Abstract
Rotavirus (RV) is the primary cause of severe dehydrating gastroenteritis and acute diarrheal disease in infants and young children. Previous studies have revealed that genistein can inhibit the infectivity of enveloped or nonenveloped viruses. Although the biological properties of genistein are well studied, the mechanisms of action underlying their anti-rotavirus properties have not been fully elucidated. Here, we report that genistein significantly inhibits RV-Wa replication in vitro by repressing viral RNA transcripts, and possibly viral protein synthesis. Interestingly, we also found that aquaporin 4 (AQP4) mRNA and protein expression, which was downregulated in RV-infected Caco-2 cells, can be upregulated by genistein in a time- and dose-dependent manner. Further experiments confirmed that genistein triggers CREB phosphorylation through PKA activation and subsequently promotes AQP4 gene transcription. These findings suggest that the pathophysiological mechanism of RV infection involves decreased expression of AQP4 and that genistein may be a useful candidate for developing a new anti-RV strategy by inhibiting rotavirus replication and upregulating AQP4 expression via the cAMP/PKA/CREB signaling pathway. Further studies on the effect of genistein on RV-induced diarrhea are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rotaviruses (RVs), members of the family Reoviridae, are nonenveloped, triple-layered icosahedral viruses containing a genome of 11 segments of dsRNA that code for six structural proteins (VP1, VP2, VP3, VP4, VP6, and VP7) and six nonstructural proteins (NSP1–NSP6) [1]. These viruses are the primary cause of severe dehydrating gastroenteritis in infants and young children, leading to 500,000 deaths annually [2, 3]. Clinical and epidemiological studies have shown that RV is strongly associated with clinical manifestations, including acute gastroenteritis with fever, vomiting, abdominal pain, diarrhea and dehydration [4].
Despite its significant clinical importance and several decades of research, the pathophysiological mechanisms that lead to diarrhea during RV infection are not well understood. A number of studies have demonstrated that secretory diarrhea caused by RV may result from a combination of excessive secretion of fluid and electrolytes into the intestinal lumen and reduced fluid absorption [5, 6]. The regulation of transepithelial fluid transport in the gastrointestinal tract is based on ion transport and also on water transport by aquaporin (AQP)-type water channels, which are responsible for the rapid transport of water across membranes [7]. Recently, several studies have demonstrated an association between abnormal AQP expression and development of diarrhea caused by cholera toxin [8], inflammatory bowel disease [9], 5-fluorouracil [10], bacterial pathogens [11], celiac disease [12], sennoside A, and allergy [13, 14]. However, the expression and pathophysiological role of AQPs in RV-induced diarrhea remains unclear.
At present, there are no specific antiviral drugs available for RV infection, and treatment options for RV-mediated gastroenteritis are limited to restoration and maintenance of hydration until the infection resolves. Oral rehydration and maintenance of proper fluid and electrolyte balance remain the primary treatment for children with RV gastroenteritis [15, 16]. Studies have also shown that the use of probiotics early in the course of diarrhea can reduce the duration of illness and RV shedding [17, 18]. For prevention of RV infection, vaccination seems to be the most promising strategy. Two developed and approved RV vaccines, Rotarix (GlaxoSmithKline) and RotaTeq (Merck), have substantially reduced the incidence of rotavirus diarrhea among infants and young children under 5 years of age and offers promise in reducing the burden of disease caused by RV [19, 20]. However, the fact that these are live attenuated vaccines has raised some concerns related to viral shedding and the risk of transmission, as well as to their cost, efficacy and safety [21, 22]. In addition, the recommended age restriction for vaccine administration is a serious impediment for the widespread use of these vaccines in developing countries, which have the lowest vaccine coverage and the lowest rates of timely immunization [19, 23]. Therefore, development of an RV-specific drug is important and urgently needed to reduce the severity of disease and duration of rotavirus-related hospitalization.
Genistein, an isoflavone, has been shown to possess antiviral properties against herpes simplex virus, bovine herpes virus type 1, bovine viral diarrhea virus, simian virus, and Epstein-Barr virus due to its ability to inhibit tyrosine-specific phosphorylation [24–26]. A study conducted by Andres et al. [27] showed that genistein, genistin, and acetylgenistin decreased RV infectivity to a similar degree by modulating virion attachment to host cells and by modulating a post-binding step in RV-infected MA104 cells. This antiviral effect was not due to alteration of the RV triple-layered structure, inhibition of protein tyrosine kinases or topoisomerase II, or mimicking the effect of estrogens [27]. Thus, the reason for the anti-rotaviral effect of genistein has not been determined.
Here, we report that genistein significantly inhibits RV replication in vitro by repressing viral RNA transcripts, and possibly viral protein synthesis, in a dose-dependent manner. Furthermore, we also demonstrate that AQP4 mRNA and protein expression, which are downregulated in RV-infected Caco-2 cells, can be upregulated by genistein in a time- and dose-dependent manner. Further experiments confirmed that genistein triggers CREB phosphorylation through PKA activation and subsequent promote AQP4 gene transcription. These results support the conclusion that the pathophysiological mechanism of RV infection involves decreased expression of AQP4 and that genistein may be a useful candidate for developing a new anti-RV strategy for treatment and prevention of rotavirus disease in children.
Materials and methods
Cell and rotavirus culture
MA104, HT-29, SW620, and Caco-2 cells were obtained from the American Type Culture Collection (ATCC) and grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10 % fetal bovine serum (Thermo) in an atmosphere of 5 % carbon dioxide. For all experiments, functionally endotoxin-free media and reagents were used. To prepare virus stocks, rotavirus Wa and SA-11 (kindly provided by Dr. Haiyang He, Institute of Immunology, PLA, Third Military Medical University, China) were pretreated with crystalline trypsin (10 mg/mL, Gibco) for 60 minutes at 37 °C. Subsequently, MA104 cells were incubated with rotavirus for 1 h at 37 °C in a humidified 5 % CO2 atmosphere with rocking every 20 min to redistribute the inoculum. Excess rotavirus was removed by washing with serum-free DMEM. The MA104 cells were maintained for 2–3 days in serum-free DMEM containing 0.5 μg of trypsin per mL. The cells were harvested when 80–95 % cytopathic effect (CPE) was observed and then frozen and thawed three times at −20 °C. The suspension was centrifuged at 2000×g for 10 min to obtain a supernatant containing rotavirus, which was stored at −70 °C.
Cytotoxicity of genistein
The cytotoxicity of genistein (Sigma, diluted in dimethyl sulphoxide) was determined using a micro-titration assay. Briefly, Caco-2 cells cultured in 96-well plates (Corning) at 8000 cells per well were treated with various concentrations of genistein at 37 °C for 3 days. During the incubation, the cells were examined using an inverted optical microscope (Nikon) for cellular morphologic alterations. In addition, cell viability was also quantified using a Cell Counting Kit (CCK-8) (Dojindo) as per the manufacturer’s instructions. The optical density (OD) was measured at 450 nm using a microplate reader (Gene). The relative cell viability rate was determined for each concentration as [(OD experiment − OD blank)/(OD control − OD blank) × 100]. All experiments were repeated three times independently.
Titration of rotavirus
The infectivity titer of rotavirus was determined on Caco-2 cells using endpoint dilution and CCK-8 assays. Briefly, cells seeded in a 96-well plate at a density of 8000 cells per well were incubated with different dilutions of the harvested RV supernatant (serially diluted tenfold in fresh DMEM in the rage of 100-106) at 37 °C for 3 days. Based on cellular morphologic alterations, the cytopathic effect (CPE) was examined using an inverted optical microscope (Nikon). In addition, cell viability was tested by treatment with 10 μL of the CCK-8 solution for 4 h at 37 °C. The OD value in the wells was measured at a wavelength of 450 nm using a microplate reader. Virus titers were calculated by the Reed and Muench method [28] and expressed as 50 % tissue culture infective dose (TCID50) per mL.
Measurement of the antiviral effect of genistein
The potential antiviral activity of genistein was assayed as follows: (1) Caco-2 cell monolayers in 12-well culture plates (Corning) was incubated with RV-Wa at 100 TCID50 for 2 h at 37 °C to allow attachment to the cell surface. After incubation, the unbound virus was removed by washing with fresh DMEM, the cells were treated for 2 h with different concentrations of genistein, and after washing to remove the genistein, were incubated for 12 h at 37 °C. (2) Caco-2 cell monolayers were incubated with different concentrations of genistein for 2 h at 37 °C, followed by washing with DMEM and incubation with RV-Wa for 2 h at 37 °C. After washing to remove the virus, cells were incubated for a further 12 h at 37 °C. (3) Caco-2 cell monolayers were incubated with RV-Wa for 2 h at 37 °C, the virus was removed by washing, and different concentrations of genistein were added. Incubation was continued for 12 h at 37 °C. In all cases, after the final incubation period, virus-induced CPE of drug-treated and untreated control cells was examined using an inverted optical microscope. In addition, cell lysates were prepared by three cycles of freezing and thawing, and virus titers were calculated.
Enzyme-linked immunosorbent assay (ELISA, DRG International Inc.) was used to test the effect of genistein on the formation of infectious virions. Caco-2 cell monolayers in 6-well plates were inoculated with RV-Wa (100 TCID50) for 2 h at 37 °C. After washing to remove the inoculum, cells were overlaid with medium containing genistein at different concentrations and incubated for 12 h at 37 °C. After incubation, genistein was removed by washing, and cell lysates and culture supernatants were collected after further incubation for 12 h at 37 °C. For detection of extracellular viral antigen, the infected cell culture supernatants were serially diluted tenfold in DMEM and used to detect RV-Wa antigen using the ELISA kit according to the manufacturer’s instructions. For detection of intracellular virus antigen, RV-Wa-infected Caco-2 cells were resuspended in fresh DMEM and were lysed by three freeze-thaw cycles at −20 °C. The supernatants were collected and used for detection of RV antigen according to the manufacturer’s instructions. The test was considered positive if the OD at 450 nm in the well containing RV antigen minus the OD 450 of control wells was ≥0.1.
Indirect immunofluorescent assay (IFA)
Immunofluorescence analysis of RV-Wa protein expression during rotavirus infection and genistein treatment of Caco-2 cells was performed by seeding cells on glass cover slips and growing them until they reached 50 % confluence. Briefly, Caco-2 cell monolayers on cover slips were inoculated with RV-Wa (100 TCID50) for 2 h at 37 °C. After washing to remove the inoculum, cells were overlaid with a medium containing 80 μM genistein or were mock-treated with DMEM and incubated for 12 h and 36 h at 37 °C, respectively. After washing with 1× phosphate-buffered saline (PBS), cells were fixed in 4 % paraformaldehyde for 20 min and permeabilized in 1 % Triton X-100 for 30 min at room temperature. After three washes in PBS containing 1 % BSA and 50 mM NH4Cl, the cells were blocked with 5 % BSA for 2 h at room temperature and then incubated overnight at 4 °C with antibody against RV VP-6 protein (1:200, Santa Cruz). After washing three times with PBST, cells were then incubated with FITC-conjugated goat anti-mouse IgG Dylight 488 (1:200, Thermo) for 2 h at room temperature, followed by a 15-min incubation with DRAQ5 (1:1000, Cell Signaling) at room temperature. The triple-stained cells were washed three times with PBST and photographed a Leica TCS SP5 laser confocal microscope.
Total RNA and rotavirus RNA extraction and quantitative real-time PCR
Caco-2 cell monolayers in 6-well plates were inoculated with RV-Wa (100 TCID50) for 2 h at 37 °C. After washing to remove the inoculum, cells were overlaid with a medium containing genistein at different concentrations and incubated for 12 h at 37 °C. Total RNA was extracted from the mock- and RV-Wa-infected Caco-2 cells using TRIzol Reagent (Invitrogen). Rotavirus RNA was extracted from 200 μL of the culture supernatant using a PureLink® Viral RNA/DNA Mini Kit (Invitrogen). First-strand cDNAs were prepared from 5 µg of total/viral RNA using SuperScript III reverse transcriptase (Invitrogen) and used as a template. Real-time RT-PCR was performed with the specific primers listed in Table 1 in an ABI7500 Real-Time PCR System using a Real-Time SYBR Master Mix Kit (Applied Biosystems) following the manufacturer’s introductions. The cycle threshold signal was used to measure RT-PCR signals. At the end of the PCR, a dissociation curve was produced to examine the specificity of the PCR product. The levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were used as an endogenous reference to normalize the quantities of target mRNA.
Electrophoresis and immunoblotting
Briefly, cell samples from each group were washed once with ice-cold PBS and lysed in 150 µL of RIPA buffer containing 50 μg/mL PMSF (Sigma) or phosphatase inhibitor (Sigma). Protein concentrations were determined using a NanoDrop Spectrophotometer (Gene). Total proteins were separated by 10 % SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore). The membranes were blocked with 2.5 % nonfat dry milk (Merck) in PBST for 120 min at room temperature. Primary antibodies against the following proteins were used: rotavirus VP-6 (Santa Cruz), AQP1 (Abcam), AQP3 (Abcam), AQP4 (Abcam), AQP8 (Santa Cruz), p-CREB (Cell Signaling), CREB (Cell Signaling) and GAPDH (Santa Cruz). The membranes were immunoblotted overnight at 4 °C, followed by incubation with a goat anti-rabbit/mouse IgG (H+L)/TRITC secondary antibody (Invitrogen). Signals were detected using an LI-COR Odyssey Infrared Imaging System (Gene). All experiments were repeated three times.
Determination of adenylate cyclase (AC) activity
AC activity was assayed by a modification of the procedure described by Aurbach and Chase [29]. Briefly, Caco-2 cell monolayers in 6-well plates were inoculated with RV-Wa (100 TCID50) or mock infected with DMEM for 2 h at 37 °C. After washing to remove the inoculum, cells were overlaid with a medium containing genistein (80 µM) or an AC activator forskolin (50 μM, sigma) and incubated for 12 h at 37 °C. Mock-infected cells were treated with DMEM. After washing with PBS, cells were recovered with a cell scraper, sonicated, and then centrifuged (2200×g for 10 min at 4 °C). The supernatant was discarded, and the pellets obtained by this centrifugation were resuspended in 2 mL of Tris buffer (pH 7.5) and homogenized again. The homogenate was assayed for AC activity. AC activity was measured by determining the rate of formation of cyclic AMP (cAMP) from ATP according to the instructions of the manufacturer of the cAMP immunoassay kit (R&D Systems).
Determination of protein kinase A (PKA) activity
The medium used to culture RV-infected Caco-2 cells in a 6-well plate for 12 h was replaced with medium containing genistein (80 µM), PKA agonist 8-Br cAMP (1 mM), PKA inhibitor H-89 or H-89 + genistein and incubated for 12 h at 37 °C. Mock-infected cells were treated with DMEM. After 60 min, the cells were washed with PBS and then collected in PKA extraction buffer (20 mM MOPS, 50 mM β-glycerophosphate, 50 mM sodium fluoride, 1 mM sodium vanadate, 5 mM EGTA, 2 mM EDTA, 1 % NP-40, 1 mM DTT, 1 mM benzamidine, 1 mM PMSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin). The suspension was kept on ice for 10 min. Cytoplasmic proteins were harvested by sonication and centrifugation (12,000 × g for 15 min at 4 °C). The supernatant was recovered, and its PKA activity was assayed using a PKA kinase activity assay kit according to the manufacturer’s instructions (Stressgen).
Statistical analysis
Where appropriate, data were expressed as mean ± standard deviation (SD) of triplicate cultures. For real-time RT-PCR, the relative expression level of each gene was calculated by the ΔΔ Ct method. Results are shown as fold changes from the control group. For western blot, protein levels were normalized to GAPDH by using Quantity One software. Statistical significance was assessed by two-tailed Student’s t-test when comparing two groups, and by one-way ANOVA for comparisons of multiple groups. Statistical analysis was performed using Statistical Product and Service Solutions (SPSS) software (Windows 13.0). Statistical significance was defined as p < 0.05.
Results
Cell viability
To assess the possible cytotoxic effects of genistein treatment on Caco-2 cell viability, cells were incubated with different concentrations (0, 5, 10, 20, 40, 80, 160 and 320 μM) of genistein using the CCK-8 assay. The results showed that the cell viability (Fig. 1a) did not differ significantly at concentrations lower than 160 μM when compared to the control untreated cells. In our present study, the potential antiviral activity of genistein was performed only for the concentrations lower than 160 μM.
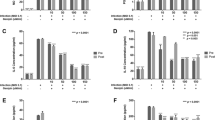
The effect of genistein on the percentage of viable cells and its antiviral effect on rotavirus-infected cells. (a) Caco-2 cell monolayers in 96-well plates were treated for 72 h at 37 °C with genistein at the indicated concentrations (0, 5, 10, 20, 40, 80, 160, and 320 μM). Cell viability was determined using CCK-8 assay. (b) Caco-2 cell monolayers in 12-well culture plates were incubated with RV-Wa at 100 TCID50 for 2 h at 37 °C, unbound virus was removed by washing with fresh DMEM, and cells were treated with different concentrations of genistein for 2 h and then incubated for 12 h at 37 °C after washing to remove the genistein. Cell lysates were prepared by freezing and thawing and virus titers were measured. (c) Caco-2 cell monolayers were incubated with different concentrations of genistein for 2 h at 37 °C followed by washing with DMEM and incubation with RV-Wa for 2 h at 37 °C. After washing to remove the virus, cells were incubated for a further 12 h at 37 °C. Cell lysates were prepared by freezing and thawing and virus titers were measured. (d) Caco-2 cell monolayers were incubated with RV-Wa for 2 h at 37 °C, and the virus was then removed by washing and different concentrations of genistein were added. Incubation was continued for 12 h at 37 °C. Cell lysates were prepared by freezing and thawing, and virus titers were measured. (e) Rotavirus-strain-dependent and cell-line-independent inhibition of rotavirus infectivity by genistein treatment. MA104, HT-29 or SW620 cell monolayers in 96-well plates were inoculated with rotavirus Wa or SA-11 for 2 h at 37 °C. After removing the inoculum, cells were overlaid with DMEM containing genistein at the indicated concentrations (20, 40 and 80 μM). After a 12h-incubation at 37 °C, the number of infected cells was determined by indirect immunofluorescent assay (IFA). Data shown are expressed as mean ± standard deviation of triplicate cultures, and three independent experiments were carried out. *, p < 0.05; **, p < 0.01
Genistein has no effect on rotavirus binding and entry but strongly inhibits rotavirus replication and synthesis of viral protein
To assess whether genistein treatment could inhibit rotavirus infectivity, three different strategies were followed to test the inhibitory activity of genistein on infection of Caco-2 cells by RV-Wa. As shown in Fig. 1b, c and d, for the virus titration assay, the experimental approach in which cells were treated with genistein after virus attachment for 2 h at 37 °C proved to be the most effective way to affect RV replication. Moreover, we also found a statistically significant reduction in replication by sustained genistein treatment in a dose-dependent manner after RV-Wa infection (Fig. 1d). These results indicate that genistein had no effect on RV binding and entry but may strongly inhibit RV-Wa replication.
To determine whether the inhibitory effect of genistein treatment on virus replication was dependent on the rotavirus strain used, MA104, HT-29 and SW620 cell lines were inoculated with rotavirus strains Wa or SA-11. Cell monolayers in 96-well culture plates were incubated with the rotavirus Wa or SA-11 at 100 TCID50 for 2 h at 37 °C. After washing with DMEM to remove the viral inoculum, various concentrations of genistein were added and maintained until 12 h postinfection. The number of infected cells was determined for each concentration of genistein using IFA. It was found that the inhibitory effect of genistein was different for each rotavirus strain when the assay was conducted on a particular cell line (Fig. 1e). However, similar levels of inhibition were observed when one particular rotavirus strain was assayed in different cell lines (Fig. 1e). These results indicate that the inhibitory effect of genistein was, to a certain extent, dependent on the rotavirus strain and independent of the cell line.
In order to confirm that genistein inhibited rotavirus replication when added after RV infection, we also evaluated rotavirus antigen, RV RNA and protein expression by ELISA, real-time PCR, and western blot, respectively. Consistent with the virus titration results, the experimental approach in which cells were treated with genistein after virus attachment for 2 h at 37 °C significantly reduced intracellular rotavirus RNA and antigen levels (Fig. 2a, b and c), as well as the expression level of the RV VP-6 protein (Fig. 2d). By measuring RNA levels, we found that genistein inhibited rotavirus Wa replication in a time-dependent and dose-dependent manner (Fig. 2a and c). An indirect immunofluorescence staining assay confirmed that genistein suppressed RV-Wa replication and synthesis of viral protein in Caco-2 cells compared to control (Fig. 2e).
Genistein inhibits rotavirus replication and synthesis of viral protein in RV-Wa-infected Caco-2 cells. Caco-2 cell monolayers in 6-well plates were incubated with RV-Wa for 2 h at 37 °C, and the virus was then removed by washing, and different concentrations of genistein were added. Incubation was continued for 12 h at 37 °C. (a) Rotavirus Wa RNA was extracted from the cells and detected by real-time RT-PCR. Intracellular rotavirus RNA levels, normalized to GAPDH mRNA, are expressed as % of control (without genistein treatment, which is defined as 100 %). (b) After incubation, genistein was removed, and cell lysates and culture supernatants were collected after a further incubation for 12 h at 37 °C. RV antigen levels were determined by ELISA. The intracellular and extracellular RV antigen is expressed as OD450. (c) Caco-2 cell monolayers in 6-well plates were incubated with RV-Wa for 2 h at 37 °C, and the virus was then removed by washing, and the infected cells were treated with 80 μM genistein. Incubation was continued for 12 h at 37 °C. Control cells were mock-treated with DMEM. Intracellular RNA was extracted at 12, 24, 36 h, and detected by real-time RT-PCR. Intracellular rotavirus RNA levels, normalized to GAPDH mRNA, are expressed as % of control (mock-treated group, which is defined as 100 at 36 h postinfection). (d) Caco-2 cell monolayers in 6-well plates were incubated with RV-Wa for 2 h at 37 °C, and the virus was then removed by washing, and different concentrations (20 and 80 μM) of genistein were added. Incubation was continued for 12 h at 37 °C. Cell lysates were harvested in RIPA buffer containing PMSF. The amount of VP-6 protein was determined by western blotting, correcting for the GAPDH level. The band intensity was determined using Quantity One software. (e) Caco-2 cell monolayers on cover slips were inoculated with RV-Wa (100 TCID50) for 2 h at 37 °C. After washing to remove the inoculum, cells were overlaid with a medium containing 80 μM genistein or mock-treated with DMEM and incubated for 12 h or 36 h, respectively, at 37 °C. Cells were fixed and analyzed by IFA using antibody against RV VP-6 protein (green); the nucleus (blue); bar, 50 or 100 μm. Data shown are expressed as mean ± standard deviation of triplicate cultures, and three independent experiments were carried out (*, p < 0.05; **, p < 0.01; ***, p < 0.001)
AQP mRNA and protein expression levels after rotavirus infection
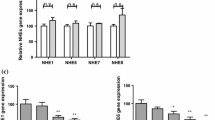
Previous studies have shown that AQPs play an important role in infectious diarrhea [8, 11]. AQP mRNA and protein expression was measured after rotavirus Wa or SA-11 infection. The mRNA and protein expression levels of AQP1, AQP3, AQP4 and AQP8 were determined in mock-infected and rotavirus-Wa- or SA-11-treated Caco-2 cells using real-time RT-PCR and western blot analysis, respectively. As shown in Fig. 3a and b, there were no significant differences in the expression levels of AQP1, AQP3 and AQP8 between mock-infected and RV-infected Caco-2 cells. However, AQP4 mRNA and protein expression level was significantly reduced in RV-Wa-infected Caco-2 cells in comparison with mock-infected cells. As illustrated in Fig. 3a and c, for RV-SA-11-treated Caco-2 cells, AQP1, and AQP4 mRNA and protein expression levels were significantly reduced in Caco-2 cells after RV-SA-11 exposure in comparison with mock-treated Caco-2 cells. In addition, after incubation with RV-Wa, cell lysates were harvested at 0, 6, 12, 18, 24, 36 h, and the AQP4 protein expression level was measured by western blotting. As shown in Fig. 3d, the AQP4 protein expression level started to decrease significantly at 6 h after RV-Wa infection.
Changes in AQP (1, 3, 4 and 8) mRNA and protein levels in Wa- or SA-11-rotavirus- infected Caco-2 cells. Caco-2 cell monolayers in 6-well plates were incubated with rotaviruses Wa or SA-11 (100 TCID50) or mock-infected with DMEM for 2 h at 37 °C, and the virus was then removed by washing, followed by incubation with fresh DMEM for a further 12 h at 37 °C. (a) Total RNA was extracted from the cells, and the AQP mRNA level was determined using a quantitative real-time RT-PCR assay. (b) and (c) Whole-cell lysates were harvested in RIPA buffer containing PMSF. The protein levels of AQPs (1, 3, 4 and 8) were determined by western blotting, correcting for the GAPDH level. (d) Caco-2 cell monolayers in 60-mm dishes were incubated with RV-Wa (100 TCID50), cell lysates were harvested at 0, 6, 12, 18, 24, 36 h, and the AQP4 protein expression level was measured by western blotting at the indicated times, correcting for the GAPDH level. The band intensity was quantified using Quantity One software. Each column represents the mean ± standard deviation of three independent experiments. n.s., not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001
Genistein upregulates AQP4 expression in rotavirus-Wa-infected Caco-2 cells
Since AQP4 mRNA and protein expression levels were significantly reduced in RV-infected Caco-2 cells, we investigated whether genistein could restore AQP4 expression in RV-Wa-infected Caco-2 cells. Caco-2 cell monolayers in 6-well plates were inoculated with RV-Wa (100 TCID50) for 2 h at 37 °C. After washing to remove the inoculum, cells were overlaid with a medium containing genistein at different concentrations and incubated for 12 h at 37 °C. After this incubation, genistein was removed by washing, and cell lysates and total RNA were collected after further incubation for 12 h at 37 °C. As illustrated in Fig. 4a, AQP4 mRNA expression was significantly increased in RV-Wa-infected cells that were treated with genistein. Moreover, we also found that genistein treatment resulted in increased AQP4 mRNA expression in a time-dependent and dose-dependent manner (Fig. 4a and b). AQP4 protein expression was also detected by western blot. Similarly, genistein treatment resulted in increased expression of AQP4 protein in RV-Wa-infected cells (Fig. 4c). These results provide further evidence that genistein treatment can restore AQPs expression and may reduce secretion of fluid into the intestinal lumen, leading to a reduction in the incidence and severity of RV-induced diarrhea.
Genistein upregulates the expression level of AQP4 in RV-infected Caco-2 cells. (a) Caco-2 cell monolayers in 6-well plates were incubated with the RV-Wa (100 TCID50) or mock-infected with DMEM for 2 h at 37 °C, unbound virus was removed by washing and different concentrations of genistein (0, 20, 40, 80 μM) were added. Incubation was continued for 12 h at 37 °C. Total RNA was extracted from the cells, and the AQP4 mRNA level was determined using a quantitative real-time RT-PCR assay. (b) Caco-2 cell monolayers in 60-mm dishes were incubated with RV-Wa (100 TCID50) or mock infected with DMEM for 2 h at 37 °C, the unbound virus was removed by washing, and the cells were treated with 80 μM genistein. Total RNA was extracted from the cells at 0, 6, 12, and 24 h, and the AQP4 mRNA expression level was measured by a quantitative real-time RT-PCR assay at the indicated times. (c) Caco-2 cell monolayers in 60-mm dishes were incubated with RV-Wa (100 TCID50) or mock-infected with DMEM for 2 h at 37 °C, the unbound virus was removed by washing, and the cells were treated with 80 μM genistein for 12 h at 37 °C. Cell lysates were harvested from the cells at 0, 12, and 24 h, and the AQP4 protein expression level was measured by western blotting at the indicated times, correcting for the GAPDH level. The band intensity was quantified using Quantity One software. Each column represents the mean ± standard deviation of three independent experiments (*, p < 0.05)
Effect of genistein on intracellular AC and PKA activity in rotavirus-infected Caco-2 cells
Previous studies have demonstrated that changes in PKA activity can affect the expression of AQPs [30, 31]. We therefore examined whether genistein regulates AQP4 expression by activating PKA. As shown in Fig. 5a, AC activity increased significantly to approximately 1.38 times that of the control at a rate similar to that seen with forskolin (50 μM), which is known to activate AC. After treatment with genistein, as shown in Fig. 5b, PKA activity also increased significantly to approximately 1.59 times that of the control; this increase was similar to that seen with the addition of 8-Br cAMP, which is known to activate PKA. When cells were treated with H-89, a common PKA inhibitor, PKA activity decreased significantly relative to that of the control. However, no significant difference was found when cells were treated with both genistein and H-89. These results indicated that the regulation of AQP4 expression by genistein occurred via changes in cellular PKA activity in RV-infected Caco-2 cells.
Genistein-enhanced AQP4 expression is mediated via activation of the cAMP/PKA/CREB cascade in RV-infected Caco-2 cells. (a) Caco-2 cell monolayers in 6-well plates were incubated with RV-Wa (100 TCID50) or mock infected with DMEM for 2 h at 37 °C, and the virus was then removed by washing, and the cells were treated with DMEM, 80 μM genistein or the AC activator forskolin (50 μM) for 12 h at 37 °C. Mock-infected cells were treated with DMEM. AC activity was assayed by determining the rate of formation of cAMP from ATP and presented using the mean of the control cells as 100 %. (b) Caco-2 cell monolayers in 6-well plates were incubated with RV-Wa (100 TCID50) or mock-infected with DMEM for 2 h at 37 °C, the unbound virus was removed by washing, and the cells were treated with DMEM, 80 μM genistein, PKA agonist 8-Br cAMP (1 mM), PKA inhibitor H-89 or H-89 + genistein. Incubation was continued for 12 h at 37 °C. Mock-infected cells were treated with DMEM. Cells were collected in PKA extraction buffer, and the intracellular PKA activity was determined. (c) Caco-2 cell monolayers in 6-well plates were incubated with RV-Wa (100 TCID50) or mock-infected with DMEM for 2 h at 37 °C, the unbound virus was removed by washing, and different concentrations of genistein (0, 20, 40, 80 μM) were added. Mock-infected cells were treated with DMEM. Incubation was continued for 12 h at 37 °C. Cell lysates were harvested from the cells and the CREB, and p-CREB protein expression levels were determined by western blotting. (d) Caco-2 cell monolayers in 6-well plates were infected with RV-Wa or mock infected for 2 h at 37 °C. Unbound virus was removed by washing, and the cells were treated with DMEM, PKA inhibitor H-89, 80 μM genistein, or H-89 + genistein. Mock-infected cells were also treated with 80 μM genistein. Incubation was continued for 12 h at 37 °C. Cell lysates were harvested from the cells, and the CREB, p-CREB, AQP4 and GAPDH protein expression levels were determined by western blotting. Quantification of three independent western blot experiments were done with the mean level of p-CREB normalized to total CREB. The AQP4 protein expression level was corrected for the GAPDH level. The data show the mean ± SD. n.s., not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001)
Genistein-stimulated AQP4 expression is mediated via CREB in rotavirus-infected Caco-2 cells
To determine the role of CREB in mediating the effect of genistein on AQP4 expression, we first investigated whether activation of PKA by genistein results in higher CREB phosphorylation. Caco-2 cell monolayers in 6-well plates were inoculated with RV-Wa (100 TCID50) for 2 h at 37 °C. After washing to remove the inoculum, cells were overlaid with a medium containing genistein at different concentrations, H-89, or H-89 + genistein and incubated for 12 h at 37 °C. After incubation, genistein was removed by washing, cell lysates were harvested, and the level of CREB phosphorylation was assayed by determining the ratio of p-CREB to CREB protein after incubation for a further 12 h at 37 °C. Western blot analysis revealed that after the addition of 20, 40 or 80 µM genistein, CREB phosphorylation in RV-Wa-infected Caco-2 cells increased significantly in comparison with the control, which is consistent with the increase observed in the AQP4 expression level induced by genistein (Fig. 5c). Pretreatment of RV-infected cells with the PKA inhibitor H-89 completely abolished the CREB phosphorylation induced by genistein treatment (Fig. 5d), suggesting that PKA mediates the genistein-stimulated phosphorylation of CREB. These results suggest that genistein upregulates AQP4 expression via the cAMP/PKA/CREB signaling pathway in RV-infected Caco-2 cells.
Discussion
Rotavirus is the leading cause of severe secretory diarrhea in infants and young children worldwide, resulting in an estimated 0.5 million deaths annually in children under age 5 years, which represents about one-third of deaths attributed to dehydration [1, 2]. Rotavirus infection represents a significant disease burden in developing countries, such as those in South Asia. Despite its clinical importance and several decades of research, knowledge of the pathophysiological mechanisms leading to this life-threatening disease remains limited.
The mechanisms by which intestinal rotavirus infection produces diarrhea remain to be fully elucidated. Studies have shown that rotavirus-induced secretory diarrhea results from a combination of excessive secretion of fluid and electrolytes into the intestinal lumen and reduced fluid absorption [5, 6]. Excessive fluid secretion is mainly caused by active chloride secretion into the intestinal lumen, which drives secondary movement of sodium and water [32, 33]. During this process, it is believed that rotaviral non-structural protein (NSP4), a secreted viral enterotoxin, activates calcium-activated chloride channel(s) (CaCCs) and inhibits the Na+/glucose-cotransporter SGLT1 at the luminal membrane of enterocytes [34–36]. Currently, a number of sodium and chloride ion channels have been identified in RV-induced secretory diarrhea, such as epithelial Na+ channel (ENaC), TMEM16A, and sodium-potassium-chloride cotransporter (NKCC1) [37]. However, the role of these channels in RV-induced diarrhea remains unclear.
Four AQP subtypes (AQPs 1, 3, 4 and 8) have been reported to be expressed in the colon and play an important role in several physiological and pathological processes [38–40]. An increasing body of evidence indicates an association between abnormal AQPs expression and pathological diarrhea development [8–14]. However, the expression and pathophysiological role of AQPs in RV infection have not been fully established. In the present study, the expression levels of AQP mRNA and protein were measured in rotavirus-infected Caco-2 cells by real-time PCR and western blotting, respectively. We found no significant differences in the expression levels of AQP1, AQP3 and AQP8 between mock-infected and RV-Wa-infected Caco-2 cells. However, AQP4 mRNA and protein expression levels were significantly reduced in Wa-rotavirus-infected Caco-2 cells in comparison with mock-infected cells. For rotavirus-SA-11-treated Caco-2 cells, AQP1 and AQP4 mRNA and protein expression levels were significantly reduced in Caco-2 cells after RV-SA-11 infection in comparison with mock-treated Caco-2 cells. The time-course of AQP4 protein expression was examined in RV-infected Caco-2 cells. We found that the expression of AQP4 protein significantly decreased after RV-Wa infection in a time-dependent manner. We therefore speculate that there may be a functional correlation between rotavirus-induced diarrhea and AQP expression in the colon. Abnormal expression of AQP4 in RV infection might result in excessive secretion of fluid and electrolytes into the intestinal lumen and reduced fluid absorption by host intestinal cells, resulting in diarrhea. Our work provides important insights into the pathophysiological mechanisms of RV-induced diarrhea.
Currently, no specific drug against rotavirus infection is available. We demonstrate here that genistein treatment of RV-infected Caco-2 cells in vitro suppresses RV-Wa replication and the release of virus particles, as reduced infectious viral titers were observed in RV-infected Caco-2 cells after treatment with genistein. Furthermore, genistein inhibited rotavirus Wa replication, as it decreased rotavirus RNA, antigen, and structural protein VP-6 expression in RV-infected Caco-2 cells. However, we did not observe an effect of genistein on RV binding and entry after incubation with RV. Due to the significant role of AQPs in RV infection, we further investigated the effect of genistein on AQP expression in RV-infected Caco-2 cells. Expression of AQP4 mRNA and protein was significantly upregulated in a dose-dependent and time-dependent manner in RV-infected Caco-2 cells when treated with genistein. These finding suggest that AQP4 may be a drug target for treatment of disease caused by RV infection.
Since genistein treatment can inhibit RV-Wa replication and viral protein synthesis, as well as upregulate AQP4 expression in RV-infected Caco-2 cells, we explored the effect of genistein on AQP4 at both the cellular and molecular level. Previous studies have demonstrated that the water channel activity of AQPs is significantly increased by protein kinase A (PKA) activators such as cAMP and forskolin [41, 42]. Some studies have shown that rotavirus NSP4 may increase the concentration of cAMP by binding to specific receptors on intestinal cells, leading to secretion of Cl− and further reducing the absorption of sodium and water [43, 44]. In the present study, however, we failed to find any significant alteration in AC and PKA activity between RV-infected and mock-infected cells. The use of genistein as an anti-RV drug may increase the AQP4 expression level in RV-infected Caco-2 cells via the cAMP/PKA/CREB pathway as follows: An increase in the intracellular genistein concentration causes the activation of AC, which leads to an increase in cAMP production. Then, the increase in the cAMP concentration in turn causes PKA activation, which promotes CREB phosphorylation at Ser133. Finally AQP4 gene transcription is promoted.
In the colon, AQP4 is localized at the basolateral membrane of surface epithelial cells and might play a role in colonic fluid transport [38]. This possibility was assessed in AQP4-knockout mice. In AQP4-deficient mice, the water content of defecated stool is higher, and a reduction of water osmotic permeability was also observed [45]. The transepithelial water permeability of the proximal colon of wild-type mice is higher than that of the distal colon of wild-type mice, and it is also higher than that of the proximal colon of AQP4 null mice. AQP4 deletion does not affect water permeability in the distal colon, which results in an increase in the water content of defecated stool [45]. These results imply that AQP4 may play an important role in colonic water absorption, particularly in the proximal colon. Furthermore, it also seems that decreased AQP expression might lead to an insufficiency of water absorption in this region of the gastrointestinal (GI) tract. Future studies investigating the enteric AQP4 expression level and water transport may aid in the development of new anti-RV drugs, applying the novel therapeutic strategy of targeting AQPs.
In summary, our results support the conclusion that the pathophysiological mechanism of rotavirus infection involves decreased expression of AQP4 and that genistein may be a useful candidate for developing a new anti-RV strategy by inhibiting rotavirus replication and upregulating AQP4 expression via the cAMP/PKA/CREB signaling pathway. Further studies on the effect of genistein on RV-induced diarrhea are warranted.
References
Jayaram H, Estes MK, Prasad BV (2004) Emerging themes in rotavirus cell entry, genome organization, transcription and replication. Virus Res 101:67–81
Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD (2012) 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 12:136–141
Parashar UD, Gibson CJ, Bresee JS, Glass RI (2006) Rotavirus and severe childhood diarrhea. Emerg Infect Dis 12:304–306
Bilcke J, Van Damme P, Van Ranst M, Hens N, Aerts M, Beutels P (2009) Estimating the incidence of symptomatic rotavirus infections: a systematic review and meta-analysis. PLoS ONE 4:e6060
Ko EA, Jin BJ, Namkung W, Ma T, Thiagarajah JR, Verkman AS (2014) Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut 63:1120–1129
Guarino A, Buccigrossi V, Armellino C (2009) Colon in acute intestinal infection. J Pediatr Gastroenterol Nutr 48(Suppl 2):S58–S62
Kunzelmann K, Mall M (2002) Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev 82:245–289
Hamabata T, Liu C, Takeda Y (2002) Positive and negative regulation of water channel aquaporins in human small intestine by cholera toxin. Microbial Pathogene 32:273–277
Hardin JA, Wallace LE, Wong JF, O’Loughlin EV, Urbanski SJ, Gall DG, MacNaughton WK, Beck PL (2004) Aquaporin expression is downregulated in a murine model of colitis and in patients with ulcerative colitis, Crohn’s disease and infectious colitis. Cell Tissue Res 318:313–323
Sakai H, Sagara A, Matsumoto K, Hasegawa S, Sato K, Nishizaki M, Shoji T, Horie S, Nakagawa T, Tokuyama S, Narita M (2013) 5-Fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines. PLOS One 8:e54788
Guttman JA, Samji FN, Li Y, Deng W, Lin A, Finlay BB (2007) Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell Microbiol 9:131–141
Laforenza U, Miceli E, Gastaldi G, Scaffino MF, Ventura U, Fontana JM, Orsenigo MN, Corazza GR (2010) Solute transporters and aquaporins are impaired in celiac disease. Biol Cell 102:457–467
Zhang Y, Wang X, Sha S, Liang S, Zhao L, Liu L, Chai N, Wang H, Wu K (2012) Berberine increases the expression of NHE3 and AQP4 in sennoside A-induced diarrhoea model. Fitoterapia 83:1014–1022
Yamamoto T, Kuramoto H, Kadowaki M (2007) Downregulation in aquaporin 4 and aquaporin 8 expression of the colon associated with the induction of allergic diarrhea in a mouse model of food allergy. Life Sci 81:115–120
Leung AK, Kellner JD, Davies HD (2005) Rotavirus gastroenteritis. Adv Ther 22:476–487
Bellemare S, Hartling L, Wiebe N, Russell K, Craig WR, McConnell D, Klassen TP (2004) Oral rehydration versus intravenous therapy for treating dehydration due to gastroenteritis in children: a meta-analysis of randomised controlled trials. BMC Med 2:11
Van Niel CW, Feudtner C, Garrison MM, Christakis DA (2002) Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 109:678–684
Sarker SA, Sultana S, Fuchs GJ, Alam NH, Azim T, Brussow H, Hammarstrom L (2005) Lactobacillus paracasei strain ST11 has no effect on rotavirus but ameliorates the outcome of nonrotavirus diarrhea in children from Bangladesh. Pediatrics 116:e221–e228
Santosham M (2010) Rotavirus vaccine–a powerful tool to combat deaths from diarrhea. New Engl J Med 362:358–360
Ward RL, Bernstein DI (2009) Rotarix: a rotavirus vaccine for the world. Clin Infect Dis 48:222–228
Anderson EJ (2008) Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect Dis 8:642–649
Parez N (2008) Rotavirus gastroenteritis: why to back up the development of new vaccines? Comp Immunol Microbiol Infect Dis 31:253–269
Clark A, Sanderson C (2009) Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 373:1543–1549
Akula SM, Hurley DJ, Wixon RL, Wang C, Chase CC (2002) Effect of genistein on replication of bovine herpesvirus type 1. Am J Vet Res 63:1124–1128
Yura Y, Yoshida H, Sato M (1993) Inhibition of herpes simplex virus replication by genistein, an inhibitor of protein-tyrosine kinase. Arch Virol 132:451–461
Andres A, Donovan SM, Kuhlenschmidt MS (2009) Soy isoflavones and virus infections. J Nutr Biochem 20:563–569
Andres A, Donovan SM, Kuhlenschmidt TB, Kuhlenschmidt MS (2007) Isoflavones at concentrations present in soy infant formula inhibit rotavirus infection in vitro. J Nutr 137:2068–2073
Flint SJ, Enquist W, Racaniello VR, Skalka AM (2009) Virological methods. In: Principles of virology. ASM Press, Washington, DC. ISBN 1-55581-443-3
Aurbach GD, Chase LR (1970) Cyclic 3′,5′-adenylic acid in bone and the mechanism of action of parathyroid hormone. Fed Proc 29:1179–1182
Wang W, Li C, Kwon TH, Knepper MA, Frokiaer J, Nielsen S (2002) AQP3, p-AQP2, and AQP2 expression is reduced in polyuric rats with hypercalcemia: prevention by cAMP-PDE inhibitors. Am J Physiol Renal Physiol 283:F1313–F1325
Woo J, Lee J, Kim MS, Jang SJ, Sidransky D, Moon C (2008) The effect of aquaporin 5 overexpression on the Ras signaling pathway. Biochem Biophys Res Commun 367:291–298
Thiagarajah JR, Broadbent T, Hsieh E, Verkman AS (2004) Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology 126:511–519
Thiagarajah JR, Verkman AS (2012) CFTR inhibitors for treating diarrhea disease. Clin Pharmacol Ther 92:287–290
Halaihel N, Lievin V, Ball JM, Estes MK, Alvarado F, Vasseur M (2000) Direct inhibitory effect of rotavirus NSP4(114-135) peptide on the Na(+)-d-glucose symporter of rabbit intestinal brush border membrane. J Virol 74:9464–9470
Morris AP, Scott JK, Ball JM, Zeng CQ, O’Neal WK, Estes MK (1999) NSP4 elicits age-dependent diarrhea and Ca(2+)mediated I(−) influx into intestinal crypts of CF mice. Am J Physiol 277:G431–G444
Hempson SJ, Matkowskyj K, Bansal A, Tsao E, Habib I, Benya R, Mackow ER, Shaw RD (2010) Rotavirus infection of murine small intestine causes colonic secretion via age restricted galanin-1 receptor expression. Gastroenterology 138:2410–2417
Ousingsawat J, Mirza M, Tian Y, Roussa E, Schreiber R, Cook DI, Kunzelmann K (2011) Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflugers Arch 461:579–589
Laforenza U (2012) Water channel proteins in the gastrointestinal tract. Mol Aspects Med 33:642–650
Ma T, Verkman AS (1999) Aquaporin water channels in gastrointestinal physiology. J Physiol 517(Pt 2):317–326
Matsuzaki T, Tajika Y, Ablimit A, Aoki T, Hagiwara H, Takata K (2004) Aquaporins in the digestive system. Med Electron Microsc 37:71–80
Han Z, Patil RV (2000) Protein kinase A-dependent phosphorylation of aquaporin-1. Biochem Biophys Res Commun 273:328–332
Wang S, Amidi F, Beall M, Gui L, Ross MG (2006) Aquaporin 3 expression in human fetal membranes and its up-regulation by cyclic adenosine monophosphate in amnion epithelial cell culture. J Soc Gynecol Investig 13:181–185
Beau I, Cotte-Laffitte J, Geniteau-Legendre M, Estes MK, Servin AL (2007) An NSP4-dependant mechanism by which rotavirus impairs lactase enzymatic activity in brush border of human enterocyte-like Caco-2 cells. Cell Microbiol 9:2254–2266
Martin-Latil S, Cotte-Laffitte J, Beau I, Quero AM, Geniteau-Legendre M, Servin AL (2004) A cyclic AMP protein kinase A-dependent mechanism by which rotavirus impairs the expression and enzyme activity of brush border-associated sucrase-isomaltase in differentiated intestinal Caco-2 cells. Cell Microbiol 6:719–731
Wang KS, Ma T, Filiz F, Verkman AS, Bastidas JA (2000) Colon water transport in transgenic mice lacking aquaporin-4 water channels. Am J Physiol Gastrointest Liver Physiol 279:G463–G470
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81173636 to L. S.; and No. 81473401 to W. Z.), and the Natural Science Foundation of Guangdong Province (S2011040005339 to L. S.). We thank Dr. Haiyang He for the provision of rotavirus and technical help. We are also grateful for useful comments on the manuscript by the colleagues of Sino-American Cancer Research Institute, Guangdong Medical College.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, H., Liao, D., Liang, L. et al. Genistein inhibits rotavirus replication and upregulates AQP4 expression in rotavirus-infected Caco-2 cells. Arch Virol 160, 1421–1433 (2015). https://doi.org/10.1007/s00705-015-2404-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2404-4