3, disrupt the protein-protein interactions which stabilise the DT. This may lead to a dilation of the desmotubule and an opening of its lumenal space, however, it remains confined by the presence of reticulons and the surrounding PD components. The actual gating effect results from increased freedom of diffusion within the DT membrane and a loss of the rigid scaffold anchoring the spokes (indicated by a detached actin monomer). By contrast, a cytosolic MP like PVX TGB1, which interacts with remorin, may directly disrupt the anchoring of the spokes on either membrane. Note that whilst the drawing is approximately to scale, the structures and true sizes of most components are unknown
Actin and myosin localised in PD seem to be involved in regulating pore aperture (Blackman and Overall 2001; Overall and Blackman 1996; Oparka 2004). Specifically, actin depolymerisation led to a widening of the neck region in some species (White et al. 1994) and to an increased SEL (Ding et al. 1996) and also seems to be used for gating by a viral MP (Su et al. 2010). The myosin ATPase inhibitor 2,3-butanedione monoxime (BDM) caused a contraction of the PD neck region (Radford and White 1998). BDM stabilises the actin-myosin-ADP-Pi complex (Herrmann et al. 1992; Zhao et al. 1995). Collectively, these data point to a role for acto-myosin in maintaining PD constriction, although the properties of myosin VIII as a molecular motor are unknown and its identification with the PD spokes remains unproven. Despite such uncertainties, the rigid scaffolding of the DT probably enables it to function as the central anchoring beam for PD constriction.
The desmotubule as a target for viral movement proteins and a factor in PD gating
In addition to disruption of DT plasma membrane contacts in the cytoplasmic sleeve, or induction of callose degradation (Epel 2009), destabilisation of the DT structure is another possible mechanism that may contribute to PD gating by viral MP. If a rigid structure (DT and spokes) is required to retain PD constriction, any protein–protein interaction that quantitatively displaces components of this scaffold could contribute to gating (Fig. 3). To disrupt the DT scaffold, viral MPs would need to affect protein–lipid interactions or protein–protein interactions within the DT membrane or lumen and therefore have to be membrane-associated themselves. They could also be expected to perturb constricted membranes in general. Intriguingly, this is exactly what is observed experimentally. Many viral MPs are membrane-associated (Lucas 2006 and see references in Martínez-Gil et al. 2009). The well-studied 30k MP of tobacco mosaic virus (TMV) associates with the ER (Heinlein et al. 1998) and induces the conversion of membrane tubules into aggregates. At early stages of TMV infection, at the height of 30k expression, this causes such extreme disruption that the tubular network transiently disappears altogether (Reichel and Beachy 1998). TMV 30k also penetrates into the DT as it localises to and remains in the central cavity of branched PD (Moore et al. 1992; Tomenius et al. 1987) and labels the sieve element ER traversing sieve plate pores in the phloem (Fitzgibbon et al. 2010). A biochemical analyses of the 30k protein has suggested that it is an integral membrane protein with two transmembrane helices (Brill et al. 2000). However, in the ilarvirus, prunus necrotic ringspot virus which encodes a single MP belonging to the 30k superfamily (Melcher 2000), extensive characterisation of its membrane interaction has shown that the single hydrophobic domain is not a transmembrane helix, but forms a kinked re-entry loop with structural similarities to fusion peptides of coated animal viruses (Martínez-Gil et al. 2009). Fusion peptides cause membrane perturbations which facilitate trans-bilayer leaflet interactions that create a hemifusion stalk in membrane fusion events leading to virus entry. They can also contribute to the creation of protein-depleted membrane patches required for fusion (Chernomordik et al. 2006). Similar work on TMV 30k now indicates that it is a similar peripheral (but strongly associated) membrane protein with the first hydrophobic region exhibiting characteristics similar to ilarviral MPs (J.A. Sánchez-Navarro, personal communication).
In viruses with a TGB of three movement proteins, TGB2 and 3 are integral membrane proteins and TGB3 recruits TGB2 to the cell periphery (Lee et al. 2010b; Morozov and Solovyev 2003; Samuels et al. 2007; Solovyev et al. 2000; Tilsner et al. 2010). After ectopic overexpression, ‘peripheral bodies’ are formed which are distended cortical ER compartments (Lee et al. 2010b; Schepetilnikov et al. 2005; Solovyev et al. 2000). Recent work on potato virus X (PVX) demonstrated that its TGB3 targets the reticulon-containing, highly curved tubules of the cortical ER, and the peripheral bodies result from a bulging of these normally constricted membranes (Lee et al. 2010b). TGB2 and 3 proteins of PVX and potato mop-top virus (PMTV) also induce the formation of membraneous ‘granules’ on the cortical ER (Cowan et al. 2002; Haupt et al. 2005; Ju et al. 2005; Krishnamurty et al. 2003; Samuels et al. 2007), which similarly are domains of locally reduced tubule constriction. In PMTV, over-expression of a GFP-TGB2/TGB3 complex at a TGB2:3 ratio similar to the virus infection results in the appearance of ‘sausage’-shaped structures on the ER (Fig. 4a; Tilsner et al. 2010). A point mutation (Y89G) within the lumenal domain of TGB3 (which has two transmembrane domains) abolishes virus movement but not TGB3-assisted PD-targeting of GFP-TGB2 (Haupt et al. 2005; Tilsner et al. 2010). However, over-expression of this mutant GFP-TGB2/TGB3(Y89G) complex does not disturb ER structure (Fig. 4b; Tilsner et al. 2010), indicating that membrane perturbation and virus movement are linked and that the function of TGB3-induced membrane perturbation must be separate from TGB2 recruitment.
Thus, whilst many details are still unclear, there is accumulating evidence that endomembrane perturbation is an important function of certain types of viral MPs. Progress in this area will depend on an increased understanding of MP–membrane interactions, including investigations of oligomerisation in lipid environments, interactions with specific membrane lipids and effects on membrane curvature and scaffolds. We have obtained preliminary data showing that the PMTV TGB2 and 3 proteins have distinct affinities for specific structural and signalling lipids that likely influence their distribution in endomembranes. Studies are currently in progress that also include the PVX TGBs, to understand how these lipid interactions influence TGB subcellular targeting (L. Torrance, G. Cowan and J. Tilsner, unpublished data).
Perturbance of the ER membrane by viral movement proteins. a A complex of the potato mop-top virus movement proteins GFP-TGB2 and TGB3, overexpressed in a ratio of ~10:1 from a bicistronic plasmid resembling the subgenomic viral mRNA, forms ‘sausage’ shaped structures on the tubular cortical ER. b Mutation of a single amino acid within the lumenal domain of the TGB3 protein (Y89G) renders the virus movement-incompetent and the distortion of the tubular ER is no longer observed (from Tilsner et al. 2010). Bars = 10 μm
The uniqueness of the DT as a PD-specific domain of the ER makes it an ideal candidate as a targeting ‘receptor’ of viral MPs. Delivery of many viral MPs to PD requires the endomembrane system (Lucas 2006), but the secretory pathway is not always involved, leading to descriptions of MP targeting as ‘unconventional’ pathways (Schepetilnikov et al. 2008). These findings could be explained by random co-transport of viral MPs and ribonucleoprotein complexes with the remodelling of the cortical ER, possibly with a tendency to associate with the tubular, cortical areas (Lee et al. 2010b), that would occasionally deliver them to the vicinity of PD (Wright and Oparka 2006). The DT probably branches off at a three-way junction similar to others in the cortical ER (Sparkes et al. 2009a, c) and both TMV 30k and membrane-associated TGB proteins associate with these vertices (Haupt et al. 2005; Reichel and Beachy 1998). Recognition of the DT by a viral MP could happen by preferential distribution into highly curved membranes (Lee et al. 2010b; Voeltz and Prinz 2007) as well as through the same specific interactions with DT components that are also responsible for disturbing DT structure.
Once viral MPs have targeted and gated PD, a diffusion-driven trafficking of membrane-bound ribonucleoprotein complexes has been proposed (Guenoune-Gelbart et al. 2008; Epel 2009; Schönknecht et al. 2008). The diffusion of membrane-anchored movement complexes would probably not only be hindered by occlusion of the cytoplasmic sleeve, but also by a rigid membrane scaffold in tissues with tight DT constriction and therefore, disruption of the DT scaffold would also be beneficial to the virus in this context. In agreement with a loosening of DT structure, Guenoune-Gelbart et al. (2008) found that 30k protein increased trafficking of lumenal GFP-fusions. Unexpectedly, diffusion of GFP fusions inserted into the ER membrane was hindered by 30k. However, this could be caused by 30k oligomers blocking the pathway (Guenoune-Gelbart et al. 2008).
Finally, it is likely that plant cells can restore PD structure after modifications by viral MPs. Despite its influence on SEL, no 30k induced disturbances of the DT or PD structure in general have been observed in MP-expressing transgenic or TMV-infected plants (Wolf et al. 1989; Moore et al. 1992). Also, PD gating is restricted to the leading edge of developing lesions (Oparka et al. 1997). Plants must be able to generate desmotubules from cortical ER tubules de novo during secondary PD formation (Ehlers and Kollmann 1996, 2001). In some cases, two DTs have been observed in the same channel (Faulkner et al. 2008; Glockmann and Kollmann 1996). Faulkner et al. (2008) developed a model whereby secondary PD can be generated by ‘twinning’ of existing pores. After PD dilation, a second DT would be generated by insertion from the cortical ER or branching of the existing DT, followed by creation of a new wall separation. This model requires de novo constriction and scaffolding of cortical ER elements or a transient loosening of DT structure which might account for PD lacking a central rod or the absence or electron-lucidiy of the DT (Botha and Cross 2000; Waigmann et al. 1997). If the cell is generally able to regenerate the DT, then ‘repair’ of virus-modified PD or compensation for the effects of stable MP expression may also be possible. The process of constricting and scaffolding a subdomain of the tubular ER is probably also a defining step in the biogenesis of PD.
Formation of specialised ER-plasma membrane adhesion sites as a central event in PD biogenesis
Differences between PD and other ER-plasma membrane contacts
The plant cortical ER has areas of close contact with all other endomembranes and the plasma membrane, which may function in the exchange of lipids and proteins (Andersson et al. 2007; Craig and Staehelin 1988; de Brito and Scorrano 2008; Lee et al. 2010a; English et al. 2009; Goetz et al. 2007; Hepler et al. 1990; Park and Blackstone 2010; Sparkes et al. 2009a, b; Staehelin 1997). These junctions are often sites of strong membrane adhesion (Andersson et al. 2007; Sparkes et al. 2009b), including non-PD ER-plasma membrane contacts that can be strong enough to withstand plasmolysis and keep the cortical ER in the vicinity of the cell wall outside of the protoplast (Oparka et al. 1994). It is unclear if there are any parallels between ER-plasma membrane adhesion sites in animals (Lee et al. 2010a; Levine and Rabouille 2005) and plants, but animals do not have myosin VIII. Myosin VIII may be a general component of plant-specific ER-plasma membrane contacts (Golomb et al. 2008; Volkmann et al. 2003) and whilst spokes have only been observed in PD, globular, electron dense proteinaceous connections can sometimes also be observed between other closely adjacent ER and plasma membrane surfaces (e.g. Fig. 8 in Hepler (1982) and Fig. 7b in Staehelin (1997)), and during the formation of PD in protoplasts regenerating the cell wall (Fig. 1 in Ehlers and Kollmann (1996)). What sets PD apart from other ER-plasma membrane contacts is the constriction and scaffolding of the ER into the DT. Establishing this structure must therefore be one of the key events during the generation of PD.
Insertion of ER tubules into the growing cell wall
Electron microscopy of dividing plant cells (Hawes et al. 1981; Hepler 1982; Segui-Simarro et al. 2004) has provided some insight into how the DT originates during cytokinesis. Vesicles containing cell wall material accumulate at the future division plane and fuse into a tubulo-vesicular network that is still extensively fenestrated. Some ER tubules become ‘entrapped’ in these gaps. Subsequently, extensive callose deposition into the tubulo-vesicular network correlates with its flattening and the closure of remaining gaps to form the cell plate, i.e. the future cell wall. Where gaps contained ER tubules, these become constricted and the contact site between ER and plasma membrane is modified into a primary PD. Mechanisms for several types of post-cytokinetic PD modifications and the de novo formation of secondary PD have been also suggested (Ehlers and Kollmann 1996, 2001; Faulkner et al. 2008, Kollmann and Glockmann 1991). In all cases, deposition of new cell wall material is thought to (further) entrap an ER tubule that either already crosses the cell wall or is newly inserted into pores.
Whilst the term entrapment accurately describes the spatial positions of ER and cell wall-forming vesicles, it may be misleading in that it suggests a rather passive role of the ER in this process. Live-cell imaging has revealed that the cortical ER network is highly dynamic (Boevink et al. 1998; Sparkes et al. 2009a, c, 2010). Tubules and three-way junctions are constantly being remodelled and at the same time are also being swept along by cytoplasmic streaming. New tubules can extend, undergo ‘searching’ motions and either retract or form new three-way junctions. In other words, there is no reason that tubules would need to become ‘entrapped’ between cell plate-forming vesicles and if they did, that they would have to become a DT; mechanisms exist that would permit their ‘escape’. On the other hand, the ER network at the forming cell plate may actually play a role in guiding the orientation of the fusing Golgi-derived vesicles and supplying the calcium required for the membrane fusions (Bednarek and Falbel 2002; Gupton et al. 2002; Hawes et al. 1981; Hepler 1982). Cytokinetic ER has also been interpreted as a store for proteins that are transferred to the cell plate forming vesicles at specific stages in a COPII-dependent manner (Prekeris and Gould 2008). All of this points to a very active role of the ER in generating PD that might warrant a description of ER tubules being ‘inserted into’, rather than ‘entrapped by’ the forming cell plate.
Possible triggers for the initiation of desmotubule formation
The study of membrane trafficking during plant cytokinesis has focussed mostly on the delivery of plasma membrane and cell wall material, which now appears to not only derive from secretory vesicles carrying newly synthesised lipids and polysaccharides, but also from endocytotic re-use of existing wall and plasma membrane material (Bednarek and Falbel 2002; Backues et al. 2007). The ER has received considerably less attention in this context (Prekeris and Gould 2008), which is partly due to the difficulty of distinguishing different membrane compartments of the cell plate at the light microcope level where most protein localisations have been carried out. Therefore, the triggers that initiate DT formation remain completely speculative. They might for instance include: (1) proximity of an ER tubule to an area of plasma membrane with a distinctive composition, perhaps due to the callose-enrichment of the underlying cytokinetic wall. (2) Contact of the ER with the membrane fusion machinery involved in closing the gaps in the cell plate. (3) General ‘priming’ of the ER network for PD formation during cytokinesis by expression of proteins that might make its surface ‘sticky’ for the cell wall membrane, similar to Golgi attachment to the cortical ER (Sparkes et al. 2009b). The contribution of recycled plasma membrane material makes it possible that the cell plate membrane is not very different from the mature plasma membrane at other ER adhesion sites, and this is further supported by the localisation of myosin VIII at the cell plate (Reichelt et al. 1999; Volkmann et al. 2003). The distinctive feature of the underlying wall however, which is shared by the wall collar around PD, is its high callose content. In this context, it would be interesting to study the expression and localisation of the PD callose binding protein, PDCB1 (Simpson et al. 2009), and its homologs in more detail during cytokinesis.
General mechanisms of ER remodelling in plants
Because so little is known about the remodelling of the ER during cytokinesis, conclusions about its transformation into the DT can currently only be drawn in very broad terms based on the principles of ER remodelling in general. The cortical ER network is not dynamic in its entirety. Computer analysis of time-lapse imaging and direct manipulation of the ER by laser trapping have identified static ‘anchor points’ that do not necessarily match the tubular three-way junctions and probably correspond to plasma membrane adhesion sites (Sparkes et al. 2009a, b, c). Thus, the cortical ER is able to both maintain its overall structure despite constant remodelling and mass flow of lipids and proteins, and to transform specific areas into static contact or adhesion sites with other membranes (Levine and Rabouille 2005; Park and Blackstone 2010; Sparkes et al. 2009a), including PD. In contrast to the plasma membrane, the ER membrane consists mostly of unsaturated glycero-phospholipids (van Meer and Sprong 2004; van Meer et al. 2008) which makes it overall more flexible and probably precludes lateral heterogeneity due to Lo–Ld phase separation. The sphingolipids and sterols responsible for raft formation in the plasma membrane are however synthesised along the secretory pathway including the ER and small quantities of some of these lipids are present in ER membranes where they may assist in packaging transmembrane cargo proteins for co-export through the Golgi apparatus (Fagone and Jackowski 2009; Haucke and Di Paolo 2007; Holthuis and Levine 2005; Laloi et al. 2007; van Meer and Sprong 2004).
Rather than through lipid phase separation, lateral heterogeneity of endomembranes occurs through a complex and highly dynamic network of protein–lipid interactions. The induction of membrane curvature by proteins and asymmetries in bilayer composition has already been discussed above. However, curvature is not simply a shape imposed on a ‘passive’ membrane by an ‘active’ protein, but in turn also targets proteins to the membrane, including the cytoskeleton (Hurley and Meyer 2001; Sprong et al. 2001; Stahelin 2009; Takano et al. 2008; Xue et al. 2009), which can lead to further changes in bilayer conformation accompanied by lipid segregation (Mitra et al. 2004; Ronchi et al. 2008; Roux et al. 2005; Sorre et al. 2009; Takano et al. 2008). For example, the small GTPase Arf1 distributes evenly between flat and curved membranes, but its GTPase activating factor Arf1-GAP is restricted to curved membranes with a radius ≤35 nm. Thus, active Arf1-GTP is restricted to flat membranes, where it recruits the golgin GMAP-210 which tethers membranes with a radius ≤50 nm through its amphipathic lipid sensor packing domain. In this way, specificity is achieved for asymmetric tethering between a flat and a curved membrane (Ambroggio et al. 2010; Drin et al. 2008). In another example, lipids with inverted cone shapes (large head groups), for instance phosphoinositides, inhibit membrane fusion/fission if they are located in the apposing leaflets, but promote it if they are in the distal leaflets of appressed bilayers (Chernomordik et al. 2006; Janmey and Kinnunen 2006; van Meer et al. 2008; van Meer and Sprong 2004). Cone-shaped lipids like diacylglycerol act in the opposite manner and are probably required for vesicle budding and fusion (Haucke and Di Paolo 2007).
In the emerging picture of endomembrane remodelling, changes in membrane conformation are both structure and targeting ‘signal’, both signalling event and signalling output at the same time. In other words, membrane transport, architecture and dynamics are inseparable and distinctions between, for instance, curvature-inducing, curvature-targeted and curvature-sensing proteins are often arbitrary (Stahelin 2009). Thus, the dynamic but ordered architecture of the cortical ER is maintained through dynamic equilibria between membrane-remodelling processes and membrane flux. Similar processes can be expected to be involved in inducing constriction and plasma membrane-tethering of the DT during PD biogenesis. Although at present, the details of this transformation are unknown, it seems likely that there could be common factors shared with the establishment of plasma membrane-attached ER ‘anchor points’.
Possible roles for phosphoinositide signalling and small GTPases in PD biogenesis
Spatially and temporally, the most dynamic lipids are phosphoinositides, i.e. phosphatidylinositol (PI) and its phosphorylated derivatives. PI has a myo-inositol head group linked to diacylglycerol via a phosphate linker. The myo-inositol has five free hydroxyl groups that can be reversibly phosphorylated, resulting in a large variety of PI phosphates (PIPs; Munnik and Testerink 2009; Thole and Nielsen 2008; Xue et al. 2009). PIPs, as well as their immediate metabolites phosphatidic acid (lacking the myo-inositol head) and diacylglycerol are important signalling molecules because of their fast, locally restricted turnover and ability to recruit proteins, including actin. PIPs therefore are involved in key steps of cellular membrane remodelling (Downes et al. 2005; Fischer et al. 2004; Hurley and Meyer 2001; Pleskot et al. 2010; Sprong et al. 2001; Stahelin 2009; Testerink and Munnik 2005; Xue et al. 2009). In the course of their involvement in regulatory processes, PIPs can accumulate at specific subcellular localisations. For instance, PI(3)P is found at the leading edges of the expanding cell plate, and PI(4)P and PI(4,5)P2 accumulate at the tip of growing root hairs, with PI(4,5)P2 also distributed in a spiral pattern along the sides (reviewed in Backues et al. 2007). Recent advances in live cell imaging have enabled monitoring of dynamic PIP changes (Downes et al. 2005; van Leeuwen et al. 2007; Vermeer et al. 2006, 2009) but also other lipids including sterols (Hölttä-Vuori et al. 2008) and will prove useful to study endomembrane remodelling at the cell plate in combination with fluorescent protein fusions of newly discovered factors involved in shaping the ER (Hu et al. 2009).
Small GTPases of the Rab, Rop and Arf families also play roles in phospholipid-mediated membrane trafficking and reorganisation (Bednarek and Falbel 2002; Rutherford and Moore 2002; Zhang and McCormick 2010) and a Rab11 homolog has been localised to PD during TMV infection (Escobar et al. 2003). This protein could be involved in the host cell’s response restoring PD function (see above) and consequentially also during cytokinetic PD formation. Indeed, Rab11 is required for cytokinesis in Caenorhabditis elegans (Bednarek and Falbel 2002; Prekeris and Gould 2008). New insights into PD biogenesis are therefore likely to emerge from studies of the roles of phosphoinositide signalling and small GTPases during cytokinesis.
Conclusions and outlook
Whilst confocal fluorescence microscopy has successfully identified a microtubule-binding protein, MAP65-5, that may be involved in PD formation at the cell plate (Van Damme et al. 2004), a clear distinction between events concerning the cell plate-forming vesicles and the remodelling of the ER into the DT will require higher resolution. Lipid- and protein-specific immuno-electron microscopy (Downes et al. 2005) may be required in some instances, but new ‘super resolution’ light microscopy techniques and correlative light/electron microscopy are also poised to have a large impact on unravelling the events that lead to PD formation (Fitzgibbon et al. 2010; Huang 2010; Perinetti et al. 2009 and see review by Bell and Oparka in this issue). PD regeneration in protoplasts may be an easier system for such studies than cytokinesis in meristematic tissues. On the other hand, characterisation of membrane topology, lipid-binding, membrane shaping and oligomerisation properties of new PD component proteins in vitro and in vivo (Boutant et al. 2010; Downes et al. 2005; Hu et al. 2008; Martínez-Gil et al. 2009; Miernyk and Thelen 2008; Stöckl and Herrmann 2010; Voeltz et al. 2006) will also be indispensable to understand PD structure.
Whilst PD are more than simply membrane adhesion sites, it will be impossible to understand their structure and function at a molecular level without considering the protein–lipid interactions involved in defining the membranes that line them. A focus on their membranes can therefore inform interpretations of data obtained by a variety of different techniques and result in new approaches to studying PD.
Abbreviations
- DRM:
-
Detergent resistant membrane
- DT:
-
Desmotubule
- EM:
-
Electron microscopy
- ER:
-
Endoplasmic reticulum
- GFP:
-
Green fluorescent protein
- GPI:
-
Glycosyl phosphatidyl inositol
- Ld :
-
Liquid-disordered
- Lo :
-
Liquid-ordered
- MP:
-
Movement protein
- PD:
-
Plasmodesma(ta)
- PDLP:
-
Plasmodesmata-located protein
- PDCB:
-
Pd callose binding protein
- PIP:
-
Phosphatidylinositol phosphate
- PMTV:
-
Potato mop-top virus
- PVX:
-
Potato virus X
- So :
-
Solid-ordered
- TMD:
-
Transmembrane domain
- TMV:
-
Tobacco mosaic virus
References
Amari K, Boutant E, Hofmann C, Schmitt-Keichinger C, Fernandez-Calvino L, Didier P, Lerich A, Mutterer J, Thomas CL, Heinlein M, Mély Y, Maule AJ, Ritzenthaler C (2010) A family of plasmodesmal proteins with receptor-like properties for plant viral movement proteins. PLoS Pathog. doi:10.1371/journal.ppat.1001119
Ambroggio E, Sorre B, Bassereau P, Goud B, Manneville JB, Antonny B (2010) ArfGAP1 generates an Arf1 gradient on continuous lipid membranes displaying flat and curved regions. EMBO J 29:292–303
Andersson MX, Goksör M, Sandelius AS (2007) Optical manipulation reveals strong attracting forces at membrane contact sites between endoplasmic reticulum and chloroplasts. J Biol Chem 282:1170–1174
Auth T, Gov NS (2009) Diffusion in a fluid membrane with a flexible cortical cytoskeleton. Biophys J 96:818–830
Avisar D, Prokhnevsky AI, Dolja VV (2008) Class VIII myosins are required for plasmodesmatal localization of a closterovirus Hsp70 homolog. J Virol 82:2836–2843
Avisar D, bu-Abied M, Belausov E, Sadot E, Hawes C, Sparkes IA (2009) A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles. Plant Physiol 150:700–709
Backues SK, Konopka CA, McMichael CM, Bednarek SY (2007) Bridging the divide between cytokinesis and cell expansion. Curr Opin Plant Biol 10:607–615
Badelt K, White RG, Overall RL, Vesk M (1994) Ultrastructural specializations in the cell wall sleeve around plasmodesmata. Am J Bot 81:1422–1427
Bagatolli LA, Ipsen JH, Simonsen AC, Mouritsen OG (2010) An outlook on organization of lipids in membranes: searching for a realistic connection with the organization of biological membranes. J Lipid Res 49:378–389
Baluska F, Samaj J, Napier R, Volkmann D (1999) Maize calreticulin localizes preferentially to plasmodesmata in root apex. Plant J 19:481–488
Baluska F, Cvrcková F, Kendrick-Jones J, Volkmann D (2001) Sink plasmodesmata as gateways for phloem unloading. Myosin VIII and calreticulin as molecular determinants of sink strength? Plant Physiol 126:39–46
Bariola P, Retelska D, Stasiak A, Kammerer R, Fleming A, Hijri M, Frank S, Farmer E (2004) Remorins form a novel family of coiled coil-forming oligomeric and filamentous proteins associated with apical, vascular and embryonic tissues in plants. Plant Mol Biol 55:579–594
Bauer M, Pelkmans L (2006) A new paradigm for membrane-organizing and -shaping scaffolds. FEBS Lett 580:5559–5564
Bednarek SY, Falbel TG (2002) Membrane trafficking during plant cytokinesis. Traffic 3:621–629
Bhat R, Panstruga R (2005) Lipid rafts in plants. Planta 223:5–19
Blackman LM, Overall RL (1998) Immunolocalisation of the cytoskeleton to plasmodesmata of Chara corallina. Plant J 14:733–741
Blackman LM, Overall RL (2001) Structure and function of plasmodesmata. Aust J Plant Physiol 28:709–727
Blackman LM, Harper JDI, Overall RL (1999) Localization of a centrin-like protein to higher plant plasmodesmata. Eur J Cell Biol 78:297–304
Boevink P, Oparka KJ, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15:441–447
Borner GHH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, MacAskill A, Napier JA, Beale MH, Lilley KS, Dupree P (2005) Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol 137:104–116
Bortolotti C, Murillo I, Fontanet P, Coca M, San Segundo B (2005) Long-distance transport of the maize pathogenesis-related PRms protein through the phloem in transgenic tobacco plants. Plant Sci 168:813–821
Botha CEJ, Cross RHM (2000) Towards reconciliation of structure with function in plasmodesmata—who is the gatekeeper? Micron 31:713–721
Botha CEJ, Hartley BJ, Cross RHM (1993) The ultrastructure and computer-enhanced digital image analysis of plasmodesmata at the kranz mesophyll-bundle sheath interface of Themeda triandra var. imberbis (Retz) A. Camus in conventionally fixed blades. Ann Bot 72:255–261
Boutant E, Didier P, Niehl A, Mély Y, Ritzenthaler C, Heinlein M (2010) Fluorescent protein recruitment assay for demonstration and analysis of in vivo protein interactions in plant cells and its application to Tobacco mosaic virus movement protein. Plant J 62:171–177
Brandizzi F, Frangne N, Marc-Martin S, Hawes C, Neuhaus JM, Paris M (2002) The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14:1077–1092
Brill LM, Nunn RS, Kahn TW, Yeager M, Beachy RN (2000) Recombinant tobacco mosaic virus movement protein is an RNA-binding, α-helical membrane protein. Proc Natl Acad Sci USA 97:7112–7117
Cantrill LC, Overall RL, Goodwin PB (1999) Cell-to-cell communication via plant endomembranes. Cell Biol Int 23:653–661
Chen MH, Sheng J, Hind G, Handa AK, Citovsky V (2000) Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J 19:913–920
Chen M-H, Tian G-W, Gafni Y, Citovsky V (2005) Effects of calreticulin on viral cell-to-cell movement. Plant Physiol 138:1866–1876
Chernomordik LV, Zimmerberg J, Kozlov MM (2006) Membranes of the world unite! J Cell Biol 175:201–207
Ciczora Y, Callens N, Penin F, Pecheur EI, Dubuisson J (2007) Transmembrane domains of hepatitis C virus envelope glycoproteins: residues involved in E1E2 heterodimerization and involvement of these domains in virus entry. J Virol 81:2372–2381
Cook ME, Graham LE, Botha CEJ, Lavin CA (1997) Comparative ultrastructure of plasmodesmata of Chara and selective bryophytes: toward an elucidation of the evolutionary origin of plant plasmodesmata. Am J Bot 84:1169–1178
Cory GOC, Cullen P (2007) Membrane curvature: the power of bananas, zeppelins and boomerangs. 17:R455–R457
Cowan GH, Lioliopoulou F, Ziegler A, Torrance L (2002) Subcellular localisation, protein interactions, and RNA binding of potato mop-top virus triple gene block proteins. Virology 298:106–115
Craig S, Staehelin LA (1988) High pressure freezing of intact plant tissues. Evaluation and characterization of novel features of the endoplasmic reticulum and associated membrane systems. Eur J Cell Biol 46:81–93
Crawford KM, Zambryski PC (2000) Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr Biol 10:1032–1040
de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456:605–610
Delmer DP, Volokita M, Solomon M, Fritz U, Delphendahl W, Herth W (1993) A monoclonal antibody recognizs a 65 kDa higher plant membrane polypeptide which undergoes cation dependent association with callose synthase in vitro and colocalizes with sites of high callose deposition in vivo. Protoplasma 176:33–42
Diener AC, Li HX, Zhou WX, Whoriskey WJ, Nes WD, Fink GR (2000) STEROL METHYLTRANSFERASE 1 controls the level of cholesterol in plants. Plant Cell 12:853–870
Ding B, Turgeon R, Parthasarathy MV (1992) Substructure of freeze-substituted plasmodesmata. Protoplasma 169:28–41
Ding B, Kwon M-O, Warnberg L (1996) Evidence that actin filaments are involved in controlling the permeability of plasmodesmata in tobacco mesophyll. Plant J 10:157–164
Dorokhov YL, Mäkinen K, Frolova OY, Merits A, Saarinen J, Kalkkinen N, Atabekov JG, Saarma M (1999) A novel function for a ubiquitous plant enzyme pectin methylesterase: the host-cell receptor for the tobacco mosaic virus movement protein. FEBS Lett 461:223–228
Dowhan W, Bogdanov M (2009) Lipid-dependent membrane protein topogenesis. Ann Rev Biochem 78:515–540
Downes CP, Gray A, Lucocq JM (2005) Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol 15:259–268
Drin G, Antonny B (2010) Amphipathic helices and membrane curvature. FEBS Lett 584:1840–1847
Drin G, Morello V, Casella JF, Gounon P, Antonny B (2008) Asymmetric tethering of flat and curved lipid membranes by a golgin. Science 320:670–673
Ehlers K, Kollmann R (1996) Formation of branched plasmodesmata in regenerating Solanum nigrum protoplasts. Planta 199:126–138
Ehlers K, Kollmann R (2001) Primary and secondary plasmodesmata: structure, origin and functioning. Protoplasma 216:1–30
Ehlers K, Schulz M, Kollmann R (1996) Subcellular localization of ubiquitin in plant protoplasts and the function of ubiquitin in selective degradation of outer-wall plasmodesmata in regenerating protoplasts. Planta 199:139–151
English AR, Zurek N, Voeltz GK (2009) Peripheral ER structure and function. Curr Opin Cell Biol 21:596–602
Epel BL (2009) Plant viruses spread by diffusion on ER-associated movement-protein-rafts through plasmodesmata gated by viral induced host β-1, 3-glucanases. Sem Cell Dev Biol 20:1074–1081
Escobar NM, Haupt S, Thow G, Boevink P, Chapman S, Oparka KJ (2003) High-throughput viral expression of cdna-green fluorescent protein fusions reveals novel subcellular addresses and identifies unique proteins that interact with plasmodesmata. Plant Cell 15:1507–1523
Fagone P, Jackowski S (2009) Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res 50:S311–S316
Farsad K, De Camilli PD (2003) Mechanisms of membrane deformation. Curr Opin Cell Biol 15:372–381
Faulkner C, Akman OE, Bell K, Jeffree C, Oparka K (2008) Peeking into pit fields: a multiple twinning model of secondary plasmodesmata formation in tobacco. Plant Cell 20:1504–1518
Faulkner CR, Blackman LM, Collings DA, Cordwell SJ, Overall RL (2009) Anti-tropomyosin antibodies co-localise with actin microfilaments and label plasmodesmata. Eur J Cell Biol 88:357–369
Fischer U, Men S, Grebe M (2004) Lipid function in plant cell polarity. Curr Opin Plant Biol 7:670–676
Fitzgibbon J, Bell K, King E, Oparka K (2010) Super-resolution imaging of plasmodesmata using 3D-structured illumination microscopy (3D-SIM). Plant Physiol. doi:10.1104/pp.110.157941
Fleurat-Lessard P, Bouché-Pillon S, Leloup C, Lucas WJ, Serrano R, Bonnemain J-L (1995) Absence of plasma membrane H+-ATPase in plasmodesmata located in pit-fields of the young reactive pulvinus of Mimosa pudica L. Protoplasma 188:180–185
Frost A, Unger VM, De Camilli P (2009) The BAR domain superfamily: membrane-molding macromolecules. Cell 137:191–196
Gechev TS, Gadjev IZ, Hille J (2004) An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell Mol Life Sci 61:1185–1197
Gechev TS, Ferwerda MA, Mehterov N, Laloi C, Qureshi MK, Hille J (2008) Arabidopsis AAL-toxin-resistant mutant atr1 shows enhanced tolerance to programmed cell death induced by reactive oxygen species. Biochem Biophys Res Commun 375:639–644
Glockmann C, Kollmann R (1996) Structure and development of cell connections in the phloem of Metasequoia glyptostroboides needles I. Ultrastructural aspects of modified primary plasmodesmata in Strasburger cells. Protoplasma 193:191–203
Goetz JG, Genty H, St-Pierre P, Dang T, Joshi B, Sauve R, Vogl W, Nabi IR (2007) Reversible interactions between smooth domains of the endoplasmic reticulum and mitochondria are regulated by physiological cytosolic Ca2+ levels. J Cell Sci 120:3553–3564
Golomb L, bu-Abied M, Belausov E, Sadot E (2008) Different subcellular localizations and functions of Arabidopsis myosin VIII. BMC Plant Biol 8:3
Grabski S, de Feijter AW, Schindler M (1993) Endoplasmic reticulum forms a dynamic continuum for lipid diffusion between contiguous soybean root cells. Plant Cell 5:25–38
Guenoune-Gelbart D, Elbaum M, Sagi G, Levy A, Epel BL (2008) Tobacco mosaic virus (TMV) replicase and movement protein function synergistically in facilitating tmv spread by lateral diffusion in the plasmodesmal desmotubule of Nicotiana benthamiana. Mol Plant Microb Interact 21:335–345
Gupton SL, Collings DA, Allen NS (2002) Endoplasmic reticulum targeted GFP reveals ER organization in tobacco NT-1 cells during cell division. Plant Physiol Biochem 44:95–105
Hancock JF (2006) Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol 7:456–462
Hansen CG, Nichols BJ (2010) Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 20:177–186
Hanzal-Bayer MF, Hancock JF (2007) Lipid rafts and membrane traffic. FEBS Lett 581:2098–2104
Haucke V, Di Paolo G (2007) Lipids and lipid modifications in the regulation of membrane traffic. Curr Opin Cell Biol 19:426–435
Haupt S, Cowan GH, Ziegler A, Roberts AG, Oparka KJ, Torrance L (2005) Two plant-viral movement proteins traffic in the endocytic recycling pathway. Plant Cell 17:164–181
Hawes CR, Juniper BE, Horne JC (1981) Low and high voltage electron microscopy of mitosis and cytokinesis in maize roots. Planta 152:397–407
Heinlein M, Padgett HS, Gens JS, Pickard BG, Casper SJ, Epel BL, Beachy RN (1998) Changing patterns of localization of the Tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell 10:1107–1120
Helms JB, Zurzolo C (2004) Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic 5:247–254
Hepler PK (1982) Endoplasmic reticulum in the formation of the cell plate and plasmodesmata. Protoplasma 111:121–133
Hepler PK, Palevitz BA, Lancelle SA, McCauley MM, Lichtscheidl L (1990) Cortical endoplasmic reticulum in plants. J Cell Sci 96:355–373
Herrmann C, Wary J, Travers F, Barman T (1992) Effect of 2, 3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry 31:12227–12232
Holmes KC, Popp D, Gebhard W, Kabsch W (1990) Atomic model of the actin filament. Nature 347:44–49
Holthuis JCM, Levine TP (2005) Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol 6:209–220
Hölttä-Vuori M, Uronen R-L, Repakova J, Salonen E, Vattulainen I, Panula P, Li Z, Bittman R, Ikonen E (2008) BODIPY-cholesterol: a new tool to visualize sterol trafficking in living cells and organisms. Traffic 9:1839–1849
Howard AR, Heppler ML, Ju HJ, Krishnamurthy K, Payton ME, Verchot-Lubicz J (2004) Potato virus X TGBp1 induces plasmodesmata gating and moves between cells in several host species whereas CP moves only in N. benthamiana leaves. Virology 328:185–197
Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, Rapoport TA, Prinz WA (2008) Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 319:1247–1250
Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C (2009) A class of dynamin-like gtpases involved in the generation of the tubular er network. Cell 138:549–561
Huang B (2010) Super-resolution optical microscopy: multiple choices. Curr Opin Chem Biol 14:10–14
Hurley JH, Meyer T (2001) Subcellular targeting by membrane lipids. Curr Opin Cell Biol 13:146–152
Itoh T, Takenawa T (2009) Mechanisms of membrane deformation by lipid-binding domains. Prog Lipid Res 48:298–305
Jacobson K, Mouritsen OG, Anderson RGW (2007) Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol 9:7–14
Janmey PA, Kinnunen PKJ (2006) Biophysical properties of lipids and dynamic membranes. Trends Cell Biol 16:538–546
Ju HJ, Samuels TD, Wang Y-S, Blancaflor E, Payton M, Mitra R, Krishnamurty K, Nelson RS, Verchot-Lubicz J (2005) The potato virus X TGBp2 movement protein associates with endoplasmic reticulum-derived vesicles during virus infection. Plant Physiol 138:1877–1895
Kierszniowska S, Seiwert B, Schulze WX (2009) Definition of Arabidopsis sterol-rich membrane microdomains by differential treatment with methyl-β-cyclodextrin and quantitative proteomics. Mol Cell Proteomics 8:612–623
Klopfenstein DR, Klumperman J, Lustig A, Kammerer RA, Oorschot V, Hauri H-P (2001) Subdomain-specific localization of CLIMP-63 (p63) in the endoplasmic reticulum is mediated by its luminal-helical segment. J Cell Biol 153:1287–1299
Knight A, Kendrick-Jones J (1993) A myosin-like protein from a higher plant. J Mol Biol 231:148–154
Kollmann R, Glockmann C (1991) Studies on graft unions III: on the mechanism of secondary formation of plasmodesmata at the graft interface. Protoplasma 165:71-85
Krishnamurty K, Mitra R, Payton ME, Verchot-Lubicz J (2002) Cell-to-cell movement of the PVX 12 k, 8 k, or coat proteins may depend on the host, leaf developmental stage, and the PVX 25 k protein. Virology 300:269–281
Krishnamurty K, Heppler M, Mitra R, Blancaflor E, Payton M, Nelson RS, Verchot-Lubicz J (2003) The Potato virus X TGBp3 protein associates with the ER network for virus cell-to-cell movement. Virology 309:269–281
Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275:1298–1300
Kusumi A, Suzuki K (2005) Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta Mol Cell Res 1746:234–251
Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki H, Murakoshi H, Kasai RS, Kondo J, Fujiwara T (2005) Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct 34:351–378
Laloi M, Perret AM, Chatre L, Melser S, Cantrel C, Vaultier MN, Zachowski A, Bathany K, Schmitter JM, Vallet M, Lessire R, Hartmann MA, Moreau P (2007) Insights into the role of specific lipids in the formation and delivery of lipid microdomains to the plasma membrane of plant cells. Plant Physiol 143:461–472
Lalonde S, Weise A, Walsh RP, Ward JM, Frommer WB (2003) Fusion to GFP blocks intercellular trafficking of the sucrose transporter SUT1 leading to accumulation in companion cells. BMC Plant Biol 3:8
Lee J-Y, Taoka K-I, Yoo B-C, Ben-Nissan G, Kim D-J, Lucas WJ (2005) Plasmodesmal-associated protein kinase in tobacco and Arabidopsis recognizes a subset of non-cell autonomous proteins. Plant Cell 17:2817–2831
Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S (2010a) An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett 584:2022–2027
Lee S-C, Wu C-H, Wang C-W (2010b) Traffic of a viral movement protein complex to the highly curved tubules of the cortical endoplasmic reticulum. Traffic 11:912–930
Lefebvre B, Timmers T, Mbengue M, Moreau S, Hervé C, Tóth K, Bittencourt-Silvestre J, Klaus D, Deslandes L, Godiard L, Murray JD, Udvardi MK, Raffaele S, Mongrand S, Cullimore J, Gamas P, Niebel A, Ott T (2010) A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc Natl Acad Sci USA 107:2343–2348
Lemmon MA, Flanagan JM, Hunt JF, Adair BD, Bormann BJ, Dempsey CE, Engelman DM (1992) Glycophorin a dimerization is driven by specific interactions between transmembrane alpha-helices. J Biol Chem 267:7683-7689
Levine T, Rabouille C (2005) Endoplasmic reticulum: one continuous network compartmentalized by extrinsic cues. Curr Opin Cell Biol 17:362–368
Levy A, Erlanger M, Rosenthal M, Epel B (2007) A plasmodesmata-associated β-1, 3-glucanase in Arabidopsis. Plant J 49:669–682
Li JF, Nebenführ A (2007) Organelle targeting of myosin XI is mediated by two globular tail subdomains with separate cargo binding sites. J Biol Chem 282:20593–20602
Li J-F, Nebenführ A (2008) The tail that wags the dog: the globular tail domain defines the function of myosin V/XI. Traffic 9:290–298
Li X-D, Jung HS, Mabuchi K, Craig R, Ikebe M (2006) The globular tail domain of myosin Va functions as an inhibitor of the myosin Va motor. J Biol Chem 281:21789–21798
Lichtenberg D, Goñi FM, Heerklotz H (2005) Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci 30:430–436
Lichtscheidl IK, Lancelle SA, Hepler PK (1990) Actin-endoplasmic reticulum complexes in Drosera. Their structural relationship with plasmalemma, nucleus, and organelles in cells prepared by high pressure freezing. Protoplasma 155:116–126
Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327:46–50
Liu J, Taylor DW, Krementsova EB, Trybus KM, Taylor KA (2006) Three-dimensional structure of the myosin V inhibited state by cryoelectron tomography. Nature 442:208–211
Lucas WJ (2006) Plant viral movement proteins: agents for cell-to-cell trafficking of viral geneomes. Virology 344:169–184
Lucas WJ, Ham BK, Kim JY (2009) Plasmodesmata—bridging the gap between neighboring plant cells. Trends Cell Biol 19:495–503
Luedeke C, Frei SB, Sbalzarini I, Schwarz H, Spang A, Barral Y (2005) Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J Cell Biol 169:897–908
Martens HJ, Roberts AG, Oparka KJ, Schulz A (2006) Quantification of plasmodesmatal endoplasmic reticulum coupling between sieve elements and companion cells using fluorescence redistribution after photobleaching. Plant Physiol 142:471–480
Martínez-Gil L, Sanchez-Navarro JA, Cruz A, Pallas V, Perez-Gil J, Mingarro I (2009) Plant virus cell-to-cell movement is not dependent on the transmembrane disposition of its movement protein. J Virol 83:5535–5543
Maule AJ (2008) Plasmodesmata: structure, function and biogenesis. Curr Opin Plant Biol 11:680–686
Melcher U (2000) The ‘30 K’ superfamily of viral movement proteins. J Gen Virol 81:257–266
Meng L, Wong JH, Feldman LJ, Lemaux PG, Buchanan BB (2010) A membrane-associated thioredoxin required for plant growth moves from cell to cell, suggestive of a role in intercellular communication. Proc Natl Acad Sci USA 107:3900–3905
Miernyk JA, Thelen JJ (2008) Biochemical approaches for discovering protein–protein interactions. Plant J 53:597–609
Mitra K, Ubarretxena-Belandia I, Taguchi T, Warren G, Engelman DM (2004) Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc Natl Acad Sci USA 101:4083–4088
Mongrand S, Morel J, Laroche J, Claverol S, Carde J, Hartmann MA, Bonneu M, Simon-Plas F, Lessire R, Bessoule JJ (2004) Lipid rafts in higher plant cells—purification and characterization of triton X-100-insoluble microdomains from tobacco plasma membrane. J Biol Chem 279:36277–36286
Moore PJ, Fenczik CA, Deom CM, Beachy RN (1992) Developmental changes in plasmodesmata in transgenic tobacco expressing the movement protein of tobacco mosaic virus. Protoplasma 170:115–127
Morozov SY, Solovyev AG (2003) Triple gene block: modular design of a multifunctional machine for plant virus movement. J Gen Virol 84:1351–1366
Mouritsen OG (2010) The liquid-ordered state comes of age. Biochim Biophys Acta 1798:1286–1288
Munnik T, Testerink C (2009) Plant phospholipid signaling: “in a nutshell”. J Lipid Res 50:S260–S265
Murillo I, Cavallarin L, San Segundo B (1997) The maize pathogenesis-related PRms protein localizes to plasmodesmata in maize radicles. Plant Cell 9:145–156
Nziengui H, Bouhidel K, Pillon D, Der C, Marty F, Schoefs B (2007) Reticulon-like proteins in Arabidopsis thaliana: structural organization and ER localization. FEBS Lett 581:3356–3362
Oparka K (2004) Getting the message across: how do plant cells exchange macromolecular complexes? Trends Plant Sci 9:33–41
Oparka KJ, Turgeon R (1999) Sieve elements and companion cells-traffic control centers of the phloem. Plant Cell 11:739–750
Oparka KJ, Prior DAM, Crawford JW (1994) Behavior of plasma-membrane, cortical ER and plasmodesmata during plasmolysis of onion epidermal-cells. Plant Cell Environ 17:163–171
Oparka KJ, Prior DA, Santa Cruz S, Padgett HS, Beachy RN (1997) Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus (TMV). Plant J 12:781–789
Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts IM, Pradel KS, Imlau A, Kotlizky G, Sauer N, Epel BL (1999) Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97:743–754
Overall RL, Blackman LM (1996) A model of the macro-molecular structure of plasmodesmata. Trends Plant Sci 1:307–311
Overall RL, Wolfe J, Gunning BES (1982) Intercellular communication in Azolla roots: I. Ultrastructure of plasmodesmata. Protoplasma 111:134–150
Park SH, Blackstone C (2010) Further assembly required: construction and dynamics of the endoplasmic reticulum network. EMBO Rep 11:515–521
Parton RG, Richards AA (2003) Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4:724-738
Perinetti G, Müller T, Spaar A, Polishchuk R, Luini A, Egner A (2009) Correlation of 4Pi and electron microscopy to study transport through single Golgi stacks in living cells with super resolution. Traffic 10:379–391
Pike LJ (2006) Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J Lipid Res 47:1597–1598
Pike LJ (2009) The challenge of lipid rafts. J Lipid Res 50:S323–S328
Pleskot R, Potocký M, Pejchar P, Linek J, Bezvoda R, Martinec J, Valentova O, Novotna Z, Zárský V (2010) Mutual regulation of plant phospholipase D and the actin cytoskeleton. Plant J 62:494–507
Praefcke GJK, McMahon HT (2004) The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 5:133–147
Prekeris R, Gould GW (2008) Breaking up is hard to do—membrane traffic in cytokinesis. J Cell Sci 121:1569–1576
Radford JE, White RG (1998) Localization of a myosin-like protein to plasmodesmata. Plant J 14:743–750
Raffaele S, Bayer E, Lafarge D, Cluzet S, German Retana S, Boubekeur T, Leborgne-Castel N, Carde JP, Lherminier J, Noirot E, Satiat-Jeunemaitre B, Laroche-Traineau J, Moreau P, Ott T, Maule AJ, Reymond P, Simon-Plas F, Farmer EE, Bessoule JJ, Mongrand S (2009) Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement. Plant Cell 21:1541–1555
Rayment I, Rypniewski WR, Schmidt-Bäse K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM (1993) Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261:50–58
Reichel C, Beachy RN (1998) Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum. Proc Natl Acad Sci USA 95113:11169–11174
Reichelt S, Knight AE, Hodge TP, Baluska F, Samaj J, Volkmann D, Kendrick-Jones J (1999) Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic cell wall. Plant J 19:555–567
Rinne PLH, Kaikuranta PM, van der Schoot C (2001) The shoot apical meristem restores its symplastic organisation during chilling-induced release from dormancy. Plant J 26:249–264
Robards AW (1968a) A new interpretation of plasmodesmatal ultrastructure. Planta 82:200–210
Robards AW (1968b) Desmotubule—a plasmodesmatal substructure. Nature 218:784
Roberts AG, Oparka KJ (2003) Plasmodesmata and the control of symplastic transport. Plant Cell Environ 26:103–124
Robinson-Beers K, Evert RF (1991) Fine structure of plasmodesmata in mature leaves of sugarcane. Planta 184:307–318
Ronchi P, Colombo S, Francolini M, Borgese N (2008) Transmembrane domain-dependent partitioning of membrane proteins within the endoplasmic reticulum. J Cell Biol 181:105–118
Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B (2005) Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J 24:1537–1545
Rutherford S, Moore I (2002) The arabidopsis rab GTPase family: another enigma variation. Curr Opin Plant Biol 5:518–528
Sagi G, Katz A, Guenoune-Gelbart D, Epel BL (2005) Class 1 reversibly glycosylated polypeptides are plasmodesmal-associated proteins delivered to plasmodesmata via the Golgi apparatus. Plant Cell 17:1788–1800
Sakamoto T, Wang F, Schmitz S, Xu Y, Xu Q, Molloy JE, Veigel C, Sellers JR (2003) Neck length and processivity of myosin V. J Biol Chem 278:29201–29207
Samuels TD, Ju H-J, Ye C-M, Motes CM, Blancaflor EB, Verchot-Lubicz J (2007) Subcellular targeting and interactions among the Potato virus X TGB proteins. Virology 367:375–389
Schepetilnikov MV, Manske U, Solovyev AG, Zamyatnin AA Jr, Schiemann J, Morozov SYu (2005) The hydrophobic segment of Potato virus X TGBp3 is a major determinant of the protein intracellular trafficking. J Gen Virol 86:2379–2391
Schepetilnikov MV, Solovyev AG, Gorshkova EN, Schiemann J, Prokhnevsky AI, Dolja VV, Morozov SY (2008) Intracellular targeting of a hordeiviral membrane-spanning movement protein: sequence requirements and involvement of an unconventional mechanism. J Virol 82:1284–1293
Schönknecht G, Brown JE, Verchot-Lubicz J (2008) Plasmodesmata transport of GFP alone or fused to potato virus X TGBp1 is diffusion driven. Protoplasma 232:143–152
Schulz A (1995) Plasmodesmal widening accompanies the short-term increase in symplasmic phloem unloading in pea root tips under osmotic stress. Protoplasma 188:22–37
Segui-Simarro JM, Austin JR II, White EA, Staehelin LA (2004) Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell 16:836–856
Senes A, Engel DE, De Grado WF (2004) Folding of helical membrane proteins: the role of polar, GxxxG-like and proline motifs. Curr Opin Struct Biol 14:465–479
Shahollari B, Peskan-Berghofer T, Oelmuller R (2004) Receptor kinases with leucine-rich repeats are enriched in Triton X-100 insoluble plasma membrane microdomains from plants. Physiol Plant 122:397–403
Shibata Y, Voss C, Rist JM, Hu J, Rapoport TA, Prinz WA, Voeltz GK (2008) The reticulon and Dp1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J Biol Chem 283:18892–18904
Shibata Y, Hu J, Kozlov MM, Rapoport TA (2009) Mechanisms shaping the membranes of cellular organelles. Ann Rev Cell Dev Biol 25:329–354
Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ (2009) An arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell 21:581–594
Simpson-Holley M, Ellis D, Fisher D, Elton D, McCauley J, Digard PA (2002) Functional link between the actin cytoskeleton and lipid rafts during budding of filamentous influenza virions. Virology 301:212–225
Solovyev AG, Stroganova TA, Zamyatnin AA, Fedorkin ON, Schiemann J, Morozov SY (2000) Subcellular sorting of small membrane-associated triple gene block proteins: TGBp3-assisted targeting of TGBp2. Virology 269:113–127
Sorre B, Callan-Jones A, Manneville JB, Nassoy P, Joanny JF, Prost J, Goud B, Bassereau P (2009) Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc Natl Acad Sci USA 106:5622–5626
Sparkes IA (2010) Motoring around the plant cell: insights from plant myosins. Biochem Soc Trans 38:833–838
Sparkes IA, Frigerio L, Tolley N, Hawes C (2009a) The plant endoplasmic reticulum: a cell-wide web. Biochem J 423:145–155
Sparkes IA, Ketelaar T, de Ruijter NCA, Hawes C (2009b) Grab a Golgi: laser trapping of Golgi bodies reveals in vivo interactions with the endoplasmic reticulum. Traffic 10:567–571
Sparkes IA, Runions J, Hawes C, Griffing L (2009c) Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 21:3937–3949
Sparkes IA, Tolley N, Aller I, Svozil J, Osterrieder A, Botchway S, Mueller C, Frigerio L, Hawes C (2010) Five arabidopsis reticulon isoforms share endoplasmic reticulum location, topology, and membrane-shaping properties. Plant Cell 22:1333–1343
Sprong H, van der Sluijs P, van Meer G (2001) How proteins move lipids and lipids move proteins. Nat Rev Mol Cell Biol 2:504–513
Staehelin LA (1997) The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J 11:1151–1165
Stahelin RV (2009) Lipid binding domains: more than simple lipid effectors. J Lipid Res 50:S299–S304
Stöckl MT, Herrmann A (2010) Detection of lipid domains in model and cell membranes by fluorescence lifetime imaging microscopy. Biochim Biophys Acta 1798:1444–1456
Su S, Liu Z, Chen C, Zhang Y, Wang X, Zhu L, Miao L, Wang XC, Yuan M (2010) Cucumber mosaic virus movement protein severs actin filaments to increase the plasmodesmal size exclusion limit in tobacco. Plant Cell 22:1373–1387
Takano K, Toyooka K, Suetsugu S (2008) EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization. EMBO J 27:2817–2828
Testerink C, Munnik T (2005) Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 10:368–375
Thole JM, Nielsen E (2008) Phosphoinositides in plants: novel functions in membrane trafficking. Curr Opin Plant Biol 11:620–631
Thomas CL, Bayer E, Ritzenthaler C, Fernandez-Calvino L, Maule AJ (2008) Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol 6:e7
Tian Q, Olsen L, Sun B, Lid SE, Brown RC, Lemmon BE, Fosnes K, Gruis D, Opsahl-Sorteberg HG, Otegui MS, Olsen OA (2007) Subcellular localization and functional domain studies of DEFECTIVE KERNEL1 in maize and Arabidopsis suggest a model for aleurone cell fate specification involving CRINKLY4 and SUPERNUMERARY ALEURONE LAYER1. Plant Cell 19:3127–3145
Tilney LG, Cooke TJ, Connelly PS, Tilney MS (1991) The structure of plasmodesmata as revealed by plasmolysis, detergent extraction, and protease digestion. J Cell Biol 112:739–747
Tilsner J, Cowan GH, Roberts AG, Chapman SN, Ziegler A, Savenkov E, Torrance L (2010) Plasmodesmal targeting and intercellular movement of potato mop-top pomovirus is mediated by a membrane anchored tyrosine-based motif on the lumenal side of the endoplasmic reticulum and the C-terminal transmembrane domain in the TGB3 movement protein. Virology 402:41–51
Tolley N, Sparkes IA, Hunter PR, Craddock CP, Nuttall J, Roberts LM, Hawes C, Pedrazzini E, Frigerio L (2010) Overexpression of a plant reticulon remodels the lumen of the cortical endoplasmic reticulum but does not perturb protein transport. Traffic 8:94–102
Tomenius K, Clapham D, Meshi T (1987) Localization by immunogold cytochemistry of the virus-coded 30 K protein in plasmodesmata of leaves infected with tobacco mosaic virus. Virology 160:363–371
Turner A, Wells B, Roberts K (1994) Plasmodesmata of maize root tips: structure and composition. J Cell Sci 107:3351–3361
Vale RD (2003) Myosin V motor proteins. J Cell Biol 163:445–450
Van Damme D, Bouget F-Y, Van Poucke K, Inzé D, Geelen D (2004) Molecular dissection of plant cytokinesis and phragmoplast structure: a survey of GFP-tagged proteins. Plant J 40:386–398
van Leeuwen W, Vermeer JEM, Gadella TWJ Jr, Munnik T (2007) Visualization of phosphatidylinositol 4, 5-bisphosphate in the plasma membrane of suspension-cultured tobacco BY-2 cells and whole Arabidopsis seedlings. Plant J 52:1014–1026
van Meer G, Sprong H (2004) Membrane lipids and vesicular traffic. Curr Opin Cell Biol 16:373–378
van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124
Vedrenne C, Hauri HP (2006) Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic 7:639–646
Vermeer JEM, van Leeuwen W, Tobeña-Santamaria R, Laxalt AM, Jones DR, Divecha N, Gadella TWJ Jr, Munnik T (2006) Visualization of PtdIns3P dynamics in living plant cells. Plant J 47:687–700
Vermeer JEM, Thole JM, Goedhart J, Nielsen E, Munnik T, Gadella TWJ Jr (2009) Imaging phosphatidylinositol 4-phosphate dynamics in living plant cells. Plant J 57:356–372
Voeltz GK, Prinz WA (2007) Sheets, ribbons and tubules—how organelles get their shape. Nat Rev Mol Cell Biol 8:258–264
Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA (2006) A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124:573–586
Volkmann D, Mori T, Tirlapur UK, Konig K, Fujiwara T, Kendrick-Jones J, Baluska F (2003) Unconventional myosins of the plant-specific class VIII: endocytosis, cytokinesis, plasmodesmata/pit-fields, and cell-to-cell coupling. Cell Biol Int 27:289–291
von Heijne G (2006) Membrane–protein topology. Nat Rev Mol Cell Biol 7:909–918
Waigmann E, Turner A, Peart J, Roberts K, Zambryski P (1997) Ultrastructural analysis of leaf trichome plasmodesmata reveals major differences from mesophyll plasmodesmata. Planta 203:75–84
Wang W, Yang X, Tangchaiburana S, Ndeh R, Markham JE, Tsegaye Y, Dunn TM, Wang GL, Bellizzi M, Parsons JF, Morrissey D, Bravo JE, Lynch DV, Xiao S (2008) An inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. Plant Cell 20:3163–3179
White RG, Badelt K, Overall RL, Vesk M (1994) Actin associated with plasmodesmata. Protoplasma 180:169–184
Wolf S, Deom CM, Beachy RN, Lucas WJ (1989) Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science 246:377–379
Wright KM, Oparka KJ (2006) The ER within plasmodesmata. In: Robinson DG (ed) The plant endoplasmic reticulum. Springer, Berlin, pp 279–308
Xue H, Chen X, Mei Y (2009) Function and regulation of phospholipid signalling in plants. Biochem J 421:145–156
Yahalom A, Warmbrodt RD, Laird DW, Traub O, Revel J-P, Willecke K, Epel BL (1991) Maize mesocotyl plasmodesmata proteins cross-react with connexin gap junction protein antibodies. Plant Cell 3:407–417
Yahalom A, Lando R, Katz A, Epel BL (1998) A calcium-dependent protein kinase is associated with maize mesocotyl plasmodesmata. J Plant Physiol 153:354–362
Zavaliev R, Sagi G, Gera A, Epel BL (2010) The constitutive expression of Arabidopsis plasmodesmal-associated class 1 reversibly glycosylated polypeptide impairs plant development and virus spread. J Exp Bot 61:131–142
Zhang Y, McCormick S (2010) The regulation of vesicle trafficking by small GTPases and phospholipids during pollen tube growth. Sex Plant Reprod 23:87–93
Zhao L, Naber N, Cooke R (1995) Muscle cross-bridges bound to actin are disordered in the presence of 2, 3-butanedione monoxime. Biophys J 68:1980–1990
Acknowledgements
We are grateful to Chris Hawes (Oxford, UK) for supplying Fig. 2a and to Christophe Ritzenthaler (Strasbourg, France), Christine Faulkner and Andy J. Maule (Norwich, UK) and Jesús A. Sánchez-Navarro (Valencia, Spain) for sharing unpublished results. We apologise to all colleagues whose work we were unable to cite due to space limitations. Funding by the Fundación Séneca, Spain (postdoctoral fellowship to K. Amari) and Scottish Government’s Rural and Environmental Research and Analysis Directorate (to SCRI) is gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Karl Oparka
Rights and permissions
About this article
Cite this article
Tilsner, J., Amari, K. & Torrance, L. Plasmodesmata viewed as specialised membrane adhesion sites. Protoplasma 248, 39–60 (2011). https://doi.org/10.1007/s00709-010-0217-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0217-6