Abstract
The aim of the study was to determine the expression profiles of GABAA, GABAB, and GAT1 using RT-PCR and the immunoreactivity of GAT1 via immunohistochemical and immunofluorescence assays in CDV-infected brain tissue of dogs. For this purpose, dogs with CDV and dogs without CDV were selected. The mRNA transcript levels of GABAA, GABAB, and GAT1 were significantly downregulated in brain tissue in the CDV-infected group as compared with that in non-CDV-infected brain tissue in the control group (p < 0.01, p < 0.001). In addition, the immunoreactivity of GAT1 in CDV-infected brain tissue was significantly lower than in the uninfected group (p < 0.05). We conclude that one of the main causes of myoclonus in CDV infections may be the blockage of postsynaptic inhibition in neurons or a lack of metabolism of GABA. In addition, a GABA neurotransmission imbalance could play a role in demyelination in CDV infections.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
γ-Aminobutyric acid (GABA) is an important inhibitory neurotransmitter in the central nervous system. There are two types of GABA receptors: GABAA (an ionotropic receptor) and GABAB (a metabotropic receptor) [1,2,3,4]. GABA is delivered to synapses and binds to either GABAA or GABAB receptors. GABAA receptors play a role in the fast response of GABA via the Cl- channel, whereas GABAB receptors lead to slower responses of GABA via G-proteins and secondary messengers. GABAA receptors contain several subunits, including α, β, γ, δ, ε, π, and θ [5]. The most important of these subunits is the α subunit because it modulates the affinity of GABA [6]. GABAB receptors contain two subunits: GABAB1 and GABAB2 [7]. The GABAB1 subunit must be active in order to perform all the functions of GABAB receptors [8, 9]. The GABAB2 subunit plays an important role in surface trafficking and G-protein coupling [10, 11]. Genetic mutations of GABA receptors could be crucial risk factors for multiple sclerosis (MS). A previous study has indicated that GABA levels are lower in patients with MS. In MS caused by viruses, since viral infection can cause granule cell loss in the cerebellum, GABA receptor binding could be decreased [12,13,14].
γ-Aminobutyric acid transporter 1 (GAT1), regulated by SLC6A1, plays an important role in GABA transmission in the brain and it is part of GABA signaling. GAT1 is a sodium- and chloride-dependent transporter located in GABAergic axons and nerve terminals [15]. Instability in GABAergic neurotransmission is observed in some neurological diseases, such as epilepsy, Alzheimer’s disease, and strokes [16, 17]. Recent studies have suggested that mutations in SLC6A1 are related to epilepsy syndromes with myoclonic atonic epilepsy and intellectual disability [17,18,19].
Canine distemper virus (CDV), which belongs to the family Paramyxoviridae, is an infectious pathogen of carnivores, including Canidae, Procyonidae, Felidae, Mustelidae, and Mephitidae [20,21,22,23]. Infection is associated with severe clinical symptoms, including respiratory, gastrointestinal, and neurological symptoms [24,25,26]. Previous studies have found a relatively high mortality rate in CDV infections [27,28,29,30]. In recent years, there has been a strategy of routine vaccination against CDV infections [31], and vaccination with a modified live vaccine has decreased CDV infections in dogs and other carnivores. However, the vaccine is not 100% effective against CDV in susceptible species [32], with CDV infections occurring in some vaccinated animals [29].
CDV infections lead to immunosuppression and demyelinating leukoencephalitis (DL). Neuropathological changes in DL and human demyelinating diseases, such as multiple sclerosis, have morphological similarities [26]. Canine distemper is an important animal model to reveal the pathogenesis of myelin-loss-related immune-mediated mechanisms [33]. Previous studies have clarified the relationship between demyelination in MS and GABA receptors in detail [34,35,36,37,38,39]. However, to date, no studies have examined the relationship between GABA receptors and demyelination caused by CDV infection in dogs. Revealing this relationship may help to understand the mechanism of demyelination in CDV infections.
In the present study, we investigated histopathological changes and expression levels of GABAA, GABAB, and GAT1 in the brain tissue of dogs with and without CDV infection, using real-time polymerase chain reaction (RT-PCR), immunohistochemical, and immunofluorescence methods, respectively.
Materials and methods
Animals
This study included a total of 10 dogs (4 males and 6 females) of various breeds, weighing 8 ± 2 kg. The CDV-infected group (n = 5) comprised Kangal crossbreed dogs (3 females and 2 males) with involuntary twitching of their legs and facial muscles, and they were positive for the CDV antigen (Ag) when tested using a rapid test kit (catalog no. RG1303DD; CDV Ag Antigen Rapid Test Kit®, BioNote, Korea). The ages of the control and CDV-infected animals are shown in Table 1. If treatment was declined, the dogs were euthanized with the consent of the owners. The control group (n = 5) comprised golden “retriever” and Rottweiler dogs (3 females and 2 males) that had died as a result of trauma and were negative for CDV using the rapid test kit. Brain tissue was removed and placed in a formaldehyde solution for histological and immunohistochemical analysis and frozen at -80 °C for later analysis using the RT-PCR method. We investigated the same area of the brain for all experiments. The experiments were performed in accordance with the approved ethical rules of Atatürk University (protocol no. 2018/31).
Total RNA isolation and cDNA synthesis
Total RNA isolation from cortex and cerebellum parts of the brain tissue of dogs was carried out using TRIzol Reagent (catalog no. 15596026; Invitrogen, USA) according to the manufacturer’s instructions, and the RNA concentration was measured using a NanoDrop Epoch Microplate Spectrophotometer (USA). The quality of the total RNA preparation in terms of DNA contamination was then evaluated using gel electrophoresis. cDNA synthesis from total RNA was performed using a QuantiTect Reverse Transcription Kit (catalog no. 330411; QIAGEN, Germany) according to the manufacturer’s instructions [40, 41].
Detection of CDV
The RT-PCR method was used to detect CDV using a ROTOR-GENE Q 5plex HRM Real-Time PCR Detection System (QIAGEN, Germany). The primers used to detect CDV have been described previously [42] (Table 1). The RT-PCR was performed in a 25-µl reaction mix with 2x QuaniTect SYBR Green PCR Master Mix (catalog no. 330500; QIAGEN, Germany), according to the manufacturer’s instructions. The reaction mixture contained 12.5 µl of 2x QuaniTect SYBR Green PCR Master Mix (catalog no. 330500; QIAGEN, Germany), which contained HotStart DNA Taq polymerase, 0.75 µl (5 µM) of each primer, 1 µl (150 ng/µL) of cDNA as a template, and 10 µl of RNase and DNase-free water. The cycling parameters were as follows: 94 °C for 5 min; 30 cycles at 94 °C for 30 s, 55°C for 30 s, and 72 °C for 30 s; and 72 °C for 5 min [43].
Determination of mRNA transcript levels of GABAA, GABAB, and GAT1
The RT-PCR method was used to measure the mRNA transcript level of GABAA, GABAB, and GAT1 in brain tissue using a ROTOR-GENE Q 5plex HRM Real-Time PCR Detection System (QIAGEN, Germany). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control gene. RT-PCR primers were designed according to the sequence of Canine lupus familiaris using the primer design program Oligo 6.0 and Primer 5.0. All primer sequences and reaction conditions are shown in Table 2. The RT-PCR was performed in a volume of 25 µl containing 12.5 µl of 2x QuaniTect SYBR Green PCR Master Mix (catalog no. 330500; QIAGEN, Germany), which contained HotStart DNA Taq polymerase, 1 µl (5 µM) of each primer, 1 µl (150 ng/µL) of cDNA as a template, and 9.5 µl of RNase and DNase-free water. A reaction carried out without a cDNA sample was used as a negative control. The specificity of PCR amplification was confirmed by agarose gel electrophoresis and a melting curve analysis [40, 41]. The relative fold change in the gene expression level was assessed using the 2-ΔΔCT method [44].
Histopathological examination
Dog cerebral cortex and cerebellums were fixed in 10% buffered formalin solution for histopathological, immunohistochemistry, and immunofluorescence procedures, then passed through xylene and a graded series of ethanol, embedded in paraffin, and sectioned at 5-μm thickness. Slides were stained by hematoxylin and eosin for histopathological analysis. Sections were stained with Luxol fast blue (Blue Optica, catalog no. BO 04-200812) to determine demyelination according to the procedure of the manufacturer.
Determination of CDV infection using immunohistochemistry staining
Following deparaffinization and hydration, brain and cerebellum sections were stained using the streptavidin biotin-peroxidase complex (Histostain Plus Kit; Invitrogen, Camarillo, CA) to detect CDV infection. Sections were stained with anti-CDV (Serotec, MCA1893, Oxford, UK, dilution: 1/150). Treatment with the primary antibody was performed in a humidified chamber for 1 h at room temperature, and color formation was obtained using 3-amino-9-ethylcarbazole (AEC) (Invitrogen). Sections were counterstained with Mayer’s hematoxylin and mounted with CC/Mount Aqueous Mounting Medium (C9368; Sigma-Aldrich).
Determination of GAT/1 immunoreactivity in CDV-infected and uninfected animals using immunohistochemistry staining
Following deparaffinization and hydration, the cerebral cortex and cerebellum sections were treated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. For antigen retrieval, sections were heated in a microwave oven for 10 min in sodium citrate buffer (10 mM; pH 6.0). Tissue sections were then left in protein block solution for 10 min to block any nonspecific binding, followed by incubation at room temperature in GABA transporter 1/GAT1 primary antibody solution (Genetex, catalog no. GTX46843, dilution: 1/100) for 20 min. Subsequent incubations were carried out according to the procedure of the EXPOSE Mouse and Rabbit Specific HRP/DAB IHC Detection Kit (Abcam, catalog no. ab236466). The color formation was obtained using 3′,3′-diaminobenzidine, and the reaction was stopped by submersion in water. Sections were counterstained with Mayer’s hematoxylin. After dehydration, the sections were placed in xylene and mounted with a coverslip [40]. The cerebral cortex and cerebellum sections of five dogs without clinical or histopathological findings of distemper were used as negative controls. GAT1 immunopositivity was scored, and the results are shown in Table 3.
Immunofluorescence staining
The cerebral cortex and cerebellum were removed, and paraffin sections were prepared as described above. The immunofluorescent staining procedure was carried out as described in our previous study [45]. Following the deparaffinization, rehydration, antigen retrieval (10 mM sodium citrate buffer, pH 6.0), and protein blocking stages, GABA transporter 1/GAT1 primary antibody (Genetex, catalog no. GTX46843, dilution: 1/100) was applied to the cerebral cortex and cerebellum sections at room temperature for 20 min. Then, the secondary antibody, mouse anti-rabbit IgG-FITC (Santa Cruz Biotechnology, catalog no. sc-2359), was applied to the sections at a dilution of 1/50, and the sections were kept in the dark for 45 min and then washed with distilled water. Sections were mounted with Fluoroshield Mounting Medium with DAPI (catalog no. ab104140) and examined using a fluorescence microscope (Zeiss Scope A1).
Statistical analysis
IBM SPSS 20 was used to perform statistical analysis. A one-way analysis of variance was used to detect statistical differences in GABAA, GABAB, and GAT1 expression in the control group and CDV-infected group. The relative fold change in mRNA level was plotted using GraphPad Prism software, version 7.0 (GraphPad Software Inc., CA, USA). The RT-PCR results are expressed as mean ± standard error of the mean. Differences were considered statistically significant at p-values of < 0.05, < 0.01, and < 0.001. Statistical differences between the cerebellums of the controls and the CDV-infected dogs were evaluated using the Mann-Whitney U test followed by the Kruskal-Wallis Test (p < 0.05). SPSS statistical software (SPSS for windows, version 20.0) was used for statistical analysis. Data are presented as mean ± standard error (SE).
Results
Detection of CDV in brain tissue
The RT-PCR method was used to detect CDV in brain tissue of dogs. Brain tissues with CDV tested CDV positive, and brain tissues without CDV tested CDV negative (Table 4).
Expression profiles of GABAA, GABAB, and GAT1
The mRNA transcript levels of GABAA, GABAB, and GAT1 were determined using the RT-PCR method. Both GABAA and GABAB expression levels were significantly downregulated in the brain tissue in the CDV-infected group as compared with that in the control group (p < 0.01, p < 0.001) (Fig. 1A, B, and C).
The mRNA transcript level of GABAA, GABAB, and GAT1 in the brains of dogs. Values represent the mean ± SE of three independent experiments for each samples. Statistical significance (*, p < 0.05; **, p < 0.01; ***, p < 0.001) was analyzed using a one-way ANOVA. A) Relative mRNA expression levels of GABAA. B) Relative mRNA expression levels of GABAB. C) Relative mRNA expression levels of GAT1
Histopathological findings
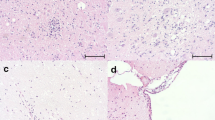
The diagnosis of CDV infection was first determined by clinical and histopathological examination. LFB staining was used to detect demyelinating lesions in CDV-infected dogs (Fig. 2D and F). In the control group, no histopathological changes were observed in the brain and cerebellum (Fig. 2A and B). Acute demyelinating lesions were characterized by severe demyelinated areas with vacuoles in the white matter of the cerebellum (Fig. 2C and D) and chromatolysis in Purkinje cells. Chronic demyelinating lesions exhibited lymphohistiocytic cell infiltration and severe demyelinated areas with vacuoles in the cerebral cortex (Fig. 2E and F).
(A-B) Normal histologic visualization of the cerebellar white matter of control dogs by hematoxylin and eosin and Luxol fast blue staining. H&E, Luxol fast blue. Bar, 50 μm. (C-F) Histopathologic imaging of the cerebral cortex and cerebellar tissues of CDV-infected dogs using hematoxylin and eosin staining. (C-D) Severe demyelinated areas (asterisk) with vacuoles (arrowhead) in acute distemper encephalitis. H&E, Luxol fast blue. Bar, 50 μm. (E-F) Chronic inflammatory lesion characterized by moderate lymphohistiocytic cells infiltration (arrow) and severe demyelinated areas with vacuoles (asterisk, arrowhead). H&E., Luxol fast blue. Bar, 100 μm
Immunolocalization of CDV infection
IHC staining of brain and cerebellum sections of dogs showed that the virus mostly infected neurons, with only a few types of glial cells staining positive for CDV (Fig. 3A–D). CDV antigen immunolabelling was observed in the nucleus and the cytoplasm of neurons in the cerebellum and cerebral cortex. Viral CDV antigens were mainly observed in neurons in the white matter of the cerebellum (Fig. 3A and B) and were also found in Purkinje cells. CDV antigens were also localized in neurons of the cerebral cortex (Fig. 3C and D).
Immunohistochemical analysis of the cerebellum and cerebral cortex of CDV-infected animals. (A-B) Intense staining in the cytoplasm of neurons (arrows) in the white matter of the cerebellum with CDV antigen. (C-D) Immunoreactivity to CDV antigen within the nucleus and cytoplasm of neurons (arrows) and glial cells (arrowhead) in the cerebral cortex. (A-C) Streptavidin-biotin-peroxidase method-AEC. (B-D) low-magnification view, 10x higher-magnification view, 20x
GAT/1 immunoreactivity in CDV-infected and uninfected animals
Within the Purkinje cells of the cerebellum in control dogs, GAT1 immunoreactivity occurred predominantly within the cytoplasm (Fig. 4A and B, Table 5). GAT1 immunoreactivity significantly decreased in cerebellar tissues of CDV-infected dogs (Fig. 4C and D, Table 5). Compared to the cerebellum, GAT1 immunoreactivity was significantly lower in the cerebral cortex (p < 0.05, Table 5). GAT1 immunoreactivity in the cerebral cortex of control dogs was higher than that of CDV-infected dogs (Fig. 5A-D, Table 5).
(A-D) Immunohistochemical and immunofluorescence analysis of the cerebellum of control dogs and CDV-infected dogs showing GAT1 immunoreactivity. (A-B) Intense GAT1 immunoreactivity surrounding Purkinje cell bodies (arrow) in the cerebellum of control dogs. (C-D) Mild GAT1 immunoreactivity surrounded the Purkinje cell bodies (arrow) in the cerebellum of CDV-infected dogs. IHC&IF. Bar, 50 μm
(A-D) Immunohistochemical and immunofluorescence analysis of the cerebral cortex of control dogs and CDV-infected dogs showing GAT1 immunoreactivity. (A-B) Intense punctate GAT1 staining in the cytoplasm of neurons (arrows) in the cerebral cortex of control dogs. (C-D) Mild punctate GAT1 staining in neurons (arrows) in the cerebral cortex of CDV-infected dogs. IHC&IF. Bar, 50 μm
Discussion
CDV leads to fatal DL in young dogs, similar to the demyelination observed in MS in humans [46]. Thus, CDV-induced DL can serve as an animal model for human demyelinating diseases such as MS [47, 48]. Axonal pathology with the accumulation of nonphosphorylated neurofilament proteins and β-amyloid precursor proteins is a hallmark of CDV‐induced DL [47, 49]. Although several studies have described the interactions between GABA receptors and MS in detail, there have been no studies on the molecular mechanisms of GABA receptors and GAT1 during CDV infections. This is an important knowledge gap in this field.
In this study, we investigated the expression profiles of GABAA, GABAB receptors and GAT1 and the immunoreactivity of GAT1 in the brain tissue of dogs with CDV. Our data demonstrated that the expression levels of GABAA, GABAB, and GAT1 were significantly downregulated in CVD-infected brain tissue as compared with that in uninfected brain tissue in the control group. In addition, the immunopositivity of GAT1 was reduced in CDV-infected brain tissue.
GABA regulates the proliferation, differentiation, and migration of oligodendrocyte precursor cells, as well as oligodendrocyte survival and myelination [50,51,52,53,54,55]. Deficits in the functional expression of GABAA receptors have been implicated in the pathogenesis of several neurological diseases [56,57,58]. For example, the expression of GABAA receptors has been shown to be downregulated in Alzheimer’s disease, Parkinson’s disease, and MS [59]. Furthermore, GABA sensitivity and GABAA expression levels were found to be attenuated in a myelin-deficient rat [60]. In addition, the expression level of GABAA has been observed to be downregulated in various infections, including HIV infection [61]. Influenza infection has been shown to result in a decrease in GABAA expression in mice [62], and the expression of GABAA receptor subunits α1 and β3 has been found to be decreased in chronic hepatitis C patients [63].
Myelin sheaths have been reported to be significantly altered in GAΒΑB-receptor-knockout mice. In the same study, the expression profiles of peripheral myelin protein-22 and myelin protein zero were also altered, and the myelin structures contained very small fibers [64]. Another study revealed that the GAΒΑB receptor is important for myelination of Schwann cells [65], while other studies have revealed that GAΒΑB receptors regulate myelination in the peripheral nervous system [66] and that they are involved in the regulation of myelin protein expression [67, 68]. Research has also demonstrated that GABAB receptors play a critical role in long-term inhibition of synaptic transmission [69].
GAT is synthesized in various membranes, including vesicle, presynaptic, and glial cell membranes, and it belongs to a family of electrogenic sodium-dependent transporters [70]. Four types of GABA transporters (GAT 1–4) have been reported, and their genes have been cloned [71, 72]. Among these receptors, GAT1 shows the highest affinity for GABA [72]. GAT1 is localized in GABAergic neurons, including the cerebellum, hippocampus, neocortex, and retina [73]. The primary role of GAT1 is the removal of GABA from the synaptic cleft and termination of GABAergic neurotransmission [74]. Thus, GAT1 plays a pivotal role in the metabolism of GABA [74]. In the present study, the immunoreactivity of GAT1 decreased in CDV-infected brain tissue, indicating that GABA transmission may be increased in the synaptic cleft. Therefore, GABA metabolism could be disrupted.
In the literature, it has been reported that GABAA and GABAB expression levels were significantly downregulated in infectious diseases and demyelinating diseases. To date, data on GABAA and GABAB receptor expression in CDV-infected dogs have not been reported. The present study is the first to report the downregulation of GABAA and GABAB in CDV-infected dogs. Regulation of GABAA and GABAB receptors may affect the speed of responses to GABA via Cl- channels, G-proteins, and secondary messengers. Thus, GABA function may be impaired, leading to an imbalance in GABAergic neurotransmission. This imbalance, together with the failure of long-term inhibition of synaptic transmission, may result in myoclonus, a neurological symptom of CDV infection. In addition, demyelination in CDV and decreased GABAA and GABAB receptor expression levels may be related. Therefore, decreased GABAA and GABAB receptor levels might contribute to the demyelination process in canine brains during CDV infection.
Myoclonus is a major neurological symptom in CDV-infected dogs. Many clinical phenotypes have been described in humans, the majority of which are associated with mutations in skeletal muscle voltage-gated chloride (CLCN1) and sodium channel (SCN4A) genes. In dogs, myotonia is related to mutations in CLCN1. Downregulation of GABAA, GABAB, and GAT1 disrupts GABA function, potentially blocking postsynaptic neuron inhibition and metabolism of GABA to glutamine by GABA transaminase for neuronal uptake. The findings of the present study suggest that one of the main causes of myoclonus in CDV infection may be the blockage of postsynaptic inhibition in neurons or a lack of metabolism of GABA.
Conclusion
In the present study, we examined the expression level of GABAA and GABAB receptors and GAT1 in CDV-infected and uninfected brain tissue from dogs, using RT-PCR, immunohistochemistry, and immunofluorescence assays. According to a literature survey, the present study is the first report in this field. The mRNA transcript levels of GABAA, GABAB and the immunoreactivity of GAT1 decreased significantly in brain tissue in the CDV-infected group as compared with that in the uninfected group. Our results suggest that one of the main causes of demyelination and myoclonus in CDV infections may be the disruption of GABA functions.
References
Bettler B, Tiao JY (2006) Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol Therap 110(3):533–543. https://doi.org/10.1016/j.pharmthera.2006.03.006
Bowery NG (2006) GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol 6(1):37–43. https://doi.org/10.1016/j.coph.2005.10.002
Couve A, Moss SJ, Pangalos MN (2000) GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci 16(4):296–312. https://doi.org/10.1006/mcne.2000.0908
Ulrich D, Bettler B (2007) GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr Opin Neurobiol 17(3):298–303. https://doi.org/10.1016/j.conb.2007.04.001
Mehta AK, Ticku MK (1999) An update on GABAA receptors. Brain Res Brain Res Rev 29(2–3):196–217
Olsen RW, Sieghart W (2008) International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev 60(3):243–260. https://doi.org/10.1124/pr.108.00505
Lopez-Bendito G, Shigemoto R, Kulik A, Vida I, Fairen A, Lujan R (2004) Distribution of metabotropic GABA receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus during prenatal and postnatal development. Hippocampus 14(7):836–848. https://doi.org/10.1002/hipo.10221
Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN (2001) Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol Cell Neurosci 17(6):1059–1070. https://doi.org/10.1006/mcne.2001.0995
Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B (2001) Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31(1):47–58. https://doi.org/10.1016/s0896-6273(01)00345-2
Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, Ristig D, Schuler V, Meigel I, Lampert C, Stein T, Prezeau L, Blahos J, Pin J, Froestl W, Kuhn R, Heid J, Kaupmann K, Bettler B (2001) C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J Neurosci 21(4):1189–1202
Marshall FH, Jones KA, Kaupmann K, Bettler B (1999) GABAB receptors—the first 7TM heterodimers. Trends Pharmacol Sci 20(10):396–399
Manyam NB, Katz L, Hare TA, Gerber JC, Grossman MH (1980) Levels of γ-aminobutyric acid in cerebrospinal fluid in various neurologic disorders. Arch Neurol 37(6):352–355
Sanders VJ, Felisan S, Waddell A, Tourtellotte WW (1996) Detection of herpesviridae in postmortem multiple sclerosis brain tissue and controls by polymerase chain reaction. J Neurovirol 2(4):249–258
Gade-Andavolu R, MacMurray JP, Blake H, Muhleman D, Tourtellotte W, Comings DE (1998) Association between the gamma-aminobutyric acid A3 receptor gene and multiple sclerosis. Arch Neurol 55(4):513–516. https://doi.org/10.1001/archneur.55.4.513
Kang JQ (2017) Defects at the crossroads of GABAergic signaling in generalized genetic epilepsies. Epilepsy Res 137:9–18. https://doi.org/10.1016/j.eplepsyres.2017.08.013
Lie MEK, Al-Khawaja A, Damgaard M, Haugaard AS, Schousboe A, Clarkson AN, Wellendorph P (2017) Glial GABA transporters as modulators of ınhibitory signalling in epilepsy and stroke. Adv Neurobiol 16:137–167. https://doi.org/10.1007/978-3-319-55769-4_7
Wu Z, Guo Z, Gearing M, Chen G (2014) Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s [corrected] disease model. Nat Commun 5:4159. https://doi.org/10.1038/ncomms5159
Carvill GL, McMahon JM, Schneider A, Zemel M, Myers CT, Saykally J, Nguyen J, Robbiano A, Zara F, Specchio N, Mecarelli O, Smith RL, Leventer RJ, Moller RS, Nikanorova M, Dimova P, Jordanova A, Petrou S, Helbig I, Striano P, Weckhuysen S, Berkovic SF, Scheffer IE, Mefford HC (2015) Mutations in the GABA transporter SLC6A1 cause epilepsy with myoclonic–atonic seizures. Am J Hum Genet 96(5):808–815. https://doi.org/10.1016/j.ajhg.2015.02.016
Johannesen KM, Gardella E, Linnankivi T, Courage C, de Saint Martin A, Lehesjoki AE, Mignot C, Afenjar A, Lesca G, Abi-Warde MT, Chelly J, Piton A, Merritt JL 2nd, Rodan LH, Tan WH, Bird LM, Nespeca M, Gleeson JG, Yoo Y, Choi M, Chae JH, Czapansky-Beilman D, Reichert SC, Pendziwiat M, Verhoeven JS, Schelhaas HJ, Devinsky O, Christensen J, Specchio N, Trivisano M, Weber YG, Nava C, Keren B, Doummar D, Schaefer E, Hopkins S, Dubbs H, Shaw JE, Pisani L, Myers CT, Tang S, Tang S, Pal DK, Millichap JJ, Carvill GL, Helbig KL, Mecarelli O, Striano P, Helbig I, Rubboli G, Mefford HC, Moller RS (2018) Defining the phenotypic spectrum of SLC6A1 mutations. Epilepsia 59(2):389–402. https://doi.org/10.1111/epi.13986
Pillet S, von Messling V (2009) Canine distemper virus selectively inhibits apoptosis progression in infected immune cells. J Virol 83(12):6279–6287. https://doi.org/10.1128/jvi.00050-09
Pratakpiriya W, Seki F, Otsuki N, Sakai K, Fukuhara H, Katamoto H, Hirai T, Maenaka K, Techangamsuwan S, Lan NT, Takeda M, Yamaguchi R (2012) Nectin4 is an epithelial cell receptor for canine distemper virus and involved in neurovirulence. J Virol 86(18):10207–10210. https://doi.org/10.1128/jvi.00824-12
Guvenc T, Yarim M, Gulbahar M, Kabak Y (2008) Immunohistochemical distribution of alpha B-crystallin in the cerebellum of dogs infected with canine distemper virus. Acta Vet Hung 56(1):117–123
Yarim M, Gulbahar MY, Guvenc T, Karahan S, Harada N, Kabak YB, Karayigit MO (2010) Aromatase expression in the cerebellum of the dog infected with canine distemper virus. Berl Munch Tierarztl Wochenschr 123(7–8):301–306
Zhao N, Li M, Luo J, Wang S, Liu S, Wang S, Lyu W, Chen L, Su W, Ding H, He H (2017) Impacts of canine distemper virus infection on the giant panda population from the perspective of gut microbiota. Sci Rep 7:39954. https://doi.org/10.1038/srep39954
Feng N, Yu Y, Wang T, Wilker P, Wang J, Li Y, Sun Z, Gao Y, Xia X (2016) Fatal canine distemper virus infection of giant pandas in China. Sci Rep 6:27518. https://doi.org/10.1038/srep27518
Beineke A, Puff C, Seehusen F, Baumgartner W (2009) Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet Immunol Immunopathol 127(1–2):1–18. https://doi.org/10.1016/j.vetimm.2008.09.023
Qiu W, Zheng Y, Zhang S, Fan Q, Liu H, Zhang F, Wang W, Liao G, Hu R (2011) Canine distemper outbreak in rhesus monkeys, China. Emerg İnfect Dis 17(8):1541–1543. https://doi.org/10.3201/eid1708.101153
Roelke-Parker ME, Munson L, Packer C, Kock R, Cleaveland S, Carpenter M, O’Brien SJ, Pospischil A, Hofmann-Lehmann R, Lutz H, Mwamengele GL, Mgasa MN, Machange GA, Summers BA, Appel MJ (1996) A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379(6564):441–445. https://doi.org/10.1038/379441a0
Ek-Kommonen C, Sihvonen L, Pekkanen K, Rikula U, Nuotio L (1997) Outbreak off canine distemper in vaccinated dogs in Finland. Vet Rec 141(15):380–383. https://doi.org/10.1136/vr.141.15.380
Williams ES, Thorne ET, Appel MJ, Belitsky DW (1988) Canine distemper in black-footed ferrets (Mustela nigripes) from Wyoming. J Wildl Dis 24(3):385–398. https://doi.org/10.7589/0090-3558-24.3.385
Blixenkrone-Moller M (1989) Detection of intracellular canine distemper virus antigen in mink inoculated with an attenuated or a virulent strain of canine distemper virus. Am J Vet Res 50(9):1616–1620
Barrett T (1999) Morbillivirus infections, with special emphasis on morbilliviruses of carnivores. Vet Microbiol 69(1–2):3–13. https://doi.org/10.1016/s0378-1135(99)00080-2
Baumgärtner W, Alldinger S (2005) The pathogenesis of canine distemper virus induced demyelination a biphasic process. In: Lavi LE, Constantinescu CS (eds) Experimental models of multiple sclerosis. Springer, New York, pp 871–888
Tian J, Dang H, Wallner M, Olsen R, Kaufman DL (2018) Homotaurine, a safe blood-brain barrier permeable GABAA-R-specific agonist, ameliorates disease in mouse models of multiple sclerosis. Sci Rep 8(1):16555. https://doi.org/10.1038/s41598-018-32733-3
Van Schependom J, Vidaurre D, Costers L, Sjogard M, D’Hooghe MB, D’Haeseleer M, Wens V, De Tiege X, Goldman S, Woolrich M, Nagels G (2019) Altered transient brain dynamics in multiple sclerosis: treatment or pathology? Hum Brain Mapp. https://doi.org/10.1002/hbm.24737
Kular L, Needhamsen M, Adzemovic MZ, Kramarova T, Gomez-Cabrero D, Ewing E, Piket E, Tegner J, Beck S, Piehl F, Brundin L, Jagodic M (2019) Neuronal methylome reveals CREB-associated neuro-axonal impairment in multiple sclerosis. Clin Epigenet 11(1):86. https://doi.org/10.1186/s13148-019-0678-1
Kiljan S, Prins M, Baselmans BM, Bol J, Schenk GJ, van Dam AM (2019) Enhanced GABAergic ımmunoreactivity in hippocampal neurons and astroglia of multiple sclerosis patients. J Neuropathol Exp Neurol 78(6):480–491. https://doi.org/10.1093/jnen/nlz028
Habermacher C, Angulo MC, Benamer N (2019) Glutamate versus GABA in neuron-oligodendroglia communication. Glia. https://doi.org/10.1002/glia.23618
Hundehege P, Fernandez-Orth J, Romer P, Ruck T, Muntefering T, Eichler S, Cerina M, Epping L, Albrecht S, Menke AF, Birkner K, Gobel K, Budde T, Zipp F, Wiendl H, Gorji A, Bittner S, Meuth SG (2018) Targeting voltage-dependent calcium channels with pregabalin exerts a direct neuroprotective effect in an animal model of multiple sclerosis. Neuro-Signals 26(1):77–93. https://doi.org/10.1159/000495425
Comakli S, Ozdemir S (2019) Comparative evaluation of the ımmune responses in cattle mammary tissues naturally ınfected with bovine parainfluenza virus Type 3 and bovine alphaherpesvirus-1. Pathogens (Basel, Switzerland) 8:1. https://doi.org/10.3390/pathogens8010026
Ozdemir S, Comakli S (2018) Investigation of the interaction between bta-miR-222 and the estrogen receptor alpha gene in the bovine ovarium. Reprod Biol 18(3):259–266. https://doi.org/10.1016/j.repbio.2018.06.006
Liu DF, Jiang YT, Yang TK, Lin H, Liu CG, Chai HL, Wang C, Cui Y, Jiang XF, Ma XQ, Liu DC, Hua YP, Qu LD, Zhang HY (2011) Establishment of the duplex PCR for the detection of canine distemper virus and canine parvovirus. Chin Pre Vet Med 34:211–213
Liu D, Liu F, Guo D, Hu X, Li Z, Li Z, Ma J, Liu C (2019) One-step triplex PCR/RT-PCR to detect canine distemper virus, canine parvovirus and canine kobuvirus. J Vet Med Sci 81(7):1040–1042. https://doi.org/10.1292/jvms.17-0442
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Çomaklı S, Sağlam YS, Timurkan MÖ (2019) Comparative detection of bovine herpesvirus-1 using antigen ELISA, immunohistochemistry and immunofluorescence methods in cattle with pneumonia. Turk J Vet Anim Sci 43(3):306–313
Klemens J, Ciurkiewicz M, Chludzinski E, Iseringhausen M, Klotz D, Pfankuche VM, Ulrich R, Herder V, Puff C, Baumgartner W, Beineke A (2019) Neurotoxic potential of reactive astrocytes in canine distemper demyelinating leukoencephalitis. Sci Rep 9(1):11689. https://doi.org/10.1038/s41598-019-48146-9
Seehusen F, Baumgartner W (2010) Axonal pathology and loss precede demyelination and accompany chronic lesions in a spontaneously occurring animal model of multiple sclerosis. Brain Pathol (Zurich, Switzerland) 20(3):551–559. https://doi.org/10.1111/j.1750-3639.2009.00332.x
Spitzbarth I, Baumgartner W, Beineke A (2012) The role of pro- and anti-inflammatory cytokines in the pathogenesis of spontaneous canine CNS diseases. Vet Immunol Immunopathol 147(1–2):6–24. https://doi.org/10.1016/j.vetimm.2012.04.005
Imbschweiler I, Seehusen F, Peck CT, Omar M, Baumgartner W, Wewetzer K (2012) Increased p75 neurotrophin receptor expression in the canine distemper virus model of multiple sclerosis identifies aldynoglial Schwann cells that emerge in response to axonal damage. Glia 60(3):358–371. https://doi.org/10.1002/glia.22270
Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C (2010) P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia 58(6):730–740. https://doi.org/10.1002/glia.20958
Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C (2013) A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61(5):732–749. https://doi.org/10.1002/glia.22469
Fannon J, Tarmier W, Fulton D (2015) Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 63(6):1021–1035. https://doi.org/10.1002/glia.22799
Hamilton NB, Clarke LE, Arancibia-Carcamo IL, Kougioumtzidou E, Matthey M, Karadottir R, Whiteley L, Bergersen LH, Richardson WD, Attwell D (2017) Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia 65(2):309–321. https://doi.org/10.1002/glia.23093
Miguel-Hidalgo JJ (2018) Molecular neuropathology of astrocytes and oligodendrocytes in alcohol use disorders. Front Mol Neurosci 11:78. https://doi.org/10.3389/fnmol.2018.00078
Yung SY, Gokhan S, Jurcsak J, Molero AE, Abrajano JJ, Mehler MF (2002) Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species. Proc Natl Acad Sci USA 99(25):16273–16278. https://doi.org/10.1073/pnas.232586699
Watanabe M, Fukuda A (2015) Development and regulation of chloride homeostasis in the central nervous system. Front Cell Neurosci 9:371. https://doi.org/10.3389/fncel.2015.00371
Rudolph U, Knoflach F (2011) Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discovery 10(9):685–697. https://doi.org/10.1038/nrd3502
Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J (2014) Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15(10):637–654. https://doi.org/10.1038/nrn3819
Luchetti S, Huitinga I, Swaab DF (2011) Neurosteroid and GABA-A receptor alterations in Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. Neuroscience 191:6–21. https://doi.org/10.1016/j.neuroscience.2011.04.010
Lim JY, Utzschneider DA, Sakatani K, Kocsis JD (1993) The attenuation of GABA sensitivity in the maturing myelin-deficient rat optic nerve. Brain Res Dev Brain Res 72(1):15–20. https://doi.org/10.1016/0165-3806(93)90155-4
Buzhdygan T, Lisinicchia J, Patel V, Johnson K, Neugebauer V, Paessler S, Jennings K, Gelman B (2016) Neuropsychological, neurovirological and neuroimmune aspects of abnormal GABAergic transmission in HIV ınfection. J Neuroimmune Pharmacol 11(2):279–293. https://doi.org/10.1007/s11481-016-9652-2
Sanders RD, Grover V, Goulding J, Godlee A, Gurney S, Snelgrove R, Ma D, Singh S, Maze M, Hussell T (2015) Immune cell expression of GABAA receptors and the effects of diazepam on influenza infection. J Neuroimmunol 282:97–103. https://doi.org/10.1016/j.jneuroim.2015.04.001
Sidorkiewicz M, Brocka M, Bronis M, Grek M, Jozwiak B, Piekarska A, Bartkowiak J (2012) The altered expression of alpha1 and beta3 subunits of the gamma-aminobutyric acid A receptor is related to the hepatitis C virus infection. Eur J Clin Microbiol İnfect Dis 31(7):1537–1542. https://doi.org/10.1007/s10096-011-1475-8
Magnaghi V, Ballabio M, Camozzi F, Colleoni M, Consoli A, Gassmann M, Lauria G, Motta M, Procacci P, Trovato AE, Bettler B (2008) Altered peripheral myelination in mice lacking GABAB receptors. Mol Cell Neurosci 37(3):599–609. https://doi.org/10.1016/j.mcn.2007.12.009
Faroni A, Melfi S, Castelnovo LF, Bonalume V, Colleoni D, Magni P, Arauzo-Bravo MJ, Reinbold R, Magnaghi V (2019) GABA-B1 receptor-null Schwann cells exhibit compromised ın vitro myelination. Mol Neurobiol 56(2):1461–1474. https://doi.org/10.1007/s12035-018-1158-x
Faroni A, Castelnovo LF, Procacci P, Caffino L, Fumagalli F, Melfi S, Gambarotta G, Bettler B, Wrabetz L, Magnaghi V (2014) Deletion of GABA-B receptor in Schwann cells regulates remak bundles and small nociceptive C-fibers. Glia 62(4):548–565. https://doi.org/10.1002/glia.22625
Magnaghi V (2007) GABA and neuroactive steroid interactions in glia: new roles for old players? Curr Neuropharmacol 5(1):47–64
Magnaghi V, Ballabio M, Cavarretta IT, Froestl W, Lambert JJ, Zucchi I, Melcangi RC (2004) GABAB receptors in Schwann cells influence proliferation and myelin protein expression. Eur J Neurosci 19(10):2641–2649. https://doi.org/10.1111/j.0953-816X.2004.03368.x
Jones TL, Sweitzer SM, Peters MC, Wilson SP, Yeomans DC (2005) GABAB receptors on central terminals of C-afferents mediate intersegmental Adelta-afferent evoked hypoalgesia. Eur J Pain (London, England) 9(3):233–242. https://doi.org/10.1016/j.ejpain.2004.06.004
Kanner BI (2006) Structure and function of sodium-coupled GABA and glutamate transporters. J Membr Biol 213(2):89–100. https://doi.org/10.1007/s00232-006-0877-5
Liu QR, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N (1993) Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain [corrected]. The Biol Chem 268(3):2106–2112
Chiu CS, Jensen K, Sokolova I, Wang D, Li M, Deshpande P, Davidson N, Mody I, Quick MW, Quake SR, Lester HA (2002) Number, density, and surface/cytoplasmic distribution of GABA transporters at presynaptic structures of knock-in mice carrying GABA transporter subtype 1-green fluorescent protein fusions. J Neurosci 22(23):10251–10266
Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI (1990) Cloning and expression of a rat brain GABA transporter. Science (New York, NY) 249(4974):1303–1306. https://doi.org/10.1126/science.1975955
Keros S, Hablitz JJ (2005) Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol 94(3):2073–2085. https://doi.org/10.1152/jn.00520.2005
Acknowledgements
We thank Ataturk University Eastern Anatolia Advanced Technology Application and Research Center (DAYTAM), Prof. Dr. Tolga Güvenç, and Dr. Mustafa Özkaraca for their support. We did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SÇ designed and organized the study. SÇ, SÖ and ŞD contributed to the planning, designing and analyses of the experiments, data collection and quality control. SÇ and SÖ performed the statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Ana Cristina Bratanich.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Çomakli, S., Özdemir, S. & Değirmençay, Ş. Canine distemper virus induces downregulation of GABAA,GABAB, and GAT1 expression in brain tissue of dogs. Arch Virol 165, 1321–1331 (2020). https://doi.org/10.1007/s00705-020-04617-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-020-04617-3