Abstract
The Lentibulariaceae are highly evolved and specialized carnivorous angiosperms displaying not only unusual morphology and embryology but also specific changes in the genome and chromosomes as large as bacterial chromosomes. Comparative study of the morphology and detailed anatomy of the ovule in the genera Genlisea, Utricularia, and Pinguicula should shed new light on the phylogeny of this family. The clade Genlisea + Utricularia is sister to the genus Pinguicula, which is considered the most primitive taxon within Lentibulariaceae. Thus we should expect the ovules of Genlisea to be more similar to those of the more closely related genus Utricularia than to Pinguicula. Surprisingly, the ovules of Genlisea retain characters (free funiculus, ES remaining in the ovule) in common with Pinguicula, presumably inherited from a common ancestor. Genlisea ovules have only one main character in common with subgenus Polypompholyx (Utricularia): a well-developed funiculus. There are differences between the ovules of the subgenera Genlisea and Tayloria. In subgenus Genlisea the micropyle tends to be closer to the funiculus and the ovule forms an unusual jacket-like nutritive tissue of integumental origin. The most specialized ovules in Lentibulariaceae evolved in the genus Utricularia. The special chalazal nutritive tissue in Genlisea and Utricularia is simply a hypostase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embryological characters (gynoecium, ovule shape and structure, mega-, and microgametophyte development) are useful and important in the study of plant phylogeny. Together with molecular and paleontological data, embryological characters can be used to reconstruct the evolutionary history of angiosperms. For this we need broad embryological studies, which are very time-consuming (e.g., see Igersheim and Endress 1998; Endress and Igersheim 2000; Igersheim et al. 2001; Endress 2005). For instance, Hydatellaceae were found to be sister to Nymphaeales by a combined molecular and structural phylogenetic analysis, including also embryological features, and to be relic angiosperms (Saarela et al. 2007; Friedman 2008). According to Endress (2005, p. 750), “Ovules are especially interesting in angiosperm systematics and evolution because their classical features are macrosystematically even more constant than previously assumed.” The structure and development of the ovules of basal angiosperms like Amborellaceae, Chloranthaceae, Magnoliales, and Nymphaeales are especially relevant to an understanding of angiosperm phylogeny and have been well studied recently (e.g., Igersheim and Endress 1997; Yamada et al. 2001a, b, 2003). Our knowledge of angiosperm ovule anatomy is still insufficient. The Lentibulariaceae represent highly evolved and specialized carnivorous angiosperms displaying not only unusual morphology (“relaxed morphology”, Rutishauser and Isler 2001; Ellison and Gotelli 2009) but also specific changes in the genome and chromosomes as large as bacterial chromosomes (Müller et al. 2004, 2006; Jobson et al. 2004; Laakkonen et al. 2006; Greilhuber et al. 2006). Here we examine ovules of Lentibulariaceae in detail.
The small genus Genlisea comprises perhaps of two dozen species classified in two subgenera: Tayloria (three species: Genlisea lobata Fromm-Trinta, Genlisea uncinata P. Taylor & Fromm-Trinta, Genlisea violacea St.-Hil. and some yet-unnamed species such as Genlisea sp. ‘Itacambira Beauty’, all restricted to Brazil) and Genlisea (18 species, Afro-American distribution; Taylor and Fromm-Trinta 1983; Fisher et al 2000; Fig. 1). The present range of this genus is from South and Central America to Africa including Madagascar (Fromm-Trinta 1977, 1979; Fisher et al. 2000). In contrast to Genlisea, the related genus Utricularia (Jobson and Albert 2002; Jobson et al. 2003; Müller et al. 2002, 2004) not only has more complicated vegetative morphology (e.g., Brugger and Rutishauser 1989) but is also much richer in number of species (ca. 223; e.g., see Lowrie et al. 2008) and has a worldwide distribution (Taylor 1989). Unlike in Utricularia (e.g., Kamieński 1877, 1878; Merz 1897; Merl 1915; Khan 1954, 1992; Płachno and Świątek 2008), the embryology of Genlisea is poorly known and was studied only in the late nineteenth (Kamieński 1890) and early twentieth centuries (Merl 1915).
Comparison of the morphology and detailed anatomy of Genlisea ovules with the genera Utricularia and Pinguicula should shed new light on the evolution of Lentibulariaceae. Molecular studies (Jobson and Albert 2002; Jobson et al. 2003; Müller et al. 2000, 2004) have shown the clade Genlisea + Utricularia to be sister to the genus Pinguicula (Fig. 1), which is considered the most primitive taxon among the Lentibulariaceae family (Taylor 1989; Müller et al. 2006). Thus we should expect the ovules of Genlisea to be more similar to those of the more related genus Utricularia than to Pinguicula. According to some authors, subgenus Polypompholyx is the most primitive taxon within the genus Utricularia (Taylor 1989; Müller and Borsch 2005). Some features of pollen architecture (Taylor 1989; Lobreau-Callen et al. 1999) and trap structure of members of subgenus Polypompholyx are similar to Genlisea (Reifenrath et al. 2006). Thus the ovules of members of Polypompholyx should have characters similar to those in Genlisea. In this study we test these two hypotheses.
Materials and methods
Plants of Genlisea aurea St.-Hil. (subgen. Genlisea, Fisher et al. 2000) were obtained from Chapada dos Guimaraes and Itacambira (Mato Grosso state, Brazil); additional plant material (fixed flowers) was given to us from the Bonn Botanical Garden (accession no. 8546). Flowers of G. lobata Fromm-Trinta (subgen. Tayloria) were obtained from Espirito Santo (Mato Grosso state, Brazil) and Genlisea hispidula Stapf (subgen. Genlisea) from Africa. We also examined flowers of the genera Pinguicula (subgen. Isolba: Pinguicula agnata Casper; subgen. Pinguicula: Pinguicula moctezumae Zamudio & R.Z.Ortega and Pinguicula moranensis H.B.K. cultivars) and Utricularia (subgen. Bivalvaria: Utricularia sandersonii Oliv. Utricularia livida Mey.; subgen. Utricularia: Utricularia longifolia Gardner) obtained from the greenhouse collections of Kamil Pásek and from the Botanical Garden of the Jagiellonian University in Cracow, Poland.
Light and electron microscopy
Part of the material was fixed in acetic alcohol, embedded in paraffin, microtome-sectioned 10 µm thick, and stained with Heidenhain’s hematoxylin and alcian blue. The PAS reaction was used to detect water-insoluble polysaccharides with 1,2-glycol groups, such as starch (Wędzony 1996). For electron microscopy, placentas with ovules were isolated from ovaries and fixed in 2.5% formaldehyde and 2.5% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.0) for 2 days. The material was postfixed in 1% OsO4 in the cacodylate buffer for 24 h at ∼4°C, rinsed in the same buffer, treated with 1% uranyl acetate in distilled water for 1 h, dehydrated with acetone and embedded in Epon 812 (Fullam, Latham, NY, USA). Semithin sections were stained with methylene blue and examined with an Olympus BX60 microscope. Ultrathin sections were cut on a Leica ultracut UCT ultramicrotome. After contrasting with uranyl acetate and lead citrate, the sections were examined in a Hitachi H500 electron microscope. The procedures for preparing samples for SEM were as described earlier (Płachno et al. 2005a, b). Flowers were hand-sectioned with a razor blade and fixed as for TEM. The dried tissues were sputter-coated with gold and viewed in a HITACHI S-4700 microscope (Scanning Microscopy Laboratory of Biological and Geological Sciences, Jagiellonian University). Additionally, whole ovules were examined with epifluorescence microscope.
Results
G. aurea
G. aurea forms a stalked, approximately spherical basal placenta covered with numerous (several dozen) ovules. The apex of the placenta is sterile and lacks ovules. The placenta consists mainly of highly vacuolated parenchyma cells. Their nuclei do not contain protein inclusions. Placental cells possess small chloroplasts (not shown). The ovule is tenuinucellate and unitegmic, ca. 150 µm long. The integument consists of two or three cell layers. It is partially fused with the funiculus, but the apex of the integument is free (Fig. 2a, b). The free part of the funiculus is prominent. The apex of the integument is curved, so that the micropyle is near the funiculus (Fig. 2b, c). The ovule is anatropous or hemianatropous, however the chalaza, embryo sac, and micropyle lie in a slightly curved line. A few cells of the funicular epidermis may be papillose, probably forming an obturator. A cup-shaped hypostase occurs at the chalazal pole of the ovule, (Figs. 2d and 3a). It directly borders and surrounds the antipodes. The hypostase cell walls are very thick and layered. The nucleus is prominent; the cytoplasm is dense, with small mitochondria. The wall of the antipodals is thin and covered by wall ingrowths (Fig. 3b). Wall ingrowths also occur on the side of the central cell on the border with antipodals. The mitochondria of antipodals are active.
Ultrastructure and anatomy of ovule of Genlisea aurea. Transverse sections of hypostase and antipodals (a), bar = 2 µm. b transverse section of antipodals note prominent nucleus (N) and wall ingrowths (arrows), bar = 0.8 µm. c Whole ovule showing curved embryo sac (Es) and micropylar nutritive tissue forming a jacket-like structure (arrows), bar = 20 µm. d semithin section through ovule showing central cell (Cc) and nutritive cells with prominent thick cell wall (NT), bar = 8 µm. e and f strong fluorescence of cell walls of nutritive cells (NT) under UV light, bar = 43 and 31 µm
Some cells of the integument have thick, prominent cell walls, and cover the ES near the egg cell at the micropylar pole (Fig. 3c, d), but not at the micropylar apex of the ES (near micropylar part of synergids). These thick-walled cells form a jacket-like structure. The thick cell walls exhibit strong fluorescence under UV light (Fig. 3e, f) and also strong staining with the PAS reaction. The anticlinal walls of these cells are very thick, but they have plasmodesmata (Fig. 4). Plasmodesmata also occur between these cells and the parenchymal cells of the next integumental layer. The thick-walled cells have a prominent nucleus but the cytoplasmic content varies among the cells. Some of them have dense cytoplasm with small lipid bodies or more translucent cytoplasm with various small vesicles or vacuoles (Fig. 4). Between the embryo sac and the integumental cells are collapsed cells with some remains of the protoplast (Fig. 4).
G. hispidula
The ovules are numerous (Fig. 5a). The micropyle is close to the funiculus (Fig. 5b). The ovule is tenuinucellate and unitegmic, ca. 180 µm long. The funiculus is well visible at the base of the ovule. In the micropylar part of the ovule is a massive (several-layered) nutritive tissue of integumental origin, which forms a thick jacket around the embryo sac (Fig. 5c). The nutritive tissue cells have thicker walls than other integumental cells. Close to the central part of the embryo sac is a well-developed integumental tapetum (Fig. 5c). It consists of one layer of radially extended cells. At the chalazal pole of the ovule is a well-developed hypostase (Fig. 5c). Collapsed nucellar cells are distributed between the embryo sac and the integumental cells.
G. lobata
G. lobata forms a stalked spherical placenta covered with numerous (ca. 40) small ovules (Fig. 6a). The apex of the placenta is sterile and lacks ovules (not shown). The placenta consists mainly of highly vacuolated parenchymatic cells. The nucleus of the placental parenchymatic cells contains a protein inclusion. The placental cells possess plastids with starch (Fig. 6b). Intracellular spaces are well developed in the placenta and below the ovules (Fig. 6c). The ovule is tenuinucellate and unitegmic, ca. 130 µm long. The integument is partially fused with the funiculus, but the apex of the integument is free (Fig. 6c, d). The free part of the funiculus is prominent and well visible at the base of the ovule. In contrast to G. aurea, in G. lobata the apex of the integument is elongated and the micropyle adheres to the placental epidermis (Fig. 6c). The embryo sac is situated mainly in the middle and chalazal parts of the ovule (Fig. 6c). The embryo sac is curved, forming an arc. The chalazal part of the embryo sac is narrow, while the micropylar part is broad. The ES has contact, laterally and at the micropylar pole, with the integumental epidermis. Neither Heidenhain’s hematoxylin and alcian blue (Fig. 6c) nor the PAS reaction revealed any special cells with very thick cell walls and dense cytoplasm near the ES. The epidermal cells of the chalazal pole of the ovule are large and prominent. The ovule may be classified as campylotropous.
Structure of placenta and ovule of Genlisea lobata. a Longitudinal section of placenta with ovules, bar = 200 µm. b Placental parenchyma cells; N nucleus with protein crystals, A plastids with starch, bar = 6 µm. c Median longitudinal section of ovule: arrow micropyle, star funiculus, Es embryo sac, bar = 50 µm. d Morphology of ovules: arrow micropyle, star funiculus, bar = 100 µm
One of the main aims of this work is to compare the structure of the ovule in Genlisea with that in Utricularia and Pinguicula. The ovule anatomy of Utricularia and Pinguicula (especially Utricularia) has been described in detail by various authors (Merz 1897; Merl 1915; Khan 1954, 1992; Płachno and Świątek 2008), so we concentrate here on their general morphology.
Pinguicula
In all examined species (Fig. 7b–d) the free part of the funiculus is well visible at the base of the ovule. The apex of the integument is elongated and the micropyle does not adhere to the funiculus.
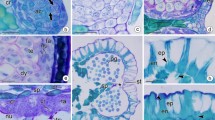
Morphology of Pinguicula and Utricularia ovules. a Longitudinal section through the placenta with ovules of P. moranensis, bar = 500 µm. b Ovule of P. moranensis: arrow micropyle, star funiculus, bar = 50 µm. c Ovule of P. moctezumae: arrow micropyle, star funiculus, bar = 100 µm. d Ovule of P. agnata: arrow micropyle, star funiculus, bar = 50 µm. e and f U. sandersonii: arrow micropyle, star funiculus, P. placenta, bar = 50 and 40 µm. g Ovules of U. livida: arrow micropyle, star funiculus, bar = 50 µm. h Ovule of U. longifolia: arrow micropyle, star funiculus, bar = 50 µm
Utricularia
The micropyle closely adheres to the raphe (Fig. 7f–h). The micropylar part of the integument borders the placenta (Fig. 7e). The embryo sac contacts the placenta (not shown).
Discussion
Ovules in Lentibulariaceae
Our work here suggests that Genlisea ovules are more similar to Pinguicula than to Utricularia. The free part of the funiculus is well visible in both Pinguicula and Genlisea. Genlisea ovules have one main character in common with Utricularia subgenus Polypompholyx: a well-developed funiculus. In the other subgenera of Utricularia the ovules are sessile. In Utricularia subgenus Polypompholyx, however, nutritive tissue makes the funiculus massive (Lang 1901; Merl 1915; Siddiqui 1978a; Płachno and Świątek 2008), unlike in Genlisea where nutritive tissue is absent from the funiculus.
There appears to be an evolutionary trend in the position of the micropyle in relation to the funiculus in Lentibulariaceae. In Pinguicula species and G. lobata the micropyle is separated from the funiculus. In Genlisea subgenus Genlisea the apex of the integument is curved so that the micropyle adheres to the funiculus. In Utricularia (subgen. Bivalvaria and Utricularia) the micropyle adheres to the funiculus. This tendency may be associated with the behavior of the embryo sac. As summarized by Farooq (1964, Table 1) the embryo sac remains inside the ovule in Pinguicula and Genlisea, but grows through the micropylar canal in Utricularia. Finally, the apex of the embryo sac is extra-ovular and has direct contact with the placenta. As shown (Płachno and Świątek 2008) this is a placental nutritive tissue, which consists of active cells and its position and structure is related to the phylogenetic position within the genus. As follows from Merl’s (1915) and our results, the nutritive tissue in the Genlisea ovule is of integumental origin. In Utricularia (sections Calpidisca and Oligocista and some other Utricularia species; Płachno and Świątek 2008, Płachno unpublished), the nutritive tissue consists of collenchymatous cells resembling those in Genlisea. Thus, the nutritive tissues in Genlisea and Utricularia are analogous because they arose from the different areas during the development of the ovule and placenta.
Within the genus Genlisea, Müller et al. (2006) and Fleischmann et al. (unpublished) consider subgenus Tayloria to be basal and subgenus Genlisea advanced. Alternatively, Płachno et al. (2007) inferred from the gland distribution pattern in digestive chambers of Genlisea traps and from the findings of a molecular study by Jobson et al. (2004) that the concentration of hairs along the vascular bundles in species of subgenus Genlisea may be primitive. According to Jobson et al. (2004), active water pumping might have occurred in the Genlisea ancestor trap and the concentration of hairs along the vascular bundles could have helped in water transport. In recent Genlisea species, however, active transport of water with suspended organisms into traps has not been observed. In light of these hypotheses, ovule anatomy and morphology are especially interesting. The micropyle position in G. lobata (subgenus Tayloria) is similar to that in Pinguicula species. In Pinguicula, micropylar nutritive tissue as in Utricularia or Genlisea does not occur, but an integumental tapetum is developed (Kopczyńska 1964 ; Farooq 1964). In G. lobata we found no micropylar nutritive tissue either. These results may suggest that species of subgenus Tayloria have more primitive characters, but further detailed studies are needed for confirmation.
According to Jobson and Albert (2002), in Utricularia the relative rates of nucleotide substitution in seven loci occurred four to 14 times faster than in Pinguicula. Also Müller et al. (2004, 2006) found the extreme DNA mutational rates in Utricularia and Genlisea in comparison to Pinguicula and moreover, that Genlisea and Utricularia exhibit substitutional rates which were the highest in the angiosperms. According to Müller et al. (2004, 2006) these changing in Lentibulariaceae genome well correspond to unusual nutritional specialization in Genlisea and Utricularia (so-called predictable prey capture hypothesis—Ellison and Gotelli 2009). Alternatively, unique molecular mutation (eg. in coxI subunit of cytochrome c oxidase) allowed to develop of morphological diversity and unique trap structures in Genlisea + Utricularia clad (Jobson and Albert 2002, Jobson et al. 2004, so-called energetics hypothesis—Ellison and Gotelli 2009). We also think that there is a positive connection between complication of the trap characters causing prey specialization and changes in Lentibulariaceae genome and moreover both hypotheses well complemented each other. However, as we show some generative characters (in case Genlisea) are more conservative than vegetative.
Special chalazal nutritive tissue or hypostase?
Several authors who studied the embryology of Lentibulariaceae have described the occurrence of a special chalazal nutritive tissue at the chalazal pole of the ovule in Genlisea (G. aurea; Merl 1915) and several species of Utricularia (e.g., Merz 1897; Lang 1901; Merl 1915; Khan 1954; Farooq 1964; Siddiqui 1978). In U. aurea, Khan (1954) described it as a group of cells which surround the base of the ES and are smaller than neighboring cells. In Utricularia uliginosa this tissue consists of thick-walled cells which are loosely arranged. At the first stage they have dense cytoplasm but later their contents are lost (Farooq 1965). According to Lang (1901), the chalazal nutritive tissue is well developed and prominent in Utricularia multifida. Merl (1915) noted nutritive tissue at the chalazal pole of the G. aurea ovule. Our results indicate that the special chalazal nutritive tissue in Genlisea and Utricularia is simply a hypostase. The position, anatomy and ultrastructure of this tissue closely correspond to the definition of the hypostase given by Batygina and Shamrov (1999). In his summary of the embryology of Lentibulariaceae, however, Khan (1992) briefly mentioned that a hypostase with lignified cell walls occurs in this family. He also mentioned chalazal nutritive tissue as another tissue type besides the hypostase in the ovule in this family. In disagreement with our results, according to Conran (1996), there is no hypostase in Lentibulariaceae.
Many authors consider that the principal function of the hypostase is to supply nutrients to gametophyte structures (e.g., Tilton 1980; Boesewinkel and Bouman 1984; Batygina and Shamrov 1999). In the case of Genlisea, the occurrence of elaborate wall labyrinths in the antipodals confirms that they are responsible for supplying nutrients from the hypostase to the central cell of the megagametophyte. Our ultrastructural study is in accordance with the work of Chamberlin et al. (1993): using autoradiographic techniques, they showed an accumulation of labeled assimilates in the integumentary tissue adjacent to the micropylar and chalazal poles of the embryo sac; they suggested that the chalazal vascular trace and two adfunicular vascular strands are the pathways for accumulation of the labeled assimilates in these regions of the ovule. In Genlisea, the funiculus does not have vascular tissue but is still the only way for metabolites to be transported from the placenta to the ovule tissue. This is unlike some Utricularia species (subgen. Bivalvaria and Utricularia) in which the embryo sac contacts the placental nutritive tissue, perhaps providing another pathway of metabolite transport directly from the placental nutritive tissue to the embryo sac (Płachno and Świątek 2008; Khan 1954, 1992).
Concluding remarks
We confirmed differences between the ovules of Genlisea subgenus Genlisea and subgenus Tayloria. Chromosome numbers also differ between these subgenera. Subgenus Tayloria contains species with low chromosome numbers; subgenus Genlisea contains high polyploids (Greilhuber et al. 2006; Płachno unpublished). Our knowledge of Genlisea cytology is still fragmented. Ovules in Genlisea retain characters in common with Pinguicula (ovules with free funiculus, ES remaining in the ovule), which were inherited from a common ancestor. However, in ovules of subgenus Genlisea the micropyle tends to be closer to the funiculus and an unusual jacket-like nutritive tissue of integumental origin is formed. The most specialized ovules in Lentibulariaceae evolved in the genus Utricularia. In this genus also the most sophisticated traps evolved within the family (Lloyd 1942; Juniper et al. 1989). Thus, in terms of both vegetative and generative characters the genus, Utricularia occupies an advanced position in the evolution in Lentibulariaceae.
Müller et al. (2006) showed that Madagascan and East African Genlisea margaretae has affinities to neotropical Genlisea species but not to other African Genlisea species studied. The recent distribution of subgenus Genlisea could be interpreted to mean that the genus Genlisea developed before the breakup of Gondwana. Thus, not only the unique Genlisea trap but also Genlisea nutritive tissue are ancient inventions.
References
Batygina TB, Shamrov II (1999) New approach to interpreting the ovular basic structures. Phytomorphology 49:223–231
Boesewinkel FD, Bouman F (1984) The seed structure. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 567–610
Brugger J, Rutishauser R (1989) Bau und Entwicklung landbewohnender Utricularia-Arten. Bot Helv 99:91–146
Conran JG (1996) The embryology and relationships of the Byblidaceae. Aust Syst Bot 9:243–254. doi:10.1071/SB9960243
Chamberlin MA, Horner HT, Palmer GR (1993) Nutrition of ovule, embryo sac and young embryo in soybean: an anatomical and autoradiographic study. Can J Bot 71:1153–1168
Ellison AM, Gotelli NJ (2009) Darwin Review Energetics and the evolution of carnivorous plants—Darwin’s‘ most wonderful plants in the world’. J Exp Bot 60:19–42 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=19213724&dopt=Abstract. doi:10.1093/jxb/ern179
Endress PK (2005) Links between embryology and evolutionary floral morphology. Curr Sci 89:749–754
Endress PK, Igersheim A (2000) Gynoecium structure and evolution in basal angiosperms. Int J Plant Sci 161:S211–S223. doi:10.1086/317572
Farooq M (1964) Studies in the Lentibulariaceae I. The embryology of Utricularia stellaris L. var. inflexa Clarke. Part I. Flower, organogeny, ovary, megasporogenesis and female gametophyte. Proc Natl Inst Sci India 30:263–279
Farooq M (1965) Studies in the Lentibulariaceae III. The embryology of Utricularia uliginosa Vahl. Phytomorphology 15:123–131
Fisher E, Porembski S, Barthlott W (2000) Revision of the genus Genlisea (Lentibulariaceae) in Africa and Madagascar with notes on ecology and phytogeography. Nord J Bot 20:291–318. doi:10.1111/j.1756-1051.2000.tb00746.x
Friedman WE (2008) Hydatellaceae are water lilies with gymnospermous tendencies. Nature 453(7191):94–97 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=18354395&dopt=Abstract. doi:10.1038/nature06733
Fromm-Trinta E (1977) Tayloria Fromm-Trinta–Nova Seçăo do gęnero Genlisea St.-Hil. (Lentibulariaceae). Boletin Museum Nacional de Rio de Janeiro. Botanica 44:1–4
Fromm-Trinta E (1979) Revisăo das espécies do gęnero Genlisea St.-Hil. (Lentibulariaceae) das regiôes sudeste e sul do Brasil. Rodriguésia (Rio de Janeiro) 31(49):17–139
Greilhuber J, Borsch T, Müller K, Worberg A, Porembski S, Barthlott W (2006) Smallest angiosperm genomes found in Lentibulariaceae, with chromosomes of bacterial size. Plant Biol 8:770–777 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=17203433&dopt=Abstract. doi:10.1055/s-2006-924101
Igersheim A, Endress PK (1997) Gynoecium diversity and systematics of the magnoliales and winteroids. Bot J Linn Soc 124:213–271. doi:10.1111/j.1095-8339.1997.tb01792.x
Igersheim A, Endress PK (1998) Gynoecium diversity and systematics of the paleoherbs. Bot J Linn Soc 127:289–370. doi:10.1111/j.1095-8339.1998.tb02102.x
Igersheim A, Buzgo M, Endress PK (2001) Gynoecium diversity and systematics of basal monocots. Bot J Linn Soc 136:1–65. doi:10.1111/j.1095-8339.2001.tb00555.x
Jobson RW, Albert VA (2002) Molecular rates parallel diversification contrasts between carnivorous plant sister lineages. Cladistics 18:127–136
Jobson RW, Playford J, Cameron KM, Albert VA (2003) Molecular phylogenetics of Lentibulariaceae inferred from plastid rps16 intron and trnL-F DNA sequences: implications for character evolution and biogeography. Syst Bot 28:157–171
Jobson RW, Nielsen R, Laakkonen L, Wikström M, Albert VA (2004) Adaptive evolution of cytochrome c oxidase: infrastructure for a carnivorous plant radiation. Proc Natl Acad Sci USA 101:18064–18068 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=15596720&dopt=Abstract. doi:10.1073/pnas.0408092101
Juniper BE, Robins RJ, Joel JM (1989) The carnivorous plants. Academic, London
Kamieński F (1877) Verleichende Untersuchungen über die Entwickelungsgeschichte der Utricularien. Bot Z 48:762–775
Kamieński F (1878) Historya zarodka pływacza pospolitego (Utricularia vulgaris L.). Rozprawy i Sprawozdania z Posiedzeń Wydziału Matematyczno-Przyrodniczego Akademii Umiejętności 5:1–8
Kamieński F (1890) Izsledowanja otnosiaszczyjasia k siemiejstwu Lentibulariaceae (Utricularieae). Zapiski Nowoross. Obszcz. Jestiestwoisp. Odessa 12:179–210
Khan R (1954) A contribution to the embryology of Utricularia flexuosa Vahl. Phytomorphology 4:80–117
Khan R (1992) Lentibulariaceae. In: Johri BM, Ambegaokar KB, Srivastava PS (eds) Comparative embryology of angiosperms II. Springer, Berlin, pp 755–762
Kopczyńska K (1964) Embryo sac development in Pinguicula vulgaris L. Acta Soc bot Pol 33:141–156
Laakkonen L, Jobson RW, Albert VA (2006) A new model for the evolution of carnivory in the bladderwort plant (Utricularia): adaptive changes in cytochrome c oxidase (COX) provide respiratory power. Plant Biol 8:758–764 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=17203431&dopt=Abstract. doi:10.1055/s-2006-924459
Lang FX (1901) Untersuchungen über Morphologie, Anatomie und Samenentwicklung von Polypompholyx und Byblis gigantea. Flora 88:149–206
Lloyd FE (1942) The carnivorous plants. Chronica Botanica, Waltham
Lobreau-Callen D, Jérémie J, Suarez-Cervera M (1999) Morphologie et ultrastructure du pollen dans le genre Utricularia L-(Lenibulariaceae). Can J Bot 77:744–767. doi:10.1139/cjb-77-5-744
Lowrie A, Cowie ID, Conran JG (2008) A new species and section of Utricularia (Lentibulariaceae) from northern Australia. Telopea (Syd) 12(1):31–46
Merl EH (1915) Beiträge zur Kenntnis der Utricularien und Genlisen. Flora 108:127–200
Merz M (1897) Untersuchungen über die Samenentwicklung der Utricularien. Flora 84:69–87
Müller K, Borsch T, Legendre L, Porembski S, Barthlott W (2000) A Phylogeny of Lentibulariaceae based on sequences of matK and adjacent non-coding regions. Am J Bot 87:145–146
Müller K, Borsch T, Legendre L, Theisen I, Barthlott W (2002) Evolution of carnivory in Lentibulariaceae: considerations based on molecular, morphological, and physiological evidence. Proceedings the 4th International Carnivorous Plant Conference Tokyo, Japan. 63–73
Müller K, Borsch T, Legendre L, Porembski S, Theisen I, Barthlott W (2004) Evolution of carnivory in Lentibulariaceae and the Lamiales. Plant Biol 6:477–490 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=15248131&dopt=Abstract. doi:10.1055/s-2004-817909
Müller K, Borsch T (2005) Phylogenetics of Utricularia (Lentibulariaceae) and molecular evolution of the trnK intron in a lineage with high substitutional rates. Plant Syst Evol 250:39–67. doi:10.1007/s00606-004-0224-1
Müller K, Borsch T, Legendre L, Porembski S, Theisen I, Barthlott W (2006) Evolution of carnivory in Lentibulariaceae and the Lamiales. Plant Biol 6:477–490. doi:10.1055/s-2004-817909
Płachno BJ, Świątek P (2008) Cytoarchitecture of Utricularia nutritive tissue. Protoplasma 23 234:25–324:25–32
Płachno BJ, Adamus K, Faber J, Kozłowski J (2005a) Feeding behaviour of carnivorous Genlisea plants in the laboratory. Acta Bot Gallica 152:159–164
Płachno BJ, Faber J, Jankun A (2005b) Cuticular discontinuities in glandular hairs of Genlisea St.-Hil. in relation to their functions. Acta Bot Gallica 152:125–130
Płachno BJ, Kozieradzka-Kiszkurno M, Świątek P (2007) Functional ultrastructure of Genlisea (Lentibulariaceae) digestive hairs. Ann Bot (Lond) 100:195–203 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=17550910&dopt=Abstract. doi:10.1093/aob/mcm109
Reifenrath K, Theisen I, Schnitzler J, Porembski S, Barthlott W (2006) Trap architecture in carnivorous Utricularia (Lentibulariaceae). Flora 201:597–605
Rutishauser R, Isler B (2001) Developmental genetics and morphological evolution of flowering plants, especially bladderworts (Utricularia): fuzzy arberian morphology complements classical morphology. Ann Bot (Lond) 88:1173–1202. doi:10.1006/anbo.2001.1498
Saarela JM, Rai HS, Doyle JA, Endress PK, Mathews S, Marchant AD, Briggs BG, Graham SW (2007) Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature 446:312–315 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=17361182&dopt=Abstract. doi:10.1038/nature05612
Siddiqui SA (1978) Studies in the Lentibulariaceae 8. The development of gametophytes in Utricularia dichotoma Labil. Flora 167:111–116
Taylor P (1989) The genus Utricularia—a taxonomic monograph. Kew B 14:1–735
Taylor P, Fromm-Trinta E (1983) Uma nova spécie para o gęnero Genlisea St.-Hil., Sect. Tayloria (Lentibulariaceae): Genlisea uncinata P. Taylor & Fromm-Trinta. Bradea 3:365–368
Tilton VR (1980) Hypostase development in Ornithogalum caudatum (Liliaceae) and notes on other types of modifications in the chalaza of angiosperm ovules. Can J Bot 58:2059–2066
Wędzony M (1996) Fluorescence microscopy for botanists. (In Polish) Dept. Plant Physiology Monographs 5. Kraków, Poland, p 128
Yamada T, Imaichi R, Kato M (2001a) Developmental morphology of ovules and seeds of Nymphaeales. Am J Bot 88:963–974 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=11410459&dopt=Abstract. doi:10.2307/2657077
Yamada T, Tobe H, Imaichi R, Kato M (2001b) Developmental morphology of the ovules of Amborella trichopoda (Amborellaceae) and Chloranthus serratus (Chloranthaceae). Bot J Linn Soc 137:277–290. doi:10.1111/j.1095-8339.2001.tb01123.x
Yamada T, Imaichi R, Kato M (2003) The outer integument and funicular outgrowth complex in the ovule of Magnolia grandiflora (Magnoliaceae). J Plant Res 116:189–198 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=12836041&dopt=Abstract. doi:10.1007/s10265-003-0086-6
Acknowledgments
The first author gratefully acknowledges the support of an award from the Foundation for Polish Sciences (Start Programme). We are grateful to Professor Wilhelm Barthlott and Dr. Inge Theisen (Botanical Institute of the University of Bonn, Bonn Botanical Garden) and to our colleagues Kamil Pásek (Czech Republic, http://www.bestcarnivorousplants.com) and Dr. Eric Schlosser for kindly providing material for this study. We particularly thank both reviewers for very helpful suggestions to made our manuscripts more clearer.
Conflicts of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Płachno, B.J., Świątek, P. Functional anatomy of the ovule in Genlisea with remarks on ovule evolution in Lentibulariaceae. Protoplasma 236, 39–48 (2009). https://doi.org/10.1007/s00709-009-0045-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-009-0045-8