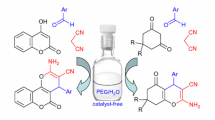
Abstract
The solvent-free cascade reaction of salicylaldehydes with two molecules of cyanoacetate results in the fast and efficient formation of substituted 2-amino-4H-chromenes in 88–98 % yields. The developed fast, solvent-free approach to substituted 2-amino-4H-chromenes—promising compounds for human inflammatory TNFα-mediated diseases and different biomedical applications—is beneficial from the viewpoint of diversity-oriented large-scale processes, and represents a facile, efficient, and environmentally benign synthetic concept for a solvent-free reaction strategy.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of “privileged medicinal scaffolds” has recently emerged as one of the guiding principles of drug discovery [1, 2]. Privileged scaffolds commonly consist of rigid ring systems, including hetero rings, that present appended residues in well-defined orientations required for target recognition [2, 3].
Functionalized chromenes have played an important role in the synthesis of promising compounds in the field of medicinal chemistry [4–6]. 2-Amino-4H-chromenes (or 2-amino-4H-benzo[b]pyranes) are of particular interest, as they belong to privileged medicinal scaffolds serving the generation of small-molecule ligands with highly pronounced spasmolitic, diuretic, anticoagulant, and antianaphylactic activities [7–9]. The current interest in 2-amino-4H-chromenes bearing nitrile functionality arises from their potential application in the treatment of human inflammatory TNFα-mediated diseases, such as rheumatoid and psoriatic arthritis, and in cancer therapy [10–13].
Recently, the progress in the field of solvent-free reactions has provided organic chemists with a new, simple and efficient synthetic method of great promise. This is connected with high efficiency and operational simplicity of the solvent-free processes [14]. The development of solvent-free organic synthesis methods has become an important research area. This is not only due to the need for more efficient and less labor-intense methodologies for the synthesis of organic compounds, but is also the consequence of the increasing importance of environmental considerations in chemistry. The elimination of volatile organic solvents in organic synthesis is also the most important goal in ‘green chemistry’.
Cascade reactions have been utilized as powerful methods to construct molecular complexity from readily available starting materials by combining two or more reactions into a single transformation [15]. As such, cascade reactions are of increasing importance in modern organic chemistry [16].
The implication of the solvent-free process in base-activated cascade reactions is highly promising, as it allows for the combination of the synthetic virtues of the conventional cascade strategy with the ecological benefits and convenience of the solvent-free procedure.
The only known solvent-free cascade reaction of salicylaldehydes with alkyl cyanoacetates has been carried out using a complex zirconium phosphate catalyst [17]. This method requires long reaction times (2–10 h) and a 60 °C reaction temperature; moreover, in the appreciable quantity of examples, the yield of substituted 2-amino-4H-chromenes was only in the range of 70 %.
The usual synthetic approaches to 2-amino-4H-chromenes from salicylaldehydes and alkyl cyanoacetates are also known and employ the reactions in alcohol catalyzed by ammonium acetate [18], or 3Ǻ molecular sieves [19]. Catalysis with ammonium acetate requires careful temperature control (5–10 °C) to ensure product selectivity, and the yields of desired product are only in the range of 40–80 % [18]. The implication of solid-phase catalysis with the use of 3Ǻ molecular sieves [19] is more convenient and results in the formation of corresponding 4H-chromene derivatives in 50–85 % yields, but requires a long reaction time (14 h). The electrocatalytic reaction of salicylaldehydes with cyanoacetates in ethanol has been carried out recently [20, 21] with the formation of substituted 2-amino-4H-chromenes in 83–91 % yields. The best yields in the range of 90–95 % were reported for the reaction of salicylaldehydes with ethyl cyanoacetates in ethanol with diethylamine as a catalyst (1.5–2.5 h) [22]. However, for this procedure [22], the reaction of salicylaldehyde with methyl cyanoacetate or other alkyl cyanoacetates was not reported. Moreover, the melting points of ethyl 2-amino-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylates were also not reported [22], thus it is not easy to estimate the purity of the compounds that were obtained by this method [22].
Recently, we have observed a solvent-free Knoevenagel reaction of aldehydes with malonitriles [23], as well as a fast (10 min), efficient, solvent-free cascade process for the transformation of salicylaldehydes and malononitrile into substituted 2-amino-4H-chromenes carried out in a mortar by grinding [24]. However, a fast and efficient, solvent-free process for the synthesis of the medicinally priviledged 2-amino-4H-chromene scaffold from salicylaldehydes and cyanoacetates is not yet known.
Thus, we were prompted to use a convenient and facile, solvent-free cascade methodology for the synthesis of the 2-amino-4H-chromene scaffold from salicylaldehydes and cyanoacetates.
Results and discussions
In the present study, we report our results on a study of solvent-free cascade transformation of salicylaldehydes 1a–1g and cyanoacetates 2a, 2b into substituted 2-amino-4H-chromenes 3a–3l under mild conditions (Scheme 1; Tables 1, 2).

First, to evaluate the synthetic potential of the procedure proposed and to optimize the general conditions, the solvent-free base-initiated cascade transformation of salicylaldehyde 1a and two equivalents of methyl cyanoacetate (2a) into 2-amino-4H-chromene 3a was studied under usual stirring conditions (method A; Table 1). The best yield of 2-amino-4H-chromene 3a (98 %) was achieved when the reaction was carried out in the presence of 10 mol% of KF at 20 °C, with a reaction time of 30 min (Table 1, entry 4).
Recently, we accomplished a solvent-free cascade and multicomponent assembling of salicylaldehydes and two equivalents of malononitrile in the presence of KF by grinding in a mortar (10 min reaction time) [24]. Thus, in the next step of our investigation, the solvent-free cascade transformation of salicylaldehyde 1a and cyanoacetate 2a into substituted 2-amino-4H-chromene 3a was accomplished by grinding in a mortar (method B; Table 2). And again, the best yield of 2-amino-4H-chromene 3a (97 %) was achieved when the reaction was carried out in the presence of 10 mol% of KF at 20 °C, but the reaction time in this case was only 15 min (Table 2, entry 5).
Under the optimal conditions thus found, salicylaldehydes 1a–1g and cyanoacetates 2a, 2b were transformed into corresponding substituted 2-amino-4H-chromenes 3a–3l in 88–98 % yields (Table 3). Generally, the yields of 2-amino-4H-chromenes 3a–3l obtained by grinding in the mortar were the same as those under usual stirring conditions, but the reaction time in this case (method B) was two times shorter (15 min instead of 30 min).
NMR data showed that the 4H-chromenes 3a–3l thus obtained were mixtures of two diastereoisomers. From a thermodynamic point of view, the more abundant isomer should have an erythro configuration (Scheme 2). A mixture of diastereomers 3b (2:1, Table 3) was crystallized from ethanol to isolate the major diastereoisomer [17]. The structure of the prevalent diastereoisomer was attributed to an erythro configuration by comparison with the data reported in the literature [22, 27].

With the above results taken into consideration and based on the mechanistic data on the solvent-free cascade process for the transformation of salicylaldehydes and malononitrile into substituted 2-amino-4H-chromenes [24], the following mechanism for the solvent-free cascade transformation of salicylaldehydes 1a–1g and cyanoacetates 2a, 2b into substituted 3a–3l is proposed. The initiation step of the catalytic cycle begins with the deprotonation of a molecule of cyanoacetate 2 by the action of potassium fluoride, which leads to the formation of an anion of cyanoacetate A (Scheme 3). The following process in the solution represents a typical cascade reaction. Knoevenagel condensation of the anion A with salicylaldehyde 1 takes place with the elimination of a hydroxide anion and the formation of Knoevenagel adduct [28], followed by cyclization and addition of the second cyanoacetate anion A, which gives the substituted 2-amino-4H-chromene 3 with regeneration of the cyanoacetate anion in the last stage. The cyanoacetate anion A continues the catalytic chain process by interaction with the next molecule of salicylaldehyde.

Thus, potassium fluoride as a catalyst can produce, under solvent-free, mild conditions, a fast and selective cascade transformation of salicylaldehydes and cyanoacetates into substituted 2-amino-4H-chromenes in excellent yields. The new, solvent-free cascade process opens an efficient and convenient potassium fluoride-catalyzed cascade to create corresponding substituted 2-amino-4H-chromenes—promising compounds for human inflammatory TNFα-mediated diseases, such as rheumatoid and psoriatic arthritis, and for application in cancer therapy. The catalytic procedure utilizes simple equipment; it is easily carried out and is valuable from the viewpoint of environmentally benign, diversity-oriented, large-scale processes. This efficient, potassium fluoride-catalyzed, solvent-free approach to substituted 2-amino-4H-chromenes represents a new synthetic concept for cascade reactions, and allows for the combination of the synthetic virtues of conventional cascade processes with ecological benefits and the convenience of a solvent-free procedure.
Experimental
All melting points were measured with a Gallenkamp melting-point apparatus. 1H and 13C NMR spectra were recorded in DMSO-d 6 and CDCl3 with a Bruker Avance II 300 spectrometer at ambient temperature. Chemical shift values are relative to Me4Si. IR spectra were recorded with a Bruker ALPHA-T FT-IR spectrometer in KBr pellets. All chemicals used in this study were commercially available.
General procedure
Salicylaldehyde (5 mmol), cyanoacetate (10 mmol), and sodium acetate or potassium fluoride (0.5 mmol) were stirred in a flask equipped with a magnetic stirrer at 20 °C for 30 min (method A) or grinded in mortar for 15 min (method B). After the reaction was finished, 2 cm3 of EtOH was added to obtain homogeneous suspension after stirring. Then ethanol was dried under reduced pressure. The solid was then rinsed through a filter with water (2 × 5 cm3) and dried.
Methyl 2-amino-4-(1-cyano-2-methoxy-2-oxoethyl)-8-ethoxy-4H-chromene-3-carboxylate (3f, C17H18N2O6)
Yield 92 %; m.p.: 149–151 °C; MS (EI, 70 eV): m/z (%) = 346 ([M]+, 3), 247 (25), 216 (8), 187 (100), 159 (59), 132 (6), 119 (8), 105 (16), 76 (41), 68 (81); IR (KBr): \( \bar{\nu } \) = 3,439, 3,320, 2,980, 2,248, 1,738, 1,683, 1,527, 1,436, 1,341, 1,094 cm−1.
Major diastereoisomer: 1H NMR (300 MHz, DMSO-d 6 ): δ = 1.38 (t, J = 7.2 Hz, 3H, CH3), 3.68 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 4.08–4.15 (m, 2H, OCH2), 4.38 (d, J = 3.8 Hz, 1H, CH), 4.52 (d, J = 3.8 Hz, 1H, CH), 6.55–6.58 (m, 1H, Ar), 7.02–7.06 (m, 2H, Ar), 7.86 (s, 2H, NH2) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 14.6, 36.5, 46.9, 50.8, 53.0, 64.3, 71.1, 113.3, 116.1, 118.9, 119.6, 124.4, 139.7, 146.4, 162.6, 165.6, 167.7 ppm.
Minor diastereoisomer: 1H NMR (300 MHz, DMSO-d 6 ): δ = 1.35 (t, J = 7.2 Hz, 3H, CH3), 3.59 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 4.08-4.17 (m, 3H, OCH2 and CH), 4.54 (d, J = 3.4 Hz, 1H, CH), 6.93 (d, J = 7.4 Hz, 1H, Ar), 7.10–7.15 (m, 2H, Ar), 7.88 (s, 2H, NH2) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 14.4, 36.8, 47.3, 50.6, 52.8, 64.1, 70.3, 113.1, 116.2, 121.2, 123.5, 124.7, 139.5, 146.1, 162.7, 165.4, 167.9 ppm.
Methyl 2-amino-6-chloro-4-(1-cyano-2-methoxy-2-oxoethyl)-4H-chromene-3-carboxylate (3g, C15H13ClN2O5)
Yield 93 %; m.p.: 126–128 °C; MS (EI, 70 eV): m/z (%) = 338 ([M]+, 1), 336 ([M]+, 3), 240 (35), 238 (71), 206 (37), 177 (20), 152 (12), 114 (35), 88 (22), 68 (51), 52 (100); IR (KBr): \( \bar{\nu } \) = 3,430, 3,312, 2,955, 2,249, 1,745, 1,686, 1,639, 1,522, 1,231, 1,078 cm−1.
Major diastereoisomer: 1H NMR (300 MHz, DMSO-d 6 ): δ = 3.68 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 4.42 (d, J = 3.8 Hz, 1H, CH), 4.56 (d, J = 3.8 Hz, 1H, CH), 7.09 (d, J = 2.1 Hz, 1H, Ar), 7.17 (d, J = 8.5 Hz, 1H, Ar), 7.40 (dd, J 1 = 8.5 Hz, J 2 = 2.1 Hz, 1H, Ar), 7.87 (s, 2H, NH2) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 36.1, 46.6, 50.8, 53.0, 70.6, 115.9, 117.9, 127.6, 128.4, 129.0, 129.2, 148.9, 162.3, 165.5, 167.4 ppm.
Minor diastereoisomer: 1H NMR (300 MHz, DMSO-d 6 ): δ = 3.60 (s, 3H, OCH3), 3.67 (s, 3H, OCH3), 4.29 (d, J = 3.2 Hz, 1H, CH), 4.58 (d, J = 3.2 Hz, 1H, CH), 7.13 (d, J = 8.6 Hz, 1H, Ar), 7.43 (dd, J 1 = 8.6 Hz, J 2 = 2.0 Hz, 1H, Ar), 7.53 (d, J = 2.0 Hz, 1H, Ar), 7.89 (s, 2H, NH2) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 36.3, 46.8, 50.7, 52.9, 69.9, 116.0, 117.6, 122.4, 123.7, 128.0, 128.2, 148.7, 162.5, 165.3, 167.6 ppm.
References
Evans BE, Rittle KE, Bock G, DiPardo RM, Freidinger FM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VG, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J (1988) J Med Chem 31:2235
Poupaert O, Carato P, Colacino E (2005) Curr Med Chem 12:877
Song Y, Zhan P, Zhang QZ, Liu XY (2013) Current Pharm Design 19:1528
Sun W, Cama LJ, Birzin ET, Warrier S, Locco L, Mosley R, Hammond ML, Rohrer SP (2006) Bioorg Med Chem Lett 16:1468
Stachulski AV, Berry NG, Low ACL, Moores S, Row E, Warhurst DC, Adagu IS, Rossignol JF (2006) J Med Chem 49:1450
Garino C, Bihel F, Pietrancosta N, Laras Y, Quéléver G, Woo I, Klein P, Bain J, Boucher JL, Kraus JL (2005) Bioorg Med Chem Lett 15:135
DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA (2004) Comb Chem High Throughput Screen 7:473
Patchett AA, Nargund RP (2000) Ann Rep Med Chem 35:289
Bonsignore L, Loy G, Secci D, Calignano A (1993) Eur J Med Chem 28:517
Skommer J, Wlodkowic D, Mättö M, Eray M, Pelkonen J (2006) Leukemia Res 30:322
Kemnitzer W, Kasibhatla S, Jiang S, Zhang H, Zhao J, Jia S, Xu L, Crogan-Grundy C, Denis R, Barriault N, Vaillancourt L, Charron S, Dodd J, Attardo G, Labrecque D, Lamothe S, Gourdeau H, Tseng B, Drewe J, Cai SX (2005) Bioorg Med Chem Lett 15:4745
Kemnitzer W, Drewe J, Jiang S, Zhang H, Wang Y, Zhao J, Jia S, Herich J, Labreque D, Storer R, Meerovitch K, Bouffard D, Rej R, Denis R, Blais C, Lamothe S, Attardo G, Gourdeau H, Tseng B, Kasibhatla S, Cai SX (2004) J Med Chem 47:6299
Gourdeau H, Leblond L, Hamelin B, Desputeau C, Dong K, Kianicka I, Custeau D, Bourdeau C, Geerts L, Cai SX, Drewe J, Labrecque D, Kasibhatla S, Tseng B (2004) Mol Cancer Ther 3:1375
Tanaka K (2003) Solvent-free organic synthesis. Wiley, Weinheim
Tietze LF (1996) Chem Rev 96:115
Grondal C, Jeanty M, Enders D (2010) Nat Chem 2:167
Curini M, Epifano F, Chimichi S, Montanari F, Nocchettic M, Rosatia O (2005) Tetrahedron Lett 46:3497
Fujimoto A, Sakurai A (1977) Synthesis 871
Yu N, Aramini JM, Germann MW, Huang Z (2000) Tetrahedron Lett 41:6993
Elinson MN, Dorofeev AS, Feducovich SK, Nasybullin RF, Gorbunov SV, Stepanov NO, Nikishin GI (2006) Tetrahedron Lett 47:7629
Feducovich SK, Elinson MN, Dorofeev AS, Gorbunov SV, Nasybullin RF, Stepanov NO, Vereshchagin AN, Nikishin GI (2008) Russ Chem Bull 2008:595
Kulkarni MA, Pandit KS, Desai UV, Lad UP, Prakash P, Wadgaonkar PP (2013) C R Chim 16:689
Elinson MN, Ilovaisky AI, Merkulova VM, Chizhov OA, Belyakov PA, Nikishin GI (2010) Tetrahedron 66:4043
Elinson MN, Medvedev MG, Ilovaisky AI, Merkulova VM, Zaimovskaya TA, Nikishin GI (2013) Mendeleev Commun 22:94
Roudier JF, Foucaud A (1984) Synthesis 159
Doshi JM, Tian D, Xing C (2006) J Med Chem 49:7731
Kokila MK, Nirmala KA, Puttaraja A, Kulkarni MV, Shivaprakash NC (1992) Acta Crystallogr C48:1619
Patai S, Israeli Y (1960) J Chem Soc 2025
Acknowledgments
The authors gratefully acknowledge the financial support of the Russian Foundation for Basic Research (Project No. 13-03-00096a).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elinson, M.N., Nasybullin, R.F., Ryzhkov, F.V. et al. Solvent-free cascade assembling of salicylaldehydes and cyanoacetates: fast and efficient approach to medicinally relevant 2-amino-4H-chromene scaffold. Monatsh Chem 145, 605–610 (2014). https://doi.org/10.1007/s00706-013-1147-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-013-1147-8