Abstract
Chemical vapor generation (CVG) of Zn was carried out in a multimode sample introduction system (MSIS) and coupled to inductively coupled plasma mass spectrometry (ICP-MS) for detection. CVG conditions were studied and 0.4% (m/v) 8-hydroxyquinoline as the reaction modifier in 0.08 mol dm–3 H2SO4 and 3.0% NaBH4 in 0.1 mol dm–3 NaOH were found optimal, providing the limits of detection and quantification of 2.8 ng cm–3 and 9.3 ng cm–3, respectively. The overall CVG efficiency of 5.3 ± 0.1% was determined from comparison with solution nebulization-ICP-MS, while 5.7 ± 0.1% was determined using 65Zn radiotracer and radiometry. The developed methodology was verified by determination of Zn in the standard reference material of fresh water (NIST 1643f). Interference study conducted with 13 hydride-forming and transition elements did not prove ability of any of the tested element to further increase the CVG efficiency, but at the same time, none of them exhibited serious interference except Ag.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vapor generation is an analyte sample introduction technique for analytical atomic spectrometry based on conversion of an element of interest to a volatile species. The main advantages of this technique are separation of the analyte from the liquid matrix, thus significantly reducing interferences during spectrometric detection, and efficient transport of the analyte to a spectrometer (100% in an ideal case) reflected in enhanced sensitivity [1]. Among the vapor generation techniques, chemical vapor generation (CVG) is a mature technique commonly used for the determination of so-called hydride-forming elements (i.e., As, Bi, Ge, Pb, Sb, Se, Sn and Te) as well as Hg, which is generated in the form of cold vapor [1, 2]. The above listed inherent advantages have motivated the scientists to expand the application of CVG to other transition elements including zinc [3,4,5,6,7] for which alternative vapor generations techniques have been also introduced in recent years, such as electrochemical vapor generation [8], photochemical vapor generation [9], or techniques based on plasma discharge [10,11,12].
Regarding CVG by means of the reaction of the acidified analyte with a reducing agent (tetrahydridoborate), the low overall CVG efficiency of Zn was noted already in the pioneering work by Luna et al. [6] and it has apparently not surpassed the nebulization efficiency of common pneumatic nebulizers in the following studies, despite the use of various modifiers (see Ref. [13] for details). The overall CVG efficiency of Zn has been convincingly estimated in only a couple of papers, for example by Peña-Vázquez et al. as 1.1% by comparison with solution nebulization [14]. Many studies relied on an indirect method of determination of the analyte remaining in the waste liquid, and the CVG efficiencies were significantly higher (50–75%) [6, 7, 15]. In our previous study, we investigated CVG of Zn in a custom-made generator. The best results in terms of CVG efficiency were achieved when no reaction coil was used (deposits of reduced Zn) and when the acidified solution of Zn(II) was mixed with NaBH4 solution inside the gas–liquid separator on a frit through which carrier Ar was introduced [13]. Using neutral to basic pH of the reaction mixture and especially 8-hydroxyquinoline as the modifier, the overall CVG efficiency was determined to be 2.5–2.7%. The determination was made via i) a direct comparison with solution nebulization efficiency and ii) 65Zn radiotracer distribution experiments. The radiotracer study also showed that the source of the low efficiency lies not in the transport losses in the gas phase but in the generation and release steps, because 93% of zinc remained in the liquid waste, most probably due to imperfect mixing of the acidified analyte with NaBH4. These observations resemble the problems associated with CVG of Ag [16]. Improved overall Ag CVG efficiency was obtained partly due to the use of dedicated reactor design permitting fast mixing of the acidified sample with NaBH4, with minimum contact with the walls of the apparatus [17].
The motivation in this paper was to employ a commercial multimode sample introduction system (MSIS™) [18] for CVG of Zn using inductively coupled plasma mass spectrometry (ICP-MS) for detection and thus substantially enhance the CVG efficiency. The design of MSIS is based on a cyclonic spray chamber with protruding inlets for introduction of NaBH4 and acidified sample permitting their fast mixing and gas–liquid separation of the volatilized analyte. In fact, the similar setups of MSIS (lab-made) were examined for CVG of various elements including Zn by Fuentes-Cid et al. [19], however, the CVG efficiency of only 0.13% was reported.
Results and discussion
Optimization of CVG conditions
CVG is based on hydrolysis of NaBH4 in neutral or acidic aqueous solution followed by hydrogen transfer from the hydridoborate intermediates to the analyte atoms. Hydrochloric acid is typically used for inducing hydrolysis, however, other acids or buffers may be used as long as they are resistant to reduction by tetrahydridoborate [1]. In the preliminary experiments with the MSIS, we tested various acids for CVG of Zn: H2SO4 (0.06 and 0.09 mol dm–3), HCl (0.13 mol dm–3), and HNO3 (0.13 mol dm–3), employing 1% (m/v) 8-hydroxyquinoline addition to Zn(II) standards and 1.5% (m/v) NaBH4 in 0.1 mol dm–3 NaOH as the reducing agent. These CVG conditions were selected to conform to our previous observation that CVG of Zn proceeds best from mild pH conditions [13]. CVG from all the tested acids was found feasible, but the best sensitivity was obtained with 0.09 mol dm–3 H2SO4 resulting in pH of the waste ranging from 9.4 to 9.9. It is evident that such conditions cannot lead to complete hydrolysis of tetrahydridoborate after merging it with acid and that the active species in reduction of Zn are likely BH4– and BH3(H2O) (or deprotonated BH3OH–), as discussed in our previous work [13]. We presume that better suitability of H2SO4 over HCl is due to slower rate of complete dissociation of H2SO4 and thus more temperate hydrolysis of NaBH4. Detailed optimization of H2SO4 concentration was carried out in the range 0.05–0.50 mol dm–3 with all other conditions kept constant (Fig. 1).
Effect of H2SO4 concentration on the corrected peak area of 66Zn/89Y, 50 ng dm–3 Zn(II) with 1.0% (m/v) 8-hydroxyquinoline taken for CVG; 1.5% (m/v) NaBH4 in 0.1 mol dm–3 NaOH; H2SO4 and reducing agent flow rates 0.5 cm3 min–1; sample volume 250 mm3; Ar flow rate 370 cm3 min–1; H2 flow rate 10 cm3 min.–1 (added from a cylinder)
The sharp maximum was achieved between 0.065 and 0.10 mol dm–3 H2SO4, wherein the responses were higher than for the other tested concentrations by at least a factor of two. Due to a fear of a sensitivity loss, concentration in the middle of the range, namely 0.08 mol dm–3, was chosen for further optimization of the method.
The concentration of NaBH4 may strongly affect the overall CVG efficiency. The trend obtained in this work is shown in Fig. 2. The highest peak area response was obtained with 4% (m/v) NaBH4 but the repeatability was rather poor. Hence, we selected 3% (m/v) NaBH4, which was reflected in only marginal loss (around 5%) in the overall CVG efficiency. The amount of evolved H2 was measured as 6.5 cm3 min−1 at the CVG conditions using 3% (m/v) NaBH4 in 0.1 mol dm–3 NaOH as the reducing agent and 0.08 mol dm–3 H2SO4. Our previous work [13] benefited from the addition of H2, which not only enabled the atomization of the generated species in the quartz tube atomizer, but also helped stabilize the generated species during volatilization and transport. When H2 fraction in the carrier gas was increased (H2 added to Ar from a cylinder) in the current setup, while keeping a constant total carrier gas flow rate (380 cm3 min–1), no effect of the presence of H2 on release, stability and transport of the generated volatile species, was identified. Hence, only Ar was used as the carrier gas in further experiments.
Effect of NaBH4 concentration prepared in 0.1 mol dm–3 NaOH on the corrected peak area response of 66Zn/89Y, 50 ng cm–3 Zn(II) in 1% (m/v) 8-hydroxyquinoline and 0.08 mol dm–3 H2SO4 taken for CVG; 0.08 mol dm−3 H2SO4 as the carrier; sample volume 250 mm3; Ar flow rate 370 cm3 min−1; H2 flow rate 10 cm3 min.–1 (added from a cylinder)
CVG of Zn typically relies on the use of various modifiers to increase the CVG efficiency. The previously published modifiers included cetyltrimethylammonium bromide (CTAB), [3, 20], diethyldithiocarbamate and 1,10-phenantroline (phen) [21], or 8-hydroxyquinoline [5, 22,23,24]. Combinations with metal ion sensitizers Ni(II) and Co(II) [3] were published too. We tested surfactants CTAB and Triton X-100 in our previous work [13] with definite negative effect from both while addition of transition metals tested during the interference study did not increased the efficiency either. The significant enhancement in the overall CVG efficiency (18-fold) was identified for 1.0% (m/v) 8-hydroxyquinoline, which, unfortunately, led also to increased blanks and blank-limited LOD of 4 ng cm–3. Using the MSIS for CVG in this work, we compared the effects of various concentrations of 1,10-phenantroline and 8-hydroxyquinoline. The highest CVG response and around 61-fold enhancement in CVG efficiency was obtained with 0.4% (m/v) 8-hydroxyquinoline while only 13-fold enhancement was obtained with 0.3% 1,10-phenantroline.
In fact, MSIS used in this work, is a spray chamber which can operate simultaneously in CVG and solution nebulization modes. Despite MSIS in our work not being equipped with the nebulizer, some analyte fraction may be still introduced to the ICP-MS in the form of aerosol that is co-generated during vigorous reaction of acid and NaBH4 and physically transported by a stream of carrier Ar. To get some insight into this fraction of aerosol, the optimization of Ar carrier gas flow rate was carried out with mixed solution of Zn (50 ng cm−3) comprising also Cs (400 ng cm−3). Cesium does not form volatile compounds and thus all detected Cs is transported only within the aerosol droplets. The experiment was carried out at a constant flow rate of Ar (370 cm3 min−1) introduced into the Ultra High Matrix Introduction (UHMI) inlet (Fig. 3). In order not to change the conditions in the plasma; the change in the flow rate of Ar introduced into the MSIS was compensated by the additional flow of Ar introduced directly upstream the UHMI inlet (not shown in Fig. 3). Maximum peak area response was obtained at 210 cm3 min−1 of carrier Ar. Moreover, this flow rate still corresponded to a minimal contribution of aerosol to the measured signal, while the 133Cs peak area response began to increase remarkably at higher flow rates. The particular value of the fraction of analyte transported in the aerosol at chosen optimal CVG conditions (Table 1) is stated in the section “Overall CVG efficiency”.
Figures of merit
At the optimal CVG conditions the LOD and LOQ (3σ and 10σ, n = 10) of 2.8 ng cm−3 (0.97 ng absolute) and 9.3 ng cm−3 (3.2 ng), respectively, were achieved. The repeatability expressed as the relative standard deviation was 1.9% (n = 7) at 150 ng cm−3 level.
Compared to LOD (4 ng cm−3) obtained in our previous work [13], a slight decrease in LOD was achieved. Comparable LODs were published by Smichowski et al. (4.6 ng cm−3; ICP-OES detection) [7] and Sun and Suo (1.6 ng cm−3; AFS detection) [5]. Lower LODs of 0.03 ng cm−3 were acquired using online preconcentration from 8 cm3 of sample on a mini column filled with 4-(2-pyridylazo)resorcinol (PAR) [4] or using generation of volatile Zn chelates (0.33 ng cm−3; AFS detection) [25].
Overall CVG efficiency
The overall CVG efficiency in our work was determined by two approaches. The first approach relies on comparison of sensitivities obtained with CVG and solution nebulization, both coupled simultaneously to ICP-MS and working in parallel. Using optimum conditions of CVG (Table 1), the overall CVG efficiency of 5.3% (uncertainty 0.1%) was achieved. The contribution of analyte introduction in the form of co-generated aerosol, derived from the 133Cs peak area responses obtained from Zn standard solutions spiked with Cs, was found 0.07% (uncertainty 0.02%) and thus negligible compared to CVG efficiency of Zn.
The second approach to determine the overall generation efficiency is based on the use of 65Zn radiotracer permitting the tracking of distribution of Zn in the setup after CVG experiments using radiometry (Table 2).
We found 6.7% (uncertainty 0.14%) of the input 65Zn activity in the part of the setup in contact with the gas phase, which means this fraction was volatilized. Around 1% was found in the PTFE transport tubing connecting the MSIS and the trapping device consisting of two charcoal columns and one syringe filter in series (connected in place of UHMI of the ICP-MS). This translates to approximately 85% transport efficiency in the gas phase. As a result, the overall CVG efficiency was 5.7% (0.1% uncertainty). The value obtained using radiometry is slightly greater than that obtained through comparison with nebulization, however, taking into account that the determination of the CVG efficiency by both approaches was made on different days and using slightly modified way of introduction into CVG system (no injection valve utilized for 65Zn introduction), this discrepancy does not appear serious.
From the total amount of input 65Zn activity a fraction 53.8% was found in the waste, of which 13.8% (uncertainty 0.1%) was found in the liquid waste and 40.0% (uncertainty 0.3%) remained deposited on the waste tubing walls. Significant fraction of 65Zn was found deposited in the CVG system (38.2%), namely 30.1% (uncertainty 0.3%) on inner surfaces of the MSIS spray chamber and 7.8% (uncertainty 0.1%) on the MSIS reaction cone, which is a “glass finger” protruding into the MSIS where the reaction of acidic sample with NaBH4 occurs; 26% of this activity (i.e., around 2% of the total 65Zn activity) was located at the very tip of the reaction cone. This value was obtained when only the tip (ca. 3 mm) of the reaction cone was selectively leached by concentrated HNO3 and the activity was measured. None or only negligible traces of 65Zn (< 0.4%) were found in the rest of the chemifold (Tygon and PTFE tubing for sample introduction, connections and inlets). The recovery of the 65Zn radiotracer introduced to the CVG system was almost 99%.
Estimating the CVG efficiency indirectly from the analyte remaining in the waste would yield a CVG efficiency of roughly 46% (Table 2). This is contradictory to the work of Nováková et al. [13] who calculated only 8% CVG efficiency from remaining Zn in the waste, because they identified a dominant fraction of activity in the waste. We presume a following explanation. We made two consecutive attempts at estimating efficiency using 65Zn radiotracer. Before the start of the first radiotracer experiment, we had been using the MSIS and waste tubing for a long time, therefore, their surfaces were heavily saturated with Zn. The first radiotracer experiment showed much lower level of activity in the MSIS (11.2%, uncertainty 0.1%) and higher activity in the waste tubing (43.6%, uncertainty 0.3%) and in the liquid waste (38.6%, uncertainty 0.2%). Estimation from the waste would result in overall CVG efficiency of approx. 18%. But prior to the second experiment the whole apparatus including the MSIS was leached with concentrated HNO3 and new tubing was used. Despite restoring the performance of CVG system by repeated introduction of 100–200 ng cm−3 Zn standards prior to 65Zn experiments, the CVG system was not likely fully saturated, which resulted in pronounced adsorption of Zn on the surfaces of MSIS, thus changing the distribution of Zn in the system and in the waste.
Interference study
With respect to the dependence in Fig. 1 where quite sharp maximum of response on concentration of H2SO4 was obtained, additional amount of any acid in the sample may be reflected in serious decrease in CVG efficiency. The similar issue was also highlighted in the previous work by Nováková et al. [13] who employed 0.1 mol dm−3 HCl for CVG and had to neutralize the acid in water CRMs prior to Zn determination.
In this work, we tested the tolerance toward HNO3 in the concentration range 0–500 mmol dm−3. Our CVG method was found relatively tolerant to the presence of HNO3 because no serious negative effect was observed at 100 mmol dm−3 HNO3 (recovery 97.0%). Addition of 200, 300, and 500 mmol dm−3 HNO3 decreased the recovery of Zn to 87.6%, 66.3%, and 59.3%, respectively. It appears that the range of suitable acidity (Fig. 1) is broader when divalent H2SO4 (0.08 mol dm−3) is used for CVG, compared to the previous work employing 0.1 mol dm−3 HCl [13].
Interference effects of selected transition metals and hydride-forming elements were studied with 100 ng dm−3 Zn(II) present in the standards. Among the transition metals, Fe(III), Co(II), Ni(II), Mn(II), Cd(II), Au(III), Ag(I), and Co(II) ions were investigated (concentration range 0–5000 ng cm−3). From hydride forming elements, As(III), Sb(III), Bi(III), Se(IV), and Te(IV) ions were chosen with a concentration range 0–1000 ng cm−3. The selection of elements was guided by our previous research [13]. (Note: the commercially supplied solutions of 1000 μg cm−3 are typically prepared in 2% HNO3 (≈0.32 mol dm−3). With respect to the examined range up to 5000 ng cm−3 of the metals, the maximum concentration of nitric acid in the solution will be less than 2 mmol dm−3, thus it would not interfere and the observed effects should by attributed only to the metals or metalloids.)
No significant interference was identified for Fe, Mn, Cd, Au, Bi, Te, and Se (Table 3). At 5000 ng cm−3 level, the referred elements all followed a similar decreasing trend with recoveries ranging between 40 and 70%. Addition of 1000 ng cm−3 Ni(II), Cu(II), and Co(II) resulted in a decrease of recovery to 83%, 76%, and 77% respectively. Arsenic and Sb did not interfere up to a concentration of 50 ng cm−3 while at 100 ng cm−3 the recovery dropped to 79 and 76%, respectively. Most significant interference was observed with Ag(I) which was the only element causing a strong memory effect. Thorough cleaning of the apparatus with 2% HNO3 was necessary to restore the peak area response from Zn to its original value. We presume that Ag(I) is reduced to Ag0 upon contact with NaBH4, deposited in the MSIS and especially on the reaction cone tip, which subsequently changes the kinetics of NaBH4 hydrolysis in a similar way as for example Pd and Au [26,27,28].
Accuracy
Accuracy of the developed method was verified using standard reference material NIST 1643f (fresh water) certified for Zn (74.4 ± 1.7 ng cm−3). This reference material is stabilized in 0.32 mol dm−3 HNO3. Based on the interference study the sample preparation required twofold dilution of the material to contain HNO3 less than 200 mmol dm−3 and addition of H2SO4 and 8-hydroxyquinoline (refer to Experimental for details). The determined concentration was 78.1 ± 1.95 ng cm−3, which is in a good agreement with the certified value, based on the Eq. (2) and expanded uncertainty (k = 2) [29].
Conclusions
This paper describes the chemical vapor generation (CVG) of Zn using sodium tetrahydridoborate in sulfuric acid and 8-hydroxyquinoline using a commercial MSIS as the reactor. The MSIS-enhanced CVG efficiency to 5.3–5.7% by facilitating a rapid reaction between the reducing agent and acid. The volatile Zn species transported to the plasma were over two orders of magnitude greater than the fraction transported by the co-generated ballast aerosol. This CVG efficiency is over 40 times higher than that achieved by Fuentes-Cid et al. [19] with a very similar MSIS design. Despite a twofold improvement in CVG efficiency compared to our previous study [13], the detection limit improved only slightly, likely it is still due to contamination in the reagents. The methodology was successfully validated using a certified reference material and showed good tolerance to additional nitric acid and various metal and metalloid ions. However, identification of the generated volatile Zn species using direct ionization in real time (DART)-MS, as done for Pd [28], was unsuccessful and remains a goal for future research.
Experimental
All reagents were of analytical purity or higher; ultrapure deionized water prepared by MilliQPLUS system (< 0.055 mS cm−1, Millipore, USA) was used throughout the measurements. Zn(II) and Cs(I) single element standards (1000 μg cm−3, prepared in 2% (m/m) HNO3, Analytika, Czech Republic) were used for preparation of standard solutions by serial dilution. Acidic carrier solution was prepared from concentrated H2SO4 (96%, Lach-Ner, Czech Republic), concentrated HCl (37%, Analpure, Analytika, Czech Republic), and HNO3 (69%, Analpure, Analytika, Czech Republic) Standards or samples contained 8-hydroxyquinoline (≥ 99%, ACS reagent, Sigma-Aldrich, USA) as the reaction modifier [13], 1,10-phenantroline (99%, ACS reagent, Sigma-Aldrich, USA) was tested as the modifier in some experiments. Solution of reducing agent NaBH4 (> 99.0% purity, for the determination of hydride formers by AAS, Honeywell, USA) was stabilized by NaOH (p.a., Lach-Ner, Czech Republic). Solution INTMIX1 (containing 10 μg cm−3 Bi, In, Sc, Tb, and Y in 5% HNO3, Analytika, Czech Republic) was used for preparation of an internal standard (IS). Argon (99.998%, Linde, Czech Republic) served as a carrier gas, H2 (99.90%, Linde, Czech Republic) was mixed with Ar in some experiments. Argon (99.9990%, SIAD, Czech Republic) and helium (99.9990%, SIAD, Czech Republic) were used as ionization and collision gas, respectively. A solution of 65Zn radioactive indicator (as 65ZnCl2 in 0.1 mol dm−3 HCl produced by National Centre for Nuclear Research—Polatom, Poland) was purchased from Lacomed, spol. s.r.o. (Czech Republic). Interferences were evaluated using solutions diluted from 1000 μg cm−3 single element standards. Apart from Ag, Au, Cd, and Cu (Merck, Germany), all the other standards were sourced from Analytika (Czech Republic). We presume the following oxidation states in solutions of standards: Fe(III), Mn(II), Cd(II), Au(III), Ni(II), Cu(II), Co(II), Ag(I), As(III), Sb(III), Bi(III), Se(IV), and Te(IV) [30].
Instrumentation
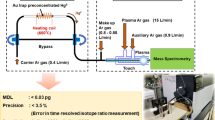
A scheme of the CVG setup in the flow injection (FI) mode is displayed in Fig. 3. Solutions of acid carrier and reducing agent were pumped separately at flow rates of 0.4 cm3 min−1 using a peristaltic pump (Reglo Digital, Ismatec, USA) and tygon tubing. Unless specified otherwise, the standard/sample containing 8-hydroxyquinoline in 0.08 mol dm−3 H2SO4 was injected into the stream of carrier (0.08 mol dm−3 H2SO4) by means of a medium pressure six port injection valve (IDEX, USA) with a sample loop of 345 mm3. A commercial MSIS™ (Agilent, USA) [18] was employed as a gas–liquid separator and for mixing the solutions of acidified sample/standard and reducing agent at the tip of a glass reaction cone protruding into the MSIS. MSIS was not operated in the dual mode with simultaneous nebulization of solution into the chamber, but only with CVG. The carrier gas (210 cm3 min−1, unless otherwise stated) was thus introduced via a tube fitted and sealed in the side inlet of the MSIS that is originally designed for a nebulizer (Fig. 3). Waste liquid was continuously removed from the bottom part of the MSIS by a second peristaltic pump (Reglo Digital, Ismatec, USA). The gas phase exiting the MSIS was transported to ICP-MS (Agilent 7900, USA) via a 150 mm long PTFE tube (i.d. 2.0 mm) and through an ultra-high matrix introduction (UHMI) port located downstream of the Scott double-pass spray chamber. The flowrate of carrier gas Ar (and possibly H2) was regulated by a mass flow controller (Cole-Parmer, USA). An IS solution (diluted to 2 ng cm−3 in 2% (m/m) HNO3) and 2% HNO3 were concurrently introduced into the spray chamber via a MicroMist nebulizer (Burgener Research Inc., Mississauga, Canada) to maintain “wet” plasma conditions in the ICP. Only for determination of the overall CVG efficiency, a manual injection valve (the same sample loop volume as used with CVG) was inserted into the 2% HNO3 channel. An IS solution was mixed with this carrier downstream of the injection valve (red line in Fig. 3).
Measurements with an ICP-MS were conducted in a time resolved analysis mode using He gas in the collision cell (4.1 cm3 min–1) and pulse mode of the detector. Isotopes 66Zn, 89Y (as internal standard), and 133Cs (aerosol) were typically monitored.
Measurement procedure and conventions
The acidified sample/standard containing 8-hydroxyquinoline was injected by the valve into the stream of the carrier 5 s after recording of the signal intensities started. The recording was stopped after the signal intensity of 66Zn (and possibly 133Cs) returned to the baseline. The transient signals (FI peaks) of 66Zn and 133Cs were integrated in MS Excel software and corrected for any sensitivity drift relative to changes in continuous response of nebulized 2 ng cm−3 89Y internal standard recorded and averaged over the same time window—a quantity 66Zn peak area / 89Y signal intensity (termed as corrected peak area 66Zn/89Y, units in s) is thus used in the figures. Averages of at least three replicates are presented. Limits of detection (LOD) and quantification (LOQ) were calculated as 3 × SD(blank) and 10 × SD(blank), respectively, divided by the slope of the calibration function.
Determination of overall CVG efficiency
The overall CVG efficiency is defined as the fraction of analyte converted to a volatile species, released from the liquid phase and transported by a carrier gas to the detector [31]. It was determined i) by comparison of sensitivities obtained with CVG and conventional solution nebulization [32,33,34,35,36,37,38,39] and ii) using 65Zn radiotracer [13].
For the former approach, Zn standards (0–200 ng cm−3) prepared in 0.08 mol dm−3 H2SO4 and comprising 0.4% 8-hydroxyquinoline were sequentially measured using FI-CVG while simultaneously nebulizing solutions of carrier liquid and IS in 2% (m/m) nitric acid. Succeeding that, Zn solutions (0–50 ng cm−3) prepared in 2% (m/m) nitric acid were introduced by FI and nebulized while CVG was in operation with 0.08 mol dm−3 H2SO4. The same volume of standards (345 mm3) was used for both sample introduction techniques. The overall CVG efficiency (εCVG) is thus calculated as the ratio of the sensitivities for CVG (aCVG) and nebulization (aneb) multiplied by nebulization efficiency (εneb) (Eq. 1).
The nebulization efficiency represents the traditional fraction of the nebulized analyte that enters the plasma and was determined as 8.0% (uncertainty 0.02%) by a dynamic mass flow approach (modified waste collection method) described in [34, 35, 38].
The second approach to determination of the overall CVG efficiency relied on application of the 65Zn radiotracer (gamma ray emitter, half-life 244 days) [13]. It allowed not only to quantify the analyte fraction converted to volatile species and the fraction remaining in the waste solution but also the analyte fraction retained in different parts of the generation setup [13, 31, 36, 40,41,42]. The commercial solution of 65Zn radiotracer was diluted with 0.08 mol dm−3 H2SO4 and it was spiked with 8-hydroxyquinoline and 200 ng cm−3 nonradioactive zinc prior to the experiments with CVG. The sample loop was removed from the CVG system and 0.5 cm3 of the radiolabeled solution was introduced by continuous aspiration from a glass sample vial followed by 0.5 cm3 of CVG blank solution. The loaded amount of 65Zn corresponded to the activity of approximately 3 kBq. Two cartridges filled with granulated activated charcoal and a 0.45 μm syringe filter (cellulose acetate) were employed to quantitatively trap the generated volatile Zn species, thus also preventing the leak of 65Zn to the atmosphere. Experiment was performed in triplicate. The cartridges packed with granulated activated carbon and the filter were replaced for every single replicate. Also, the liquid waste was collected separately for each replicate into special glass vials (around 20 cm3 volume) suitable for autosampler of the gamma counter. After three replicates, the CVG setup (channels for acid and reducing agent) was rinsed with 2 cm3 of deionized water and the generator was then dismantled. The individual parts were placed directly into the glass vials (tubing, junctions, reaction cone and charcoal columns) or leached with concentrated HNO3 (syringe filter and MSIS chamber). The automatic gamma counter (1480 Wizard 3, Perkin-Elmer) fitted with a NaI(Tl) well-type crystal was employed to quantify the activity of 65Zn in the columns, wastes of CVG, leaches and various parts of the apparatus. The measured energy ranged from 10 to 2048 keV, and the counting time was 60 s. Correction was made for the well-type detector’s geometric effect.
CRM preparation
Standard reference material NIST 1643f (fresh water) was diluted 2-times followed by addition of H2SO4 acid and 8-hydroxyquinoline to finally obtain their 0.08 mol dm−3 and 0.4% (m/v) concentrations. The Zn concentration was determined using an external calibration technique. An agreement with a certified value was assessed in accordance with Eq. 2 [29].
Data availability
The data will be made available on request.
References
D’Ulivo A, Sturgeon RE (2022) Vapor generation techniques for trace element analysis. Elsevier
Dedina J, Tsalev DL (1995) Hydride generation atomic absorption spectrometry. Wiley, Chichester
Sun H, Suo R, Lu Y (2002) Anal Chim Acta 457:305
da Costa MAP, da Silva DLF, de Souza Dias F, de Freitas Santos Júnior A, dos Santos WNL (2020) Anal Methods 12:1711–1719
Sun H-W, Suo R (2004) Anal Chim Acta 509:71
Luna AS, Sturgeon RE, de Campos RC (2000) Anal Chem 72:3523
Smichowski P, Farías S, Pérez Arisnabarreta S (2003) Analyst 128:779
Arbab-Zavar M-H, Chamsaz M, Youssefi A, Aliakbari M (2012) Anal Sci 28:717
Hu J, Yu Y, Xiao Z, Gao Y (2023) Microchem J 193:109178
Wang Z, Wang X, Wang Q, Xiong X (2019) Microchem J 149:104052
Zhu Z, Liu L, Li Y, Peng H, Liu Z, Guo W, Hu S (2014) Anal Bioanal Chem 406:7523
Greda K, Gorska M, Welna M, Jamroz P, Pohl P (2019) Talanta 199:107
Nováková E, Sembschová K, Musil S (2023) Spectrochim Acta Part B At Spectrosc 202:106645
Peña-Vázquez E, Villanueva-Alonso J, Bermejo-Barrera P (2007) J Anal At Spectrom 22:642
Zeng C, Kong J, Zhou N, Cui R, Li X (2013) Anal Methods 5:1564
Musil S, Kratzer J, Vobecký M, Benada O, Matoušek T (2010) J Anal At Spectrom 25:1618
Matoušek T, Dědina J, Vobecký M (2002) J Anal At Spectrom 17:52
McLaughlin RLJ, Brindle ID (2005) Multimode sample introduction system. US Patent US 6,891,605 B2, May 10, 2005; (2003) Chem Abstr 140:35006
Fuentes-Cid A, Villanueva-Alonso J, Peña-Vázquez E, Bermejo-Barrera P (2012) Anal Chim Acta 749:36
Villanueva-Alonso J, Suárez-García A, Peña-Vázquez E, Bermejo-Barrera P (2015) Atom Spectrosc 36:63
Villanueva-Alonso J, Peña-Vázquez E, Barciela-Alonso M, Bermejo-Barrera P (2014) Atom Spectrosc 35:7
Villanueva-Alonso J, Peña-Vázquez E, Bermejo-Barrera P (2009) Spectrochim Acta Part B At Spectrosc 64:659
Villanueva-Alonso J, Peña-Vázquez E, Bermejo-Barrera P (2012) Talanta 100C:45
Peña-Vázquez E, Villanueva-Alonso J, Bermejo-Barrera P (2008) Atom Spectrosc 29:180
Duan X, Sun R, Fang J (2016) Spectrochim Acta Part B At Spectrosc 128:11
Matoušek T, Sturgeon RE (2004) J Anal At Spectrom 19:1014
Šoukal J, Benada O, Matoušek T, Dědina J, Musil S (2017) Anal Chim Acta 977:10
Vyhnanovský J, Kratzer J, Benada O, Matoušek T, Mester Z, Sturgeon RE, Dědina J, Musil S (2018) Anal Chim Acta 1005:16
Linsinger T (2005) Application note: Comparison of a measurement result with the certified value. https://crm.jrc.ec.europa.eu/graphics/cms_docs/erm1_english.pdf. Accessed 4 April 2024
Schweitzer GK, Pesterfield LL (2010) The Aqueous Chemistry of the Elements. Oxford University Press
Matoušek T (2007) Anal Bioanal Chem 388:763
Šoukal J, Sturgeon RE, Musil S (2018) Anal Chem 90:11688
Vyhnanovský J, Sturgeon RE, Musil S (2019) Anal Chem 91:13306
Nováková E, Horová K, Červený V, Hraníček J, Musl S (2020) J Anal At Spectrom 35:1380
Vyhnanovský J, Yildiz D, Štádlerová B, Musil S (2021) Microchem J 164:105997
Sagapova L, Musil S, Kodríková B, Svoboda M, Kratzer J (2021) Anal Chim Acta 1168:338601
Jeníková E, Nováková E, Hraníček J, Musil S (2022) Anal Chim Acta 1201:339634
Šoukal J, Musil S (2022) Microchem J 172:106963
Jeníková E, Nováková E, Ruxová H, Musil S (2022) Monatsh Chemie 153:811
Musil S, Kratzer J, Vobecký M, Hovorka J, Benada O, Matoušek T (2009) Spectrochim Acta Part B At Spectrosc 64:1240
Rybínová M, Musil S, Červený V, Vobecký M, Rychlovský P (2016) Spectrochim Acta Part B At Spectrosc 123:134
Arslan Y, Matoušek T, Kratzer J, Musil S, Benada O, Vobecný M, Ataman OY, Dědina J (2011) J Anal At Spectrom 26:828
Acknowledgements
The authors would like to thank Charles University, Grant Agency of the Czech Republic (project 19-17604Y) and the Czech Academy of Sciences (Institutional Research Plan RVO: 68081715) for financial support. AH specifically thanks the Grant Agency of Charles University (project GAUK 266623) and the SVV project, EN would like to thank the Cooperatio project.
Funding
Grantová Agentura České Republiky, 19-17604Y, Stanislav Musil, Univerzita Karlova v Praze, 266623, Alexandra Hadbavníková, Akademie Věd České Republiky, 68081715, Stanislav Musil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hadbavníková, A., Musil, S., Hraníček, J. et al. Enhancing the efficiency of chemical vapor generation of zinc in a multimode sample introduction system. Monatsh Chem 155, 889–897 (2024). https://doi.org/10.1007/s00706-024-03241-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-024-03241-0