Abstract
Infection of chickens with virulent Newcastle disease virus (NDV) is associated with severe pathology and increased morbidity and mortality. The innate immune response contributes to the pathogenicity of NDV. As professional antigen-presenting cells, dendritic cells (DCs) play a unique role in innate immunity. However, the contribution of DCs to NDV infection has not been investigated in chickens. In this study, we selected two representative NDV strains, i.e., the velogenic NDV strain Chicken/Guangdong/GM/2014 (GM) and the lentogenic NDV strain La Sota, to investigate whether NDVs could infect LPS-activated chicken bone-derived marrow DCs (mature chicken BM-DCs). We compared the viral titres and innate immune responses in mature chicken BM-DCs following infection with those strains. Both NDV strains could infect mature chicken BM-DC, but the GM strain showed stronger replication capacity than the La Sota strain in mature chicken BM-DCs. Gene expression profiling showed that MDA5, LGP2, TLR3, TLR7, IFN-α, IFN-β, IFN-γ, IL-1β, IL-6, IL-18, IL-8, CCL5, IL-10, IL-12, MHC-I, and MHC-II levels were altered in mature DCs after infection with NDVs at all evaluated times postinfection. Notably, the GM strain triggered stronger innate immune responses than the La Sota strain in chicken BM-DCs. However, both strains were able to suppress the expression of some cytokines, such as IL-6 and IFN-α, in mature chicken DCs at 24 hpi. These data provide a foundation for further investigation of the role of chicken DCs in NDV infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Newcastle disease virus (NDV) is a member of the family Paramyxoviridae, genus Avulavirus, and can cause Newcastle disease (ND) [2]. Despite vaccination and culling of infected birds in most countries, ND outbreaks occur frequently, particularly in developing countries, including China [11, 48, 55]. NDVs have a negative-sense, non-segmented, single-stranded RNA genome of approximately 15.2 kb that encodes six structural proteins, including hemagglutinin-neuraminidase (HN), nucleoprotein (NP), fusion protein (F), phosphoprotein (P), matrix (M), RNA-dependent RNA polymerase (L), and two non-structural proteins (V and W proteins) [4, 5]. Based on the cleavage site of the F protein and their pathogenicity in chickens, NDV strains are classified as velogenic, mesogenic, or lentogenic [33]. Virulent strains produce a severe systemic disease with high mortality in chickens [25].
Since the first infectious NDV derived from cloned cDNA was recovered, several studies have shown that the virulence of NDV is determined by multiple genes in the viral genome [12, 34, 35]. NDV has been shown to initiate the host innate immune response in different susceptible birds, such as chickens, pigeons, ducks, and geese [23, 26, 41, 51]. In several in vivo studies, strong immune responses were observed in the spleens of chickens following infection with virulent NDV, and the expression levels of type I and II interferons (IFN-α, IFN-β, and IFN-γ), cytokines (i.e., IL-6 and IL-1β), chemokines (i.e., IL-8 and CCLi3), and inducible nitric oxide synthase (iNOS) were significantly increased [10, 13, 39, 41]. It has therefore been concluded that NDV is involved in modulation of cytokine responses in the peripheral blood and thymus of NDV-infected chickens [21, 30]. Consistent with these in vivo results, NDV has also been shown to induce cytokine expression in several cell lines, including chicken embryonic fibroblasts (CEFs) and chicken splenic leukocytes (i.e., IFNs, IL-1β, and IL-6), chicken macrophages (i.e., IFN-α and IFN-β), peripheral blood mononuclear cells (PBMCs; i.e., IFN-γ and iNOS) [1, 20, 24, 27, 41]. These findings highlight that the host innate immune response induced by NDV may be associated with its pathogenicity.
DCs are not only adept at antigen processing and presentation but also secrete cytokines, such as type I and II interferons, and activate natural killer (NK) and NKT cells in response to microbial infection [44]. The key roles of DCs in the immune response highlight their importance in defending the host against foreign pathogens, such as viruses. In the lungs, chicken DCs are involved in uptake of bacterial or viral antigens, indicating that chickens DCs may have an important role in initiation of the host innate immune response against respiratory virus infections, such as NDV and avian influenza virus (AIV) [8]. After the first functional chicken-derived DCs were produced in vitro in 2010 [49], subsequent studies have shown that highly pathogenic avian influenza viruses (HPAIVs) can elicit a strong innate immune response and enhance the activation of DCs, which may cause deregulation of the immune response [47]. However, the role of mature chicken DCs in response to NDVs is still not clear.
To investigate the role of mature chicken DCs in NDV infection, we compared the replication ability of NDV strains with differing pathogenicity and the immune response in mature chicken BM-DCs following infection with velogenic and lentogenic NDV strains. These data are expected to improve our understanding of the role of DCs in chickens following infection with NDV.
Materials and methods
Animals, viruses, and cells
Ten-day-old specific-pathogen-free (SPF) chicken eggs and 1-day-old SPF chickens were supplied by Guangdong Wens Dahuanong Biotechnology Co., Ltd. (China). Two NDV strains, the velogenic strain GM (GenBank accession number DQ486859) and the lentogenic strain La Sota (GenBank accession number AF077761), were preserved in our laboratory (National and Regional Joint Engineering Laboratory for Medicament of Zoonosis Prevention and Control, China) as described previously [27]. The viruses were propagated in 10-day-old SPF embryonated chicken eggs and stored at − 80 °C until further use. Chicken BM-DCs were isolated and cultured as described previously [28, 29]. In brief, BM-DCs were generated from 7- to 10-day-old SPF chicken bone marrow and cultured in complete RPMI-1640 medium (Gibco, CA, USA) supplemented with 10% foetal bovine serum (BI), 1% penicillin-streptomycin, and 30–50 ng of recombinant human granulocyte-macrophage colony-stimulating factor and recombinant human interleukin (IL)-4 (Pepro Tech, NJ, USA) per mL at 39 °C in an atmosphere containing 5% CO2. After 7 days of culture, these cells were stimulated with lipopolysaccharide (LPS) for 24 h. Subsequently, chicken BM-DCs were identified using an FC500 flow cytometer (Beckman Coulter, Brea, CA, USA) with PE-conjugated anti-mouse CD11c and FITC-conjugated anti-mouse CD86 monoclonal antibodies (Affymetrix eBioscience, CA, USA). Primary CEFs were obtained from 10-day-old SPF embryonated chicken eggs as described previously [42].
Infection of mature chicken BM-DCs with NDV
After stimulation with LPS for 24 h, large veils (a sign of maturation) were observed in most of the cells. These mature BM-DCs were then infected with the NDV strain GM or La Sota at a multiplicity of infection (MOI) of 0.1 in serum-free medium for 1 h at 39 °C in an atmosphere containing 5% CO2. The cells were then washed three times with phosphate-buffered saline (PBS) and cultured in growth medium containing 2% inactivated chicken serum. The supernatants and cell lysates from NDV-infected BM-DCs and mock-infected BM-DCs were harvested at 6, 12, 24, 36, and 48 h postinfection (hpi).
Western blotting
Total protein samples from the culture were extracted, separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis on 10% gels, and transferred to 0.45-μm nitrocellulose membranes (Millipore, USA). Membranes were blocked with 7.5% non-fat dry milk at 37 °C for 1 h and then incubated overnight at 4 °C with antibodies against NP (Zoonogen, Beijing, China) or mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies (Beyotime Inst Biotech, Shanghai, China). The membranes were washed three times with Tris-buffered saline containing 0.05% Tween-20 (TBST) and incubated with IRDye 800CW goat anti-rabbit or anti-mouse IgG (1:10, 000; Rockland Immunochemical, Limerick, PA, USA) at 37 °C for 1 h. Finally, the membranes were washed three times with TBST and visualized using an Odyssey Infrared Imaging system (LI-COR Biosciences, Lincoln, NE, USA).
RNA isolation and cDNA synthesis
Total RNA was collected from cells using an RNA extraction kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. All purified RNA samples were assayed for quantity and quality using an Ultrospec 2000 mass spectrophotometer (Pharmacia Biotech, Uppsala, Sweden). Subsequently, approximately 1 μg of RNA was reverse transcribed into cDNA using a SuperScript III First-Strand Synthesis System (Takara) according to the manufacturer’s protocol and stored at −20 °C until further analysis.
Growth characteristics of the two NDV strains in mature chicken BM-DCs
To compare the replication ability of the GM and La Sota strains in mature chicken BM-DCs, the viral titres in cell supernatants from BM-DCs after infection with GM or La Sota strains at 6, 12, 24, 36, and 48 hpi were determined using a hemagglutination (HA) test and expressed in terms of the 50% tissue culture infective dose (TCID50) on CEFs as previously described [27].
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis
qRT-PCR was performed to measure the RNA levels of NDV M gene and mRNA levels of pattern recognition receptors (PRRs), cytokines, chemokines, and MHC molecules using SYBR Premix Ex Taq (TaKaRa) with a 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Primers (Table 1) used in this study for qRT-PCR were selected from previous studies and synthesised by Life Technologies. The following PCR conditions were utilized: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s. To confirm the specificity of the SYBR Green PCR signal, dissociation curves were carefully analysed, and purified amplified products were cloned into the pMD19-T vector and sequenced.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of IL-6 and IL-8 in culture supernatants from NDV and mock-infected mature chicken BM-DCs at 36 hpi were determined using commercial sandwich ELISA kits (Boster, Beijing, China) according to the manufacturer’s instructions.
Statistical analysis
The data are presented as the mean ± standard deviation (SD). Statistical analysis of NDV titre data was conducted using Student’s t-test for pairwise comparisons, and differences in the expression levels of immune molecules were analysed using analysis of variance (ANOVA) with Dunn’s multiple comparison test in GraphPad Prism 5 (GraphPad, Inc., San Diego, CA, USA). The relative expression levels of the genes were calculated by normalizing the levels of the target genes to that of GAPDH using the formula 2−ΔΔCt [31]. Differences with p-values less than 0.05 were considered significant. The data were confirmed in at least three independent experiments.
Results
Confirmation of NDV infection in cultured mature chicken BM-DCs
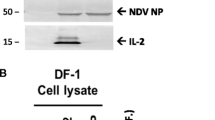
In our culture system, over 80% of the cells in LPS-treated BM-DCs displayed double-positive staining for CD11c and CD86, in flow cytometric analysis, indicating maturation of the DCs, while this was observed in only 32.6% of mock-treated BM-DCs (Fig. 1A). To investigate whether NDVs can infect mature chicken BM-DCs, we inoculated LPS-treated BM-DCs with NDV strain GM or La Sota at an MOI of 0.1 and observed morphological changes at 36 hpi. As shown in Figure 1B, an obvious cytopathic effect (CPE), including syncytium formation, and detachment, was observed in mature chicken BM-DCs following infection with the virulent GM strain; however, no obvious CPE was observed in BM-DCs after infection with the avirulent strain La Sota. Meanwhile, the expression of the NP protein of NDV was detectable at 36 hpi in NDV-infected cells, but not in mock-infected cells (Fig. 1C).
Comparison of multicycle growth kinetics of GM and La Sota strains in mature chicken BM-DCs. (A) Expression of the surface antigens CD11c and CD86 on non-stimulated and LPS-stimulated BM-DCs. (B) Morphological changes in chicken DCs infected with La Sota and GM (36 hpi; ×100). (C) Western blot analysis of NP expression in mature chicken BM-DCs infected with GM and La Sota at 36 hpi. (D) Viral titers of culture supernatants from mature chicken DCs infected with GM or La Sota at an MOI of 0.1 determined in CEFs by hemagglutination (HA) tests. (E) Viral M RNA expression measured by qRT-PCR at 6, 12, 24, and 36 h after inoculation with GM or La Sota. The data are shown as the mean ± standard deviation (SD) from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001
To compare the proliferation of different NDV strains in mature chicken DCs, the multicycle growth kinetics of the GM and La Sota strains were determined by measuring the number of TCID50 units in CEFs. The viral titer of the GM strain was 103.94 TCID50 at 12 hpi, and the highest viral titer was 107.29 TCID50 at 36 hpi, while those of the La Sota strain were 102.42 TCID50 and 103.97 TCID50, respectively (Fig. 1D). In addition, the mRNA expression levels of the M gene of the GM and La Sota strains in BM-DCs, as determined by qRT-PCR, were consistent with the results of virus titration assays (Fig. 1E). In summary, these results suggested that the NDV strains infected mature chicken BM-DCs efficiently in vitro and that the highly virulent strain GM had a significantly higher replication capacity in mature chicken DCs than the low-virulent strain La Sota.
Expression of PRRs in NDV-infected mature chicken BM-DCs
Previous studies have confirmed that PRRs, particularly Toll-like receptors (i.e., TLR3 and TLR7) and RIG-I-like receptors (i.e., MDA5 and LGP2), are involved in recognition of NDV infection [6, 46, 52]. In this study, we analysed the expression levels of PRRs (TLR3, TLR7, MDA5, and LGP2) in mature chicken BM-DCs after infection with the GM or La Sota strain at 6, 12, 24, and 36 hpi. The expression levels of MDA5 and TLR3 were increased throughout the period of infection, except that induced by GM at 6 hpi (0.23- and 0.21-fold, respectively), after infection with both viruses (Fig. 2A and C). Notably, the expression levels of MDA5 showed the highest upregulation (1316.39- and 328.61-fold; p < 0.001) at 36 hpi when induced by the GM and La Sota strain, respectively. Compared with the mock-infected control, the expression level of TLR7 was significantly increased by infection with the GM strain at 6 and 12 hpi (5.36- and 2.31-fold, respectively; p < 0.01) but was downregulated at 24 and 36 hpi (0.43- and 0.31-fold, respectively). In contrast, TRL7 was maintained at baseline levels at 6 and 12 hpi and decreased at 24 and 36 hpi (0.15- and 0.57-fold, respectively) after induction by the La Sota strain (Fig. 2D). The expression levels of LGP2 were significantly elevated at 12 and 24 hpi in response to infection by the GM or La Sota strain but were decreased in the GM-infected group at 6 hpi (0.36-fold) and the La Sota-infected group at 36 hpi (0.82-fold; Fig. 2B). Thus, our results suggest that TLR3, TLR7, MDA5, and LGP2 might be involved in the response to NDV infection in mature chicken BM-DCs, with MDA5 and TLR3 having more-important roles in this process.
Expression of mRNA for MDA5 (A), LGP2 (B), TLR3 (C), and TLR7 (D) in mature chicken BM-DCs after 6, 12, 24, and 36 h infection with GM or La Sota, was determined by qRT-PCR. The y-axis represents the fold change of the target gene in the NDV-infected groups versus that in the mock-infected group. The data are shown as the mean ± SD from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001
Expression of antiviral-related innate immune genes in NDV-infected mature chicken BM-DCs
The IFN system plays a key role in host defence systems against viral infection, such as inhibition of viral replication and modulation of innate immune responses [19, 38, 54]. In the present study, we compared the expression levels of IFN-I (IFN-α and IFN -β) and IFN-II (IFN-γ) in mature chicken BM-DCs after infection with the GM or La Sota strain. Notably, IFN-α expression induced by the GM strain was upregulated during the study period, except at 24 hpi (0.46-fold); peak expression was observed at 12 hpi (11.07-fold, p < 0.001; Fig. 3A). In contrast, moderate increases were observed at 12 hpi (1.47-fold) and 36 hpi (7.69-fold; p < 0.001) with decreases at 6 hpi (0.33-fold) and 24 hpi (0.09-fold) in La Sota-infected cells (Fig. 3A). However, the expression levels of IFN-β and IFN-γ showed different patterns in mature chicken DCs when induced by both viruses. As shown in Fig. 3B and C, compared with the mock-infected control group, IFN-β and IFN-γ were significantly upregulated at 12 hpi (45.04- and 4019.71-fold, respectively; p < 0.001), subsequently decreased at 24 hpi, and then again increased at 36 hpi (7.46- and 991.43-fold, respectively), with a peak at 12 hpi when induced by the GM strain; however, the expression levels of IFN-β and IFN-γ were slightly increased at 6 hpi, peaked at 12 hpi (11.06- and 3153.23-fold, respectively), gradually decreased at 24 hpi (0.01- and 0.14-fold, respectively), and then again increased at 36 hpi (4.52- and 610.79-fold, respectively) when induced by the La Sota strain. Thus, these results suggest that the expression levels of type I and II IFNs were altered in mature chicken BM-DCs in response to both viruses, with weaker responses for the La Sota strain than for the GM strain and stronger responses for IFN-II than for IFN-I in terms of transcript levels.
Expression of mRNA for IFN-α (A), IFN-β (B), and IFN-γ (C) in mature chicken BM-DCs 6, 12, 24, and 36 h after infection with GM or La Sota, determined by qRT-PCR. The data are shown as the mean ± SD from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. The y-axis represents the fold change of the target gene in the NDV-infected groups versus that in the mock-infected group
Expression of cytokines and chemokines in NDV-infected mature chicken BM-DCs
Cytokines and chemokines play important roles in resistance and clearance of viruses. To evaluate the cytokine and chemokine responses in mature chicken DCs after infection with the GM and La Sota strains, we investigated the expression levels of pro-inflammatory cytokines (IL-1β, IL-6, and IL-18), chemokines (IL-8 and CCL5), and anti-inflammatory cytokines (IL-10) in mature chicken BM-DCs after NDV infection by qRT-PCR. As shown in Fig. 4A and C, the expression levels of IL-6 and IL-18 induced by the GM strain were decreased at 6 hpi (0.47- and 0.36-fold, respectively), increased at 12 hpi (152.01- and 13.75-fold, respectively; p < 0.001), markedly decreased at 24 hpi (0.49- and 0.98-fold, respectively), and then again elevated at 36 hpi (439.73- and 18.86-fold, respectively; p < 0.001). Similar patterns were observed after infection with the La Sota strain, except for the expression of IL-6 at 6 hpi (5.58-fold). In addition, the expression level of IL-1β in mature chicken BM-DCs was suppressed at 6 hpi (0.52- and 0.39-fold, respectively), significantly increased at 12 hpi (64.60- and 2.91-fold, respectively; p < 0.01), gradually decreased at 24 hpi (13.76- and 2.92-fold, respectively), and then further decreased at 36 hpi (3.51- and 1.18-fold, respectively) after infection with the GM or La Sota strain (Fig. 4B). However, IL-10 was upregulated at all time points when induced by the GM or La Sota strain and peaked at 24 hpi (9.75- and 6.28-fold, respectively; p < 0.001; Fig. 4D). Compared with the mock-infected control, the expression level of IL-8 in GM- or La Sota-infected mature DCs was upregulated at 6 hpi (5.48- and 7.01-fold, respectively), markedly increased at 12 hpi (24.37- and 16.40-fold, respectively; p < 0.001), plateaued at the baseline with no significant changes at 24 hpi, and then reached a peak at 36 hpi (121.89- and 41.70-fold, respectively; p < 0.001; Fig. 4E). The expression level of CCL5 in mature DCs was upregulated throughout the experiment in response to both viruses, with greater responses observed for the GM strain than for the La Sota strain (Fig. 4F). The expression level of IL-12 was significantly increased in GM- or La Sota-infected mature chicken DCs at 6, 12, and 24 hpi but decreased at 36 hpi (Fig. 4G). Thus, the GM strain could elicit stronger cytokine responses than the La Sota strain in mature chicken DCs.
Expression of cytokines and chemokines in mature chicken BM-DCs 6, 12, 24, and 36 h after infection with GM or La Sota. The expression of mRNA for IL-6 (A), IL-1β (B), IL-18 (C), IL-10 (D), IL-8 (E), CCL5 (F), and IL-12 (G) was measured by qRT-PCR. (H) The concentrations of IL-6 and IL-8 in the supernatants were determined by ELISA at 36 hpi. The data are shown as the mean ± SD from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001
Furthermore, ELISAs showed that the concentrations of IL-6 and IL-8 in culture supernatants from NDV- and mock-infected chicken BM-DCs at 36 hpi were markedly increased in mature chicken BM-DCs in response to NDV infection, consistent with the transcription level (Fig. 4H).
Expression of MHC class I and II molecules in NDV-infected mature chicken DCs
To investigate whether MHC-I and MHC-II molecules were involved in the immune responses of mature chicken DCs following infection with NDV, we measured mRNA expression in mature chicken DCs after NDV infection. Interestingly, MHC-I mRNA was upregulated in response to the GM or La Sota strain throughout the duration of the experiment, with peak expression at 24 hpi (116.14- and 76.95-fold, respectively; p < 0.001; Fig. 5A). In addition, MHC-II mRNA expression in mature chicken DCs began to increase at 12 hpi (20.54- and 9.56-fold, respectively; p < 0.001), peaked at 24 hpi (45.18- and 16.89-fold, respectively; p < 0.001), and then gradually decreased at 36 hpi (10.88- and 16.33-fold, respectively; p < 0.001), in response to infection with the GM or La Sota strain (Fig. 5B). These data indicate that MHC-I and MHC-II may mediate host immune responses in mature chicken DCs after infection with NDV.
Expression of mRNA of MHC-I (A) and MHC-II (B) in mature chicken BM-DCs 6, 12, 24, and 36 h after infection with GM or La Sota, determined by qRT-PCR. The data are shown as the mean ± SD from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. The y-axis represents the fold change of the target gene in the NDV-infected groups versus that in the mock-infected group
Discussion
Previous studies have evaluated the immune responses of murine DCs infected with NDV [14, 15]; however, no studies have evaluated NDV infection in chicken DCs. Meanwhile, several studies have demonstrated differences in susceptibility to viral infection between immature and mature DCs [17, 43], suggesting that immature and mature DCs play different roles during virus infection. Immature DCs can capture antigens without activating T cell, while mature DCs are crucial for the sustainability of innate immune responses and initiation of adaptive immunity [18, 32]. In this study, we analysed the ability of NDV to replicate in mature chicken BM-DCs and compared the immune response of mature DCs after infection with NDV strains with different pathogenicity.
Our results indicated that NDV can replicate in mature chicken DCs and that the virulent strain GM replicated more efficiently than the avirulent strain La Sota, which was consistent with the proliferation data for these two NDVs in CEFs [27]. This could explain why CPE was observed in mature chicken DCs in response to the GM strain but not to the La Sota strain. Similar responses to AIV were also observed in chicken BM-DCs infected with high- and low-pathogenicity AIVs [47].
In DCs, a variety of PRRs can recognize pathogen-associated molecular patterns and induce activation of downstream signalling pathways, leading to the production of chemokines and cytokines and stimulating the apoptotic response [50]. These PRRs, such as TLR3/7, MDA5, and LGP2, play essential roles in facilitating the resistance and clearance of RNA viruses during the early stage of infection [53]. In the current analysis, the expression levels of MDA5, LGP2, and TLR3 were significantly increased in mature chicken BM-DCs after infection with the GM and La Sota strains compared with that in control BM-DCs from 12 to 36 hpi; these data were generally consistent with previous studies [23, 26, 41, 51]. Interestingly, the relative expression levels of these PRRs (MDA5, LGP2, and TLR3) and cytokines showed no significant changes in NDV-infected cells when compared with uninfected mature DCs at 6 hpi. To obtain mature chicken DCs, immature DCs were stimulated with LPS for 24 h. However, LPS can significantly up-regulate the expression levels of PRRs (Supplementary Material Figure S1), pro-inflammatory cytokines such as IL-1β and IL-6, and chemokines such as the IL-8-like molecules CXCLi1 and CXCLi2 [49]. During the very early stage of infection, the expression levels of immune-related genes in mature DCs were mainly affected by previous LPS stimulation. However, TLR7 was not significantly altered in mature DCs after viral infection, with the exception of that induced by the GM strain at 6 and 12 hpi, indicating that TLR7 in mature chicken DCs may play a relatively minor role against NDV infections. Therefore, MDA5, LGP2, and TLR3 but not TLR7 may be involved in the early-stage host innate immune response following infection with the GM and La Sota strains during the early phase of infection in mature chicken BM-DCs.
Our earlier study showed that the highly virulent GM strain can trigger more-robust pro-inflammatory cytokine expression than the low-virulence La Sota strain [27]. In the present study, we also observed that the GM strain induced higher expression of proinflammatory cytokines than the La Sota strain in mature chicken DCs. However, the levels of IL-18, IL-6, and IL-8 expression were decreased in NDV-infected mature DCs at 24 hpi, which was inconsistent with the results in CEFs and DEFs [24, 27]. It should be noted that the expression level of IL-10 reached a peak in mature DCs in response to both viruses at 24 hpi. Studies have shown that IL-10, an anti-inflammatory cytokine, can significantly inhibit the production of inflammatory cytokines [9, 40]. The high level of expression of IL-10 might result in the downregulation of proinflammatory cytokines in mature chicken DCs after infection with NDVs at 24 hpi. These results require further investigation in vivo.
Type I (IFN-α, IFN-β) and type II (IFN-γ) IFNs act as key mediators of apoptosis, inflammation, and antiviral activity [3]. In the current study, we observed that the highly virulent NDV strain GM induced a more potent IFN response than the low-virulence NDV strain La Sota, which is consistent with previous studies [20, 41]. Surprisingly, the expression levels of IFN-α, and IFN-β induced by both viruses were decreased at 24 hpi, possibly resulting from the high level of V protein that can inhibit the induction of type I IFN genes [22, 37]. We also observed the downregulation of IFN-γ in La Sota-infected mature DCs at 24 hpi. The IFN-γ expression level in NDV-inoculated splenocytes decreased in a stepwise pattern at 12 and 24 hpi [20]. Meanwhile, DCs can produce IFN-γ in response to IL-12 and IL-18 [45]. Furthermore, high expression of IL-10, which significantly inhibits IFN-γ production in DCs, was observed in NDV-infected mature DCs [7, 16]. These factors may contribute to low expression of IFN-γ in mature chicken DCs after infection with NDVs at 24 hpi. However, the exact mechanisms behind this observation require further investigation.
The acquired immune responses activated by MHC molecules can eliminate pathogens such as viruses. In our previous studies, we showed that the virulent NDV strains SS-10 and NH-10 increased expression of MHC-I, but suppressed expression of MHC-II in CEFs and duck embryonic fibroblasts (DEFs) during early-phase NDV infection [24]. In agreement with these findings, the expression of MHC-I was upregulated in mature DCs in response to both viruses. NDV strains SS-10 and NH-10 could suppress the expression of MHC-II in CEFs, and DEFs [23]. However, a recent study demonstrated that high expression of MHC-II induced by NDV-like particles (containing the M and HN proteins of NDV) was observed in mouse DCs, consistent with our study [36]. In the model of mature chicken DCs, both NDV strains could significantly increase the expression of MHC-I and -II, but higher expression levels were observed in the GM-infected group when compared with those in the La Sota-infected group. Therefore, these results indicated that NDV strains with differing pathogenicity differed in their ability to regulate expression of MHC-I and MHC-II at the transcription level.
Conclusion
Although both velogenic and lentogenic NDVs could replicate in chicken DCs, the velogenic NDV strain GM triggered a stronger innate immune response than the lentogenic NDV strain La Sota. Differences in the replication ability of the GM strain and the La Sota strain might play an important role in the induction of cytokines and chemokines. However, to further evaluate the immune response of DCs infected with NDV strains with different pathogenicity, future studies need to be performed using single-cell RNA sequencing methods.
Abbreviations
- NDV:
-
Newcastle disease virus
- BM-DCs:
-
Bone-marrow-derived dendritic cells
- CEFs:
-
Chicken embryo fibroblasts
- MHC:
-
Major histocompatibility complex
- PRRs:
-
Pattern recognition receptors
- TBST:
-
Tris-buffered saline containing 0.05% Tween-20
- SPF:
-
Specific-pathogen-free
References
Ahmed KA, Saxena VK, Ara A, Singh KB, Sundaresan NR, Saxena M, Rasool TJ (2007) Immune response to Newcastle disease virus in chicken lines divergently selected for cutaneous hypersensitivity. Int J Immunogenet 34:445–455
Alexander DJ (2000) Newcastle disease and other avian paramyxoviruses. Revue scientifique et technique (International Office of Epizootics) 19:443–462
Barber GN (2001) Host defense, viruses and apoptosis. Cell Death Differ 8:113–126
Chambers P, Samson AC (1982) Non-structural proteins in Newcastle disease virus-infected cells. J Gen Virol 58(Pt 1):1–12
Chambers P, Millar NS, Bingham RW, Emmerson PT (1986) Molecular cloning of complementary DNA to Newcastle disease virus, and nucleotide sequence analysis of the junction between the genes encoding the haemagglutinin-neuraminidase and the large protein. J Gen Virol 67(Pt 3):475–486
Cheng JH, Sun YJ, Zhang XR, Zhang FQ, Zhang SL, Yu SQ, Qiu XS, Tan L, Song CP, Gao S, Wu YT, Ding C (2014) Toll-like receptor 3 inhibits Newcastle disease virus replication through activation of pro-inflammatory cytokines and the type-1 interferon pathway. Adv Virol 159:2937–2948
D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G (1993) Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med 178:1041–1048
de Geus ED, Jansen CA, Vervelde L (2012) Uptake of particulate antigens in a nonmammalian lung: phenotypic and functional characterization of avian respiratory phagocytes using bacterial or viral antigens. J Immunol 188:4516–4526
de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE (1991) Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174:1209–1220
Degen WG, Daal N, Rothwell L, Kaiser P, Schijns VE (2005) Th1/Th2 polarization by viral and helminth infection in birds. Vet Microbiol 105:163–167
Dimitrov KM, Bolotin V, Muzyka D, Goraichuk IV, Solodiankin O, Gerilovych A, Stegniy B, Goujgoulova GV, Silko NY, Pantin-Jackwood MJ, Miller PJ, Afonso CL (2016) Repeated isolation of virulent Newcastle disease viruses of sub-genotype VIId from backyard chickens in Bulgaria and Ukraine between 2002 and 2013. Adv Virol 161:3345–3353
Dortmans JC, Koch G, Rottier PJ, Peeters BP (2011) Virulence of Newcastle disease virus: what is known so far? Vet Res 42:122
Ecco R, Brown C, Susta L, Cagle C, Cornax I, Pantin-Jackwood M, Miller PJ, Afonso CL (2011) In vivo transcriptional cytokine responses and association with clinical and pathological outcomes in chickens infected with different Newcastle disease virus isolates using formalin-fixed paraffin-embedded samples. Vet Immunol Immunopathol 141:221–229
Fournier P, Arnold A, Schirrmacher V (2009) Polarization of human monocyte-derived dendritic cells to DC1 by in vitro stimulation with Newcastle disease virus. J BUON 14(Suppl 1):S111–S122
Fournier P, Arnold A, Wilden H, Schirrmacher V (2012) Newcastle disease virus induces pro-inflammatory conditions and type I interferon for counter-acting Treg activity. Int J Oncol 40:840–850
Fukao T, Frucht DM, Yap G, Gadina M, O’Shea JJ, Koyasu S (2001) Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J Immunol (Baltimore, Md: 1950) 166:4446–4455
Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman RM (1998) Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol 72:2733–2737
Hopkins RA, Connolly JE (2012) The specialized roles of immature and mature dendritic cells in antigen cross-presentation. Immunol Res 53:91–107
Hou F, Liu K, Shen T, Zhou B, Cao R, Li P, Chen P (2011) Antiviral activity of rChIFN-alpha against vesicular stomatitis virus and Newcastle disease virus: a novel recombinant chicken interferon-alpha showed high antiviral activity. Res Vet Sci 91:e73–e79
Hu Z, Hu J, Hu S, Liu X, Wang X, Zhu J, Liu X (2012) Strong innate immune response and cell death in chicken splenocytes infected with genotype VIId Newcastle disease virus. Virol J 9:208
Hu Z, Hu J, Hu S, Song Q, Ding P, Zhu J, Liu X, Wang X, Liu X (2015) High levels of virus replication and an intense inflammatory response contribute to the severe pathology in lymphoid tissues caused by Newcastle disease virus genotype VIId. Adv Virol 160:639–648
Jang J, Hong SH, Choi D, Choi KS, Kang S, Kim IH (2010) Overexpression of Newcastle disease virus (NDV) V protein enhances NDV production kinetics in chicken embryo fibroblasts. Appl Microbiol Biotechnol 85:1509–1520
Kang Y, Li Y, Yuan R, Feng M, Xiang B, Sun M, Li Y, Xie P, Tan Y, Ren T (2015) Host innate immune responses of ducks infected with newcastle disease viruses of different pathogenicities. Front Microbiol 6:1283
Kang Y, Feng M, Zhao X, Dai X, Xiang B, Gao P, Li Y, Li Y, Ren T (2016) Newcastle disease virus infection in chicken embryonic fibroblasts but not duck embryonic fibroblasts is associated with elevated host innate immune response. Virol J 13:41
Kang Y, Xiang B, Yuan R, Zhao X, Feng M, Gao P, Li Y, Li Y, Ning Z, Ren T (2016) Phylogenetic and pathotypic characterization of Newcastle disease viruses circulating in South China and transmission in different birds. Front Microbiol 7:119
Li Y, Xu Q, Zhang T, Gao M, Wang Q, Han Z, Shao Y, Ma D, Liu S (2015) Host avian beta-defensin and toll-like receptor responses of pigeons following infection with pigeon paramyxovirus type 1. Appl Environ Microbiol 81:6415–6424
Li Y, Xie P, Sun M, Xiang B, Kang Y, Gao P, Zhu W, Ning Z, Ren T (2016) S1PR1 expression correlates with inflammatory responses to Newcastle disease virus infection. Infect Genet Evolut 37:37–42
Liu D, Dai M, Zhang X, Cao W, Liao M (2016) Subgroup J avian leukosis virus infection of chicken dendritic cells induces apoptosis via the aberrant expression of microRNAs. Sci Rep 6:20188
Liu D, Qiu Q, Zhang X, Dai M, Qin J, Hao J, Liao M, Cao W (2016) Infection of chicken bone marrow mononuclear cells with subgroup J avian leukosis virus inhibits dendritic cell differentiation and alters cytokine expression. Infect Genet Evolut 44:130–136
Liu WQ, Tian MX, Wang YP, Zhao Y, Zou NL, Zhao FF, Cao SJ, Wen XT, Liu P, Huang Y (2012) The different expression of immune-related cytokine genes in response to velogenic and lentogenic Newcastle disease viruses infection in chicken peripheral blood. Mol Biol Rep 39:3611–3618
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif) 25:402–408
Munz C, Steinman RM, Fujii S (2005) Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med 202:203–207
OIE (2012) Chapter 2.3.14. Newcastle disease. http://www.ieint/fileadmin/Home/eng/Health_standards/tahm/20314_NEWCASTLE_DISpdf
Panda A, Huang Z, Elankumaran S, Rockemann DD, Samal SK (2004) Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb Pathog 36:1–10
Peeters BP, de Leeuw OS, Koch G, Gielkens AL (1999) Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. J Virol 73:5001–5009
Qian J, Ding J, Yin R, Sun Y, Xue C, Xu X, Wang J, Ding C, Yu S, Liu X, Hu S, Cong Y, Ding Z (2017) Newcastle disease virus-like particles induce dendritic cell maturation and enhance viral-specific immune response. Virus Genes 53:555–564
Qiu X, Fu Q, Meng C, Yu S, Zhan Y, Dong L, Song C, Sun Y, Tan L, Hu S, Wang X, Liu X, Peng D, Liu X, Ding C (2016) Newcastle disease virus V protein targets phosphorylated STAT1 to block IFN-I signaling. PLoS One 11:e0148560
Qu H, Yang L, Meng S, Xu L, Bi Y, Jia X, Li J, Sun L, Liu W (2013) The differential antiviral activities of chicken interferon alpha (ChIFN-alpha) and ChIFN-beta are related to distinct interferon-stimulated gene expression. PLoS One 8:e59307
Rasoli M, Yeap SK, Tan SW, Moeini H, Ideris A, Bejo MH, Alitheen NB, Kaiser P, Omar AR (2014) Alteration in lymphocyte responses, cytokine and chemokine profiles in chickens infected with genotype VII and VIII velogenic Newcastle disease virus. Comp Immunol Microbiol Infect Dis 37:11–21
Rossato M, Curtale G, Tamassia N, Castellucci M, Mori L, Gasperini S, Mariotti B, De Luca M, Mirolo M, Cassatella MA, Locati M, Bazzoni F (2012) IL-10-induced microRNA-187 negatively regulates TNF-alpha, IL-6, and IL-12p40 production in TLR4-stimulated monocytes. Proc Natl Acad Sci USA 109:E3101–E3110
Rue CA, Susta L, Cornax I, Brown CC, Kapczynski DR, Suarez DL, King DJ, Miller PJ, Afonso CL (2011) Virulent Newcastle disease virus elicits a strong innate immune response in chickens. J Gen Virol 92:931–939
Shahsavandi S, Ebrahimi MM, Mohammadi A, Zarrin Lebas N (2013) Impact of chicken-origin cells on adaptation of a low pathogenic influenza virus. Cytotechnology 65:419–424
Spiegel M, Schneider K, Weber F, Weidmann M, Hufert FT (2006) Interaction of severe acute respiratory syndrome-associated coronavirus with dendritic cells. J Gen Virol 87:1953–1960
Steinman RM (1991) The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 9:271–296
Stoll S, Jonuleit H, Schmitt E, Muller G, Yamauchi H, Kurimoto M, Knop J, Enk AH (1998) Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol 28:3231–3239
Sun Y, Ding N, Ding SS, Yu S, Meng C, Chen H, Qiu X, Zhang S, Yu Y, Zhan Y, Ding C (2013) Goose RIG-I functions in innate immunity against Newcastle disease virus infections. Mol Immunol 53:321–327
Vervelde L, Reemers SS, van Haarlem DA, Post J, Claassen E, Rebel JM, Jansen CA (2013) Chicken dendritic cells are susceptible to highly pathogenic avian influenza viruses which induce strong cytokine responses. Dev Comp Immunol 39:198–206
Wang JY, Liu WH, Ren JJ, Tang P, Wu N, Wu HY, Ching CD, Liu HJ (2015) Characterization of emerging Newcastle disease virus isolates in China. Virol J 12:119
Wu Z, Rothwell L, Young JR, Kaufman J, Butter C, Kaiser P (2010) Generation and characterization of chicken bone marrow-derived dendritic cells. Immunology 129:133–145
Wu Z, Kaiser P (2011) Antigen presenting cells in a non-mammalian model system, the chicken. Immunobiology 216:1177–1183
Xu Q, Chen Y, Zhao W, Zhang T, Liu C, Qi T, Han Z, Shao Y, Ma D, Liu S (2016) Infection of goose with genotype VIId Newcastle disease virus of goose origin elicits strong immune responses at early stage. Front Microbiol 7:1587
Yan B, Zhang J, Zhang W, Wang M, Jia R, Zhu D, Liu M, Yang Q, Wu Y, Sun K, Chen X, Cheng A, Chen S (2017) GoTLR7 but not GoTLR21 mediated antiviral immune responses against low pathogenic H9N2 AIV and Newcastle disease virus infection. Immunol Lett 181:6–15
Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5:730–737
Yuk SS, Lee DH, Park JK, Tseren-Ochir EO, Kwon JH, Noh JY, Lee JB, Park SY, Choi IS, Song CS (2016) Pre-immune state induced by chicken interferon gamma inhibits the replication of H1N1 human and H9N2 avian influenza viruses in chicken embryo fibroblasts. Virol J 13:71
Zhu J, Hu S, Xu H, Liu J, Zhao Z, Wang X, Liu X (2016) Characterization of virulent Newcastle disease viruses from vaccinated chicken flocks in Eastern China. BMC Vet Res 12:113
Funding
This study was supported by the National Natural Science Foundation of China (no. 31372412, 31702213), the Chinese Special Fund for Agro-Scientific Research in the Public Interest (no. 201303033), the Specialized Research Fund for Doctoral Program of Higher Education of China (no. 20124404110016), the Science and Technology Projects of Guangdong Province (no. 2013B020224002), the Natural Science Foundation Project (no. 2016YFD0501603), and the Poultry Production Technology of Guangdong System (no. 2016LM1115).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
All animal experiments in the present study were approved by the South China Agricultural University Experimental Animal Welfare Ethics Committee (Permit number: 2016-011[20161101]).
Additional information
Handling Editor: Zhenhai Chen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiang, B., Zhu, W., Li, Y. et al. Immune responses of mature chicken bone-marrow-derived dendritic cells infected with Newcastle disease virus strains with differing pathogenicity. Arch Virol 163, 1407–1417 (2018). https://doi.org/10.1007/s00705-018-3745-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3745-6