Abstract
A novel approach for chemoselective synthesis of functionalized benzo[g]thiazolo[3,2-a]quinolones from dithioacetals, cysteamine hydrochloride, 2-hydroxy-1,4-naphthoquinone, and aromatic aldehydes in ethanol is described. All products were tested in vitro for their cytotoxic effects on lung, breast, and prostate cancer cells.
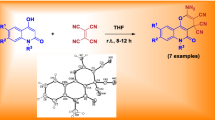
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, sulfur–nitrogen heterocyclic compounds have attracted much attention of an increasing number of pharmaceutical and agrochemical researchers, due to their versatile biological activity, unusual physical properties and also existence of these groups in a broad range of pharmaceuticals and natural products. Their unique physical properties in the molecular structure, especially from magnetism and conductivity aspects, are due to the availability of unshared pairs of electrons and the difference in electronegativity between heteroatoms and carbon [1, 2].
Among such sulfur–nitrogen containing heterocycles, thiazolo[3,2-a]pyridines are an important class of compounds with two different fused heterocycles, which are found in a broad range of compounds possessing a variety of biological activities. These compounds act as potent CDK2-cyclin A inhibitor [3], potential uterus stimulant [4], beta-amyloid production inhibitor [5], coronary dilator, antibacterial and antifungal agent [6], and scavenger of free radicals because of possessing electron acceptors and donors [7]. Also, these derivatives play an important role in the drug development for chemotherapy of various cancers, such as leukemia, lung cancer, and melanoma [8, 9]. Some important thiazolopyridine scaffolds from the biological point of view are presented in Fig. 1 [10,11,12].
Furthermore, to increase the therapeutic properties of thiazolo[3,2-a]pyridines, we synthetically attached the 1,4-naphthoquinone ring, with various biologic activities, such as antimalarial, antibacterial, cytotoxic, antifungal, antiviral, and anti-inflammatory, and unique properties, such as redox and acid–base properties to these scaffolds [13, 14].
Cancer is one of the leading causes of death worldwide and the exploration of new potential approaches for cancer treatment including the design of novel anti-cancer drugs is one of the important strategies [15]. Because of the biological importance and anti-cancer activities of thiazolo[3,2-a]pyridines, numerous procedures have been developed for the synthesis of these components [5, 7, 16,17,18,19]. Although previous studies have shown the synthesis of compounds with similar structures, developing new methods for the synthesis of sulfur-containing pharmaceutical compounds seems to be essential. Herein, we describe our results in the synthesis of several derivatives of thiazolo[3,2-a]pyridines and the evaluation of their in vitro cytotoxic activity against tumor cells, including A549 cells, MCF-7 cells, and PC3 cells using MTT assay. Finally, we showed cell death by evaluation of nucleus of cells by using DAPI staining.
Results and discussion
Chemistry
In this regard, and in continuation of our previous works based on the discovery of new heterocycles with potentially biological activities [20,21,22,23], herein we would like to report a novel efficient one-pot strategy for the synthesis of 4-nitro-5-phenyl-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione via condensation of β-nitrothiazolidine as an enamine analog, 2-hydroxy-1,4-naphthoquinone, and aromatic aldehydes under mild reaction condition in ethanol (Scheme 1). In this paper, ethanol was used as an inexpensive and environmentally friendly solvent, which is miscible with water [24, 25]. β-Nitrothiazolidine derived from the addition of cysteamine hydrochloride to 1,1-bis(methylthio)-2-nitroethene was also used. Its electron donation of nitrogen and sulfur atoms increased the electron density of the carbon at the β-position to nitrogen, which acted as the nucleophile [18, 26, 27].

The reaction mechanism, on the basis of the results obtained, is designated in Scheme 2. To form product 5, it is possible that initially the formation of β-nitrothiazolidine (6) occurs through the addition of cysteamine hydrochloride (1) to 1,1-bis(methylthio)-2-nitroethene (2) in the presence of an equivalent amount of triethylamine base for the release of cysteamine salts. Then Michael acceptor 7 occurs through Knoevenagel condensation between aromatic aldehydes 3 and 2-hydroxy-1,4-naphthoquinone (4) that is followed by loss of water molecules. β-nitrothiazolidine then adds to the Knoevenagel adduct 7 in a Michael addition to give open chain intermediate 8, which undergoes successive imine–enamine tautomerization, followed by N-cyclization via attack of the secondary amino group to the more reactive carbonyl group of 2-hydroxy-1,4-naphthoquinone to give product 5.

In this method, chromatography and recrystallization did not need purification; the mixture was filtered and the precipitate washed with EtOH (96%) to afford the pure product 5. As shown in Table 1, several functionalities present in the aryl moiety such as halogen, hydroxyl, and nitro groups in ortho or para position were tolerated and worked well with different efficiencies.
The second step was completed after 1–8 h in EtOH and afforded rge corresponding heterocyclic systems 5a–5l, in good to excellent yields (68–95%). The structures of the separated crude products were clearly deduced from their elemental analyses, IR, 1H, 13C NMR, and mass spectra. The mass spectrum of 5a displayed the molecular ion peaks at appropriate m/z = 390 with the frequency of 18%, which was in agreement with the proposed structure. The 1H NMR spectrum of 5a showed two multiplets for four of the CH2 groups (δ = 3.43–3.48, 4.75–4.88 ppm), one singlet for the CH group (5.60 ppm) and aromatic region of the spectrum (7.12–7.98 ppm) for the aromatic moieties. The 1H-decoupled 13C NMR spectrum of 5a showed 19 distinct resonances. Two signals at 180.0 and 180.9 ppm, which were assigned to two unsaturated 1,4-dicarbonyl groups, revealed the selective formation of 5a.
Cytotoxic activity
At first, to compare the anti-cancer properties between heterocycles with a variety of structures, the cytotoxic activity of the synthesized compounds of our previous papers [20, 21], including pyrrolo[2,3-d]pyrimidines 9a–9c, indenone-fused thiazolo[3,2-a]pyridines 10a–10c and benzo[g]thiazolo[3,2-a]quinoline 5e (Fig. 2), were evaluated against A549 cell, MCF-7 cell, and PC3 cell lines using the MTT colorimetric assay. The activity is expressed as 50% growth inhibitory concentration (IC50) values at 48 h and the results are presented in Table 2. We used DMSO (1%) as a negative control and etoposide as a positive control. Among the different heterocyclic compounds in terms of chemical structure, compound 5e (IC50 = 2.5 and 15 µM) showed the highest cytotoxicity against every three cell line.
To further examine the effect of substitution on the anti-cancer properties of the benzo[g]thiazolo[3,2-a]quinolines, novel synthesized derivatives 5a–5l were evaluated against A549 cells, MCF-7 cells, and PC3 cells. Compounds 5e (IC50 = 2.5 µM) and 5 h (IC50 = 20 µM), which possessed 4-hydroxy and 4-nitro substituent moieties, respectively, displayed the highest cytotoxicity on the PC3 cell line (Table 3). In the synthesis of structurally drug-like compounds, compounds with numerous hydrogen bonding possibilities are superior to other compounds. These compounds are similar to normal metabolites and can easily be mistaken for metabolic activities by biological systems. Next, we treated cells with IC50 of the 5e compound for 24 h. Finally, using DAPI staining, we showed clear morphological changes and fragmentation in the chromatin within the nucleus of treated cells, but their morphology was not altered in untreated normal cells. Our results indicated that the 5e compound induced cell death (Fig. 3).
Inverted fluorescent microscopy images of chromatin damage occurrence in the nucleus of treated cells with 5e compound (as sample) and DMSO (1%, as control), which had been stained with DAPI in different cancer cell lines. The experiments were performed three times (original microscope magnification, 40×, scale bar, 10 μm)
It was previously shown that heterocyclic compounds containing nitrogen and sulfur heteroatoms have highly effective antitumor activities on cancer cells. Herein, we found that our synthesized benzo[g]thiazolo[3,2-a]quinolines derivatives exhibited the strongest inhibitory effect on cancer cells compared with other thiazole-based heterocycles [28].
Notably, our results demonstrated among the three cancer cell lines that we used, MCF-7 and PC3 cell lines were more sensitive than the A549 cell line to 5e. Moreover, the PC3 cell line was more sensitive to the other potent anti-cancer compound 5h, compared to MCF-7 and A549 cell lines. Breast cancer and prostate cancer are the most common cancers in women and men in the world, respectively. Therefore, our findings can be helpful to design new therapeutic drugs against cancer. However, more studies will be required to show the molecular mechanisms involved in inducing cell death. Also, it is important to investigate the toxic side effects of new synthetic compounds by using in vivo experiments.
Conclusion
In this paper, we have developed a novel and efficient approach to the synthesis of heterocyclic scaffold comprising thiazole and quinoline, namely, 4-nitro-5-phenyl-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione through sequential Knoevenagel/Michael/intramolecular N-cyclization sequences in EtOH media. Since sulfur- and nitrogen-containing compounds often show different biological activities and serve important functions in applications in the pharmaceutical industry, we evaluated derivatives’ in vitro cytotoxic activity against tumor cells including A549 cells, MCF-7 cells, and PC3 cells using MTT assay. Finally, we showed cell death by evaluation of the nucleus of cells using DAPI staining. Depending on the above-mentioned results, it can be concluded that both compounds 5e and 5h have potent antitumor activity.
Experimental
1,1-Bis(methylthio)-2-nitroethene, cysteamine hydrochloride, 2-hydroxy-1,4-naphthoquinone, aromatic aldehydes, trimethylamine, and solvents were purchased from Aldrich and Merck Chemical Co. and used without further purification. IR spectra were measured with Bruker Tensor 27 spectrometer. NMR spectra were recorded with a Bruker DRX-300 Avance instrument (300 MHz for 1H and 75.4 MHz for 13C) with CDCl3 and DMSO-d6 as solvents. Chemical shifts are given in ppm (δ) relative to internal TMS, and coupling constant (J) is reported in hertz (Hz). Mass spectra were recorded with an Agilent 5975C VL MSD with Triple-Axis detector operating at an ionization potential of 70 eV. Elemental analyses for C, H, and N were performed using a Heraeus CHNO-Rapid analyzer. Melting points were measured with an electrothermal 9100 apparatus.
Exemplary procedure
A mixture of 0.113 g cysteamine hydrochloride (1 mmol), 0.165 g 1,1-bis(methylthio)-2-nitroethylene (1 mmol), 139 mm3 triethylamine (1 mmol), and 10 cm3 EtOH in a 50 cm3 round-bottomed flask fitted with a reflux condenser was heated with stirring in an oil bath at reflux temperature for 4 h. After reaction completion (monitored by TLC, ethyl acetate/n-hexane, 6:4), 101 mm3 benzaldehyde (1 mmol) and 0.174 g 2-hydroxy-1,4-naphthoquinone (1 mmol) were added to the reaction mixture and it was stirred under 80 °C for a period of time shown in Table 1. Then, the reaction mixture was cooled to room temperature and filtered to give the crude product. The solid was washed with ethanol 96% and then dried in an oven at 150 °C to give product 5a and analyzed by 1H NMR and 13C NMR. The crude product recrystallized from ethanol to afford the pure product (for CHN analyses).
4-Nitro-5-phenyl-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5a, C21H14N2O4S)
Red powder; m.p.: 245–247 °C; yield 0.304 g (78%); IR (KBr): \( \bar{v} \) = 3449, 2926, 1744 (C=O), 1664 (C=O), 1539 and 1347 (NO2), 1445, 1243 (C–N), 1006 (C–O), 727 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 7.98–8.03 (t, J = 7.5 Hz, 1H), 7.85–7.90 (t, J = 7.5 Hz, 1H), 7.81 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 7.8 Hz, 2H), 7.26–7.30 (t, J = 7.2 Hz, 2H), 7.12-7.23 (m, 1H), 5.60 (s, 1H), 4.75–4.88 (t, J = 7.5 Hz, 2H), 3.43–3.48 (t, J = 7.5 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.9 (C=O), 180.0 (C=O), 158.9 (C=C–NO2), 142.7 (C=C–N), 139.5, 134.9, 134.5, 132.1, 131.0, 128.9, 128.7, 127.8, 127.1, 125.7 (Ar), 124.7 (C=C–N), 123.0 (C–NO2), 54.2 (CH2N), 38.5 (CH2S), 29.9 (CH) ppm; MS (EI, 70 eV): m/z (%) = 390 (18, M+), 344 (84), 313 (100), 267 (26), 234 (20), 183 (57), 139 (4), 77 (13).
5-(4-Chlorophenyl)-4-nitro-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5b, C21H13ClN2O4S)
Red powder; m.p.: 272–274 °C; yield 0.386 g (91%); IR (KBr): \( \bar{v} \) = 3447, 2926, 1742 (C=O), 1679 (C=O), 1585 and 1335 (NO2), 1446, 1243 (C–N), 1006 (C–O), 727 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 7.98–8.06 (t, J = 7.59 Hz, 1H), 7.85–7.80 (t, J = 7.9 Hz, 1H), 7.82 (d, J = 8.1 Hz, 2H), 7.34 (d, J = 8.7 Hz, 4H), 5.58 (s, 1H), 4.78–4.84 (t, J = 7.8 Hz, 2H), 3.42–3.48 (t, J = 7.8 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.9 (C=O), 179.9 (C=O), 159.1 (C=C–NO2), 141.6 (C=C–N), 139.6, 134.9, 134.5, 132.4, 132.1, 131.0, 130.7, 128.8, 127.1, 125.7 (Ar), 124.1 (C=C–N), 122.7 (C–NO2), 54.2 (CH2N), 38.2 (CH2S), 29.9 (CH) ppm; MS (EI, 70 eV): m/z (%) = 425.5 (7, [M + 1]+), 424.5 (20, M+), 378 (94), 313 (100), 267 (32), 234 (24), 210 (9), 172 (5), 111 (8), 75 (16).
5-(4-Fluorophenyl)-4-nitro-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5c, C21H13FN2O4S)
Orange powder; m.p.: 214–215 °C; yield 0.334 g (82%); IR (KBr): \( \bar{v} \) = 3416, 2925, 1735 (C=O), 1647 (C=O), 1547 and 1370 (NO2), 1455, 1227 (C–N), 1016 (C–O), 718 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 7.98–8.02 (t, J = 7.5 Hz, 1H), 7.84–7.90 (t, J = 7.5 Hz, 1H), 7.81 (d, J = 6.9 Hz, 2H), 7.36 (d, J = 8.1 Hz, 2H), 7.08 (d, J = 8.1 Hz, 2H), 5.59 (s, 1H), 4.72–4.84 (t, J = 7.4 Hz, 2H), 3.40–3.47 (t, J = 7.4 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.9 (C=O), 180.0 (C=O), 162.0, 165.3 (Ar, d, 1JCF = 247.5 Hz), 159.0 (C=C–NO2), 139.6 (C=C–N), 138.9, 134.9, 132.1 (Ar), 130.8, 130.7 (Ar, d, 3JCF = 9.0 Hz), 127.1, 125.7 (Ar), 124.4 (C=C–N), 123.0 (C–NO2), 115.7, 115.4 (Ar, d, 2JCF = 21.7 Hz), 54.2 (CH2N), 38.0 (CH2S), 29.9 (CH) ppm; MS (EI, 70 eV): m/z (%) = 408 (20, M+), 362 (100), 313 (8), 267 (25), 234 (20), 207 (6), 170 (2), 139 (4), 75 (9).
Methyl 4-(4-nitro-6,11-dioxo-1,2,6,11-tetrahydro-5H-benzo[g]thiazolo[3,2-a]quinoline-5-yl)benzoate (5d, C23H16N2O6S)
Red powder; m.p.: 289–290 °C; yield 0.358 g (80%); IR (KBr): \( \bar{v} \) = 3424, 2941, 1762 (C=O), 1724 (C=O), 1677 (C=O), 1542 and 1334 (NO2), 1441, 1245 (C–N), 1108 (C–O), 720 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 7.79-7.87, 7.99-8.02 (m, 6H), 7.49 (d, J = 8.1 Hz, 2H), 5.65 (s, 1H), 4.79-4.84 (t, J = 7.5 Hz, 2H), 3.80 (s, 3H), 3.44-3.50 (t, J = 7.5 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.8 (C=O), 179.9 (C=O), 166.3 (CO2), 159.3 (C=C–NO2), 147.7 (Ar), 139.6 (C=C–N), 134.9, 134.5, 132.1, 131.0, 129.7, 129.3, 129.0, 127.1, 125.7 (Ar), 124.0 (C=C–N), 122.5 (C–NO2), 54.2 (CH2N), 52.6 (OCH3), 38.8 (CH2S), 29.9 (CH) ppm.
5-(4-Hydroxyphenyl)-4-nitro-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5e, C21H14N2O5S)
Red powder; m.p.: 310–311 °C; yield 0.365 g (90%); 1H NMR (300 MHz, DMSO-d6): δ = 9.35 (s, 1H), 7.78–8.00 (m, 4H), 7.09 (d, J = 8.1 Hz, 2H), 6.62 (d, J = 8.1 Hz, 2H), 5.47 (s, 1H), 4.76–4.81 (t, J = 7.5 Hz, 2H), 3.41-3.46 (t, J = 7.5 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.9 (C=O), 180.0 (C=O), 158.4 (C=C–NO2), 157.1 (Ar), 138.9 (C=C–N), 134.9, 134.4, 133.2, 132.0, 131.0, 129.8, 127.0, 125.7 (Ar), 125.1 (C=C–N), 123.4 (C–NO2), 115.5 (Ar), 54.1 (CH2N), 37.5 (CH2S), 29.8 (CH) ppm.
5-(2-Hydroxy-4-methoxyphenyl)-4-nitro-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5f, C22H16N2O6S)
Red powder; m.p.: 184–186 °C; yield 0.314 g (72%); 1H NMR (300 MHz, DMSO-d6): δ = 9.60 (s, 1H), 7.72-7.98 (m, 4H), 7.17 (d, J = 8.4 Hz, 1H), 6.31 (d, J = 8.4 Hz, 1H), 6.21 (s, 1H), 5.54 (s, 1H), 5.02–5.08 (t, J = 8.1 Hz, 2H), 4.43–4.52 (t, J = 8.1 Hz, 2H), 3.58 (s, 3H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 182.1 (C=O), 180.6 (C=O), 159.9 (C=C–NO2), 158.8 (C–OMe), 157.7 (C–OH), 139.7 (C=C–N), 135.0, 134.3, 133.3, 131.8, 131.2, 126.9, 125.7, 123.7 (Ar), 122.0 (C=C–N), 120.0 (C–NO2), 104.8 (Ar), 102.0 (Ar), 55.3 (OCH3), 53.5 (CH2N), 36.4 (CH2S), 29.4 (CH) ppm.
5-(4-Bromophenyl)-4-nitro-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5g, C21H13BrN2O4S)
Brown powder; m.p.: 286–288 °C; yield 0.375 g (80%); 1H NMR (300 MHz, DMSO-d6): δ = 7.97–8.03 (t, J = 7.4 Hz, 1H), 7.84-7.90 (t, J = 7.1 Hz, 1H), 7.82 (d, J = 6.9 Hz, 2H), 7.44 (d, J = 7.9 Hz, 2H), 7.29 (d, J = 8.6 Hz, 2H), 5.57 (s, 1H), 4.75–4.83 (t, J = 8.1 Hz, 2H), 3.40-3.48 (t, J = 8.1 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.4 (C=O), 179.1 (C=O), 159.2 (C=C–NO2), 142.9 (C=C–N), 139.5, 134.9, 134.5, 132.3, 131.7, 131.0, 127.1, 125.7, 124.4, 124.1 (Ar), 122.7 (C=C–N), 120.9 (C–NO2), 54.2 (CH2N), 38.3 (CH2S), 29.9 (CH) ppm; MS (EI, 70 eV): m/z (%) = 470 (11, [M + 1]+), 469 (4, M+), 424 (60), 313 (100), 267 (21), 234 (17), 210 (6), 157 (3), 113 (2), 76 (9).
4-Nitro-5-(4-nitrophenyl)-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5h, C21H13N3O6S)
Red powder; m.p.: 228 °C; yield 0.413 g (95%); 1H NMR (300 MHz, DMSO-d6): δ = 8.12 (d, J = 7.6 Hz, 2H), 7.99–8.07 (t, J = 6.8 Hz, 1H), 7.78–7.92 (m, 3H), 7.64 (d, J = 7.6 Hz, 2H), 5.73 (s, 1H), 4.79–4.88 (t, J = 7.9 Hz, 2H), 3.44–3.51 (t, J = 7.9 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.8 (C=O), 179.9 (C=O), 159.0 (C=C–NO2), 149.7 (Ar), 147.1 (Ar), 139.9 (C=C–N), 135.0, 134.5, 132.0, 130.9, 130.3, 127.1, 125.8, 124.0 (Ar), 123.5 (C=C–N), 122.3 (C–NO2), 53.5 (CH2N), 39.1 (CH2S), 29.9 (CH) ppm; MS (EI, 70 eV): m/z (%) = 435 (19, M+), 389 (62), 343 (9), 313 (100), 267 (20), 234 (15), 210 (5), 178 (2), 139 (3), 76 (7).
5-(4-Methoxyphenyl)-4-nitro-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5i, C22H16N2O5S)
Red powder; m.p.: 208–210 °C; yield 0.302 g (72%); 1H NMR (300 MHz, DMSO-d6): δ = 7.79–7.99 (m, 4H), 7.23 (d, J = 8.7 Hz, 2H), 6.79 (d, J = 8.7 Hz, 2H), 5.53 (s, 1H), 4.78–4.86 (t, J = 7.5 Hz, 2H), 3.65 (s, 3H), 3.42–3.47 (t, J = 7.5 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.5 (C=O), 179.9 (C=O), 158.9 (C=C–NO2), 157.9 (C–OCH3), 141.5 (C=C–N), 139.2, 133.6, 134.1, 131.7, 128.4, 128.0, 127.3, 125.5 (Ar), 123.5 (C=C–N), 122.8 (C–NO2), 114.2 (Ar), 55.5 (OCH3), 54.2 (CH2N), 39.1 (CH2S), 29.9 (CH) ppm.
5-(3-Fluorophenyl)-4-nitro-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5j, C21H13FN2O4S)
Orange powder; m.p.: 273–275 °C; yield 0.277 g (68%); IR (KBr): \( \bar{v} \) = 3447, 2927, 1741 (C=O), 1685 (C=O), 1539 and 1305 (NO2), 1448, 1210 (C–N), 1115 (C–O), 776 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 7.80–8.02 (m, 4H), 7.00–7.34 (m, 4H), 5.60 (s, 1H), 4.79–4.84 (t, J = 7.5 Hz, 2H), 3.43-3.48 (t, J = 7.5 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.9 (C=O), 179.9 (C=O), 164.2, 161.0 (Ar, d, 1JCF = 240 Hz), 159.2 (C=C–NO2), 145.2 (C=C–N), 139.7, 134.9, 134.5, 132.1, 131.0 (Ar), 130.7, 130.6 (Ar, d, 3JCF = 8.3 Hz), 127.1, 125.8, 125.0 (Ar), 124.0 (C=C–N), 122.6 (C–NO2), 115.7, 115.4 (d, 2JCF = 21.7 Hz, Ar), 114.5, 114.8 (d, 2JCF = 21.0 Hz, Ar), 54.2 (CH2N), 38.5 (CH2S), 29.9 (CH) ppm.
5-(3-Chlorophenyl)-4-nitro-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5k, C21H13ClN2O4S)
Red powder; m.p.: 285 °C; yield 0.390 g (92%); 1H NMR (300 MHz, DMSO-d6): δ = 7.98-8.04 (t, J = 8.2 Hz, 1H), 7.84–7.92 (t, J = 8.2 Hz, 1H), 7.82 (d, J = 6.9 Hz, 2H), 7.38 (s, 1H), 7.29–7.31 (m, 3H), 5.57 (s, 1H), 4.77–4.85 (t, J = 7.9 Hz, 2H), 3.42–3.48 (t, J = 7.9 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.9 (C=O), 179.9 (C=O), 159.2 (C=C–NO2), 144.8 (Ar), 139.7 (C=C–N), 134.9, 134.5, 133.4, 132.1, 130.9, 130.7, 128.7, 127.8, 127.6, 127.0, 125.7 (Ar), 123.9 (C=C–N), 122.6 (C–NO2), 54.2 (CH2N), 38.7 (CH2S), 29.9 (CH) ppm.
5-(3-methoxyphenyl)-4-nitro-1,2-dihydro-5H-benzo[g]thiazolo[3,2-a]quinoline-6,11-dione (5l, C22H16N2O5S)
Red powder; m.p.: 245–247 °C; yield 0.290 g (69%); IR (KBr): \( \bar{v} \) = 3448, 2925, 1743 (C=O), 1665 (C=O), 1536 and 1341 (NO2), 1440, 1199 (C–N), 1046 (C–O), 718 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 7.79–8.01 (m, 4H), 7.14–7.20 (t, J = 8.1 Hz, 1H), 6.87 (d, J = 7.8 Hz, 1H), 6.82 (s, 1H), 6.76 (d, J = 8.1 Hz, 1H), 5.57 (s, 1H), 4.74–4.84 (t, J = 7.5 Hz, 2H), 3.68 (s, 3H), 3.42–3.47 (t, J = 7.5 Hz, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 181.9 (C=O), 180.0 (C=O), 159.7 (C=C–NO2), 158.8, 144.0 (Ar), 139.5 (C=C–N), 134.9, 134.4, 132.1, 131.1, 130.0, 127.0, 125.8, 124.6 (Ar), 122.9 (C=C–N), 120.9 (C–NO2), 115.0, 112.5 (Ar), 55.4 (OCH3), 54.2 (CH2N), 38.4 (CH2S), 29.9 (CH) ppm.
Cell lines and cell culture
Human non-small cell lung cancer A549 cells, human breast cancer MCF-7 cells, and prostate cancer PC3 cells were received from Pasture Institute, Tehran, Iran. MCF-7 cells were grown in RPMI 1640 medium, A549 cells and PC3 cells were grown in DMEM medium. All media contain 10% fetal bovine serum (FBS), penicillin G, streptomycin 100 µg/cm3 and 1% l-glutamine. The cells were cultured and incubated under humidified 5% CO2 atmosphere at 37 °C.
MTT assay
The effect of compound treatment on the viability of cancer cell lines was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide or MTT) assay (MTT assay kit, Bio IDEA, CatNo:BI1017, Iran) based on the ability of live cells to cleave the tetrazolium ring to a molecule that absorbs at 4900 nm as per the manufacturer’s instructions [29]. Etoposide (was kindly provided by Dr. A. Foroumadi, Tehran Medical Science University, Iran) and dimethyl sulfoxide (DMSO) were used as the positive and negative controls, respectively [30]. Briefly, cells were plated in 96-well culture plates (5 × 103 cells/well). After 24 h incubation, the cells were treated with different concentrations of the compounds. After 48 h at 37 °C, the medium was removed and 100 mm3 of MTT reagent (1 mg/cm3) was added to each well, and cells were further incubated at 37 °C for 4 h. The MTT solution was removed, 50 mm3 of DMSO was added to each well to dissolve formazan crystals, and the plates were gently shaken for 10 min, followed by reading with an ELISA plate reader (Biotek ELx 800, USA). The 50% inhibition concentration (IC50) was defined as the concentration that inhibited cell proliferation by 50% when compared to DMSO-treated cells (as negative control).
DAPI staining assay
DAPI staining assay was used to determine chromatin changes. MCF-7 cells, PC3 cells, and A549 cells were seeded in six well plates (5 × 104 cells/well) containing 12 mm coverslips and subsequently treated for 5e compound (sample or treated cells) and DMSO (control or untreated cells) for 24 h. Cells were then fixed with 3.7% paraformaldehyde, permeabilized in 0.5% (w/v) Triton X-100, and 1% BSA (w/v) for 5 min, washed in PBS, and stained with DAPI (Sigma-Aldrich, USA). All images were taken by an inverted fluorescent microscope (Nikon Eclipse Ti-E).
References
Rakitin OA (2009) Arkivoc i:129
Feng M, Tang B, Liang H, Jiang S (2016) Curr Top Med Chem 16:1200
Park H, Hwang KY, Oh KH, Kim YH, Lee JY, Kim K (2008) Bioorg Med Chem 16:284
El-Hag Ali GAM, Khalil AK, Lamphon RQ, El-Maghraby AA (2005) Phosphorus. Sulfur Silicon Relat Elem 180:1909
Boominathan M, Nagaraj M, Maheshwaran C, Muthusubramanian S, Bhuvanesh N (2014) J Heterocycl Chem 51:244
El-Gaby MSA, Al-Sehemi AG, Mohamed YA, Ammar YA (2006) Phosphorus. Sulfur Silicon Relat Elem 181:631
Shi F, Li C, Xia M, Miao K, Zhao Y, Tu S, Zheng W, Zhang G, Ning M (2009) Bioorg Med Chem Lett 19:5565
Acheson RM, Elmore NF (1978) Adv Heterocycl Chem 23:263
Khalifa NM, Adel AH, Abd-Elmoez SI, Fathalla OA, El-Gwaad AAA (2015) Res Chem Intermed 41:2295
Aberg V, Sellstedt M, Hedenström M, Pinkner JS, Hultgren SJ, Almqvist F (2006) Bioorg Med Chem 14:7563
Park H, Hwang KY, Oh KH, Kim YH, Lee JY, Kim K (2008) Bioorg Med Chem 16:284
Ali GH, Khalil A, Ahmed AHA, El-Gaby MSA (2002) Acta Chim Slov 49:365
Voskiene A, Sapijanskaite B, Mickevicius V, Jonuskiene I, Stasevych M, Komarovska-Porokhnyavets O, Musyanovych R, Novikov V (2012) Molecules 17:14434
Ferreira SB, da Silva FD, Bezerra FAFM, Lourenco MCS, Kaiser CR, Pinto AC, Ferreira VF (2010) Arch Pharmazie 343:81
Siegel RL, Miller KD, Jemal A (2017) CA Cancer J Clin 67:7
Perrino MP, del Villar-Guerra R, Sañudo MC, Calvo LA, González-Ortega A (2010) Tetrahedron 66:2815
Chen HL, Guo HY (2012) J Chem Res 36:162
Yıldırım M, Celikel D, Evis N, Knight DW, Kariuki BM (2014) Tetrahedron 70:5674
Dotsenko VV, Bushmarinov IS, Goloveshkin AS, Chigorina EA, Frolov KA, Krivokolysko SG (2017) Phosphorus, Sulfur Silicon Relat Elem 192
Bayat M, Nasri S, Notash B (2017) Tetrahedron 73:1522
Bayat M, Nasri S (2018) J Mol Struct 1154:366
Nasri S, Hosseini FS, Bayat M (2018) Tetrahedron 74:4409
Bayat M, Hosseini F, Notash B (2016) Tetrahedron Lett 57:5439
Noyori R (2005) Chem Commun 14:1807
Talaviya S, Majmudar F (2012) J Med Sci 1:7
Fan Y, Liu S, Chen N, Shao X, Xu X, Li Z (2015) Synlett 26:393
Yildirim M, Celikel D, Dürüst Y, Knight DW, Kariuki BM (2014) Tetrahedron 70:2122
Martins P, Jesus J, Santos S, Raposo L, Roma-Rodrigues C, Baptista P, Fernandes A (2015) Molecules 20:16852
Denizot F, Lang R (1986) J Immunol Methods 89:271
Mahdavi M, Dianat S, Khavari B, Moghimi S, Abdollahi M, Safavi M, Mouradzadegun A, Kabudanian Ardestani S, Sabourian R, Emami S, Akbarzadeh T, Shafiee A, Foroumadi A (2017) Chem Biol Drug Des 89:797
Acknowledgements
We are grateful to the University of Guilan and Imam Khomeini International University, Iran, for financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayat, M., Safari, F., Nasri, S. et al. A chemoselective synthesis and biological evaluation of novel benzo[g]thiazolo[3,2-a]quinolone derivatives. Monatsh Chem 150, 703–710 (2019). https://doi.org/10.1007/s00706-018-2337-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2337-1