Abstract
Although a white spot syndrome virus (WSSV) subunit vaccine could significantly enhance the immune response and benefit the shrimp host, its practical application is currently not feasible because of drawbacks in existing expression systems. We generated a transgenic Dunaliella salina (D. salina) strain by introducing the WSSV VP28 gene to produce a novel oral WSSV subunit vaccine. Following transformation of D. salina, VP28 gene expression was assessed by reverse transcription polymerase chain reaction (RT-PCR) assays, enzyme-linked immunosorbent assays (ELISAs), and western blot analysis. The RT-PCR results indicated that the VP28 gene was successfully expressed in D. salina cells. The presence of recombinant VP28 proteins with natural bioactivity was confirmed by western blot analysis and ELISA. Animal vaccination experiments indicated that transgenic D. salina can induce protection against WSSV by oral delivery in crayfish. Our findings indicate that the VP28 gene can be successfully expressed in transgenic D. salina and can be applied as an oral vaccine to protect crayfish against WSSV. We have demonstrated that it is feasible to produce an oral vaccine using D. salina, and thereby provide a new method for controlling other viral diseases in crustaceans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White spot syndrome virus (WSSV) is the most serious pathogen that adversely impacts shrimp farms worldwide. An efficient vaccine would be a desirable and feasible method for controlling the spread of WSSV. There is growing evidence that various vaccines, such as inactivated WSSV vaccines [1, 2], recombinant viral subunit vaccines [3], DNA vaccines [4], and RNA vaccines [5, 6], can significantly enhance immune responses in the host and enhance resistance against WSSV-associated diseases.
More than 39 WSSV structural proteins have been identified [7, 8]. VP28, the main viral envelope protein, plays a pivotal role during viral infection, budding, entry, and virion assembly and has become the main target for exploitation of WSSV subunit vaccines [9, 10]. The expression of recombinant VP28 has been attempted in a variety of host systems, including Pichia pastoris [11], Brevibacillus brevis [12], Escherichia coli [13, 14], baculoviruses [15], silkworm pupae [16], and Bacillus subtilis [17, 18]. However, there are several disadvantages associated with these expression systems, including unsafe injection methods, high costs, long cultivation times, and difficulties in batch production. These disadvantages have resulted in the aforementioned expression systems not being considered feasible for practical applications in aquaculture. Thus, an optimal expression system for the production of a WSSV subunit vaccine is necessary and must be developed.
The eukaryotic green alga, Dunaliella salina, has been exploited as a novel bioreactor [19], as it has a very simple unicellular structure and can be cultured easily, rapidly, and cheaply. It has a high capacity for large-scale growth under natural conditions and can effectively accumulate protein products at high yields. D. salina lacks a rigid cell wall, making it a naturally occurring protoplast, which in turn makes it relatively easy to introduce exogenous genes. As a eukaryotic organism, it can facilitate transcription and translation to produce crude proteins [20]. Because it is photosynthetic, D. salina can be cultured in open photobioreactors or natural seawater. D. salina cells have a single, large, cup-shaped chloroplast which can be used as an alternative and effective expression system to nuclear expression, as “position effect”, “gene escaping” and “gene silencing” can be eliminated [21, 22]. Other valuable components, such as carotenoids, lipids, vitamins, and minerals, accumulate in D. salina, and therefore this alga has great potential for industrial and pharmaceutical applications [23]. As a bioreactor, D. salina is an optimal choice that provides the perfect opportunity for the production of a WSSV subunit vaccine.
Shrimp are the natural hosts for WSSV, and the WSSV structural gene can be introduced into D. salina cells by transformation; in turn, D. salina cells can be used as crude bait for shrimp. Furthermore, all these organisms live in the same seawater environment. Using these ecological interactions, we attempted to produce a live oral vaccine for controlling the spread of WSSV. This vaccine can be used more conveniently for shrimp than other vaccines, and it is expected to yield better immune protection.
Materials and methods
Algae strain and culture conditions
The UTEX-1644 strain of D. salina was purchased from the Culture Collection of Algae at the University of Texas (Austin, TX, USA). Under a light intensity of 50 μmol photon m−2 s−1, D. salina cells were cultured in modified PKS medium at 26 °C over 12-h night/day cycles [24]. At the logarithmic growth phase (105 cells ml−1), cells were harvested by centrifugation for subsequent transformation. During this phase, D. salina cells had uniform size, shape, and movement (Fig. 1).
Cloning and sequencing the VP28 gene
Prior to VP28 gene cloning, WSSV was purified and titrated from an infected Penaeus monodon shrimp using sucrose gradient centrifugation [3, 25]. WSSV genomic DNA was extracted using the phenol-chloroform method [10]. Using the viral DNA as template, PCR amplification was conducted using specific primers 5′-CA CCC GGG ATG GAT CTT TCT TTC ACT CTT TC-3′ and 5′-TC GAG CTC TTA CTC GGT CTC AGT GCC AGA-3′; the italics sequences represent SmaI and SacI endonuclease recognition sites, respectively), which were designed according to the coding region of VP28 (GenBank accession no. AF502435.1). Amplification reactions comprised 5 μl of PCR buffer (10×), 1 μl of template DNA, 2 μl of each primer (25 μM), 2 μl of dNTPs (25 μM), 1 μl of Taq DNA polymerase (5 U/μl) and dH2O to a volume of 50 μl. The thermal cycling conditions involved an initial denaturation step at 94 °C for 4 min, followed by 30 cycles at 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, and a final extension step at 72 °C for 10 min and termination at 4 °C. Amplifications were visualized by electrophoresis on 1 % (w/v) agarose gels. Amplified VP28 fragments were inserted into the pMD19-T vector (TaKaRa, Dalian, China) to yield pMD19-T-VP28 and sequenced (Sangon, Shanghai, China).
Generation of the eukaryotic expression vector
The pBI221-bar and pUΩ-GUS plasmids were obtained from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences. The pUΩ-GUS plasmid was digested using endonucleases, and the linearized pUΩ- was then collected using a DNA gel extraction kit (Axygen Biosciences, USA). Using SmaI and SacI, the VP28 fragment was excised from pMD19-T-VP28 and ligated into linearized pUΩ- to generate pUΩ-VP28. The bar box was removed from pBI221-bar by HindIII digestion and inserted into pUΩ-VP28 to produce pUΩ-VP28-bar (Fig. 2).
Schematic diagram of the pUΩ-VP28-bar vector. Bar box, bialaphos resistance gene driven by the CaMV35S promoter; Ubil, maize ubiquitin promoter; Ω, 5′ leader sequence of tobacco mosaic virus RNA; VP28, WSSV VP28 gene; Nos, nopaline synthase gene terminator. Other labels are the restriction endonuclease sites
Transformation and screening of D. salina cells
Using glass beads [24], pUΩ-VP28-bar was introduced into D. salina cells using optimized transformation parameters. Transformed D. salina cells were cultured in fresh liquid medium under dim light for 24 h and then harvested by centrifugation and plated on solid selective PKS medium containing 10 g l−1 agarose and 3 μg ml−1 phosphinothricin (PPT) to screen for positive colonies. Negative controls were prepared by treating D. salina cells using the same transformation protocol without pUΩ-VP28-bar. Independent experiments were repeated at least three times.
Reverse transcription polymerase chain reaction (RT-PCR) analysis of transformants
When the cell concentration reached 105 cells ml−1, transformed cells were collected by centrifugation and washed three times with fresh liquid medium. Following the manufacturer’s instructions, total RNA was extracted using Trizol Reagent (Invitrogen). Using the extracted total RNA as template, RT-PCR was conducted to detect VP28 gene expression. Amplification products were analyzed by electrophoresis on 10 g l−1 agarose gels. The transformed strain with the highest expression level of mRNA was selected from three transformants and used for further western blotting assays.
ELISA and western blot analysis of recombinant VP28 proteins
Proteins were extracted from the negative control and transformants using Trizol, and measured by enzyme-linked immunosorbent assay (ELISA) and western blot analysis. The concentration of proteins was determined using the Bradford assay [26]. Approximately 150 μl of total protein lysate from each transformant was used to coat ELISA plates at 37 °C overnight. After washing twice with Tris-buffered saline (TBS; 150 mM NaCl, 10 mM Tris, pH 7.4), the plate was incubated with rabbit anti-VP28 IgG in TBS for 2 h at 37 °C. Subsequently, the plate was incubated with goat anti-rabbit IgG conjugated to alkaline phosphatase (Sigma) at 37 °C for 1 h. The substrate solution was added to the plate for 20 min at 37 °C, and the absorbance at 492 nm was determined using a microplate reader.
The method of Yoganandhan et al. [27] was used for western blot detection of recombinant VP28. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. After blocking with 3 % (w/v) skim milk, membranes were incubated with rabbit anti-VP28 IgG for 2 h, and then with goat anti-rabbit IgG conjugated with alkaline phosphatase for 1.5 h at 37 °C. NBT and BCIP were used as substrates, and protein molecular weights were estimated with Band Scan 5 software.
Vaccination experiment and WSSV challenge
The oral vaccination experiment for transgenic D. salina in animals was carried out according to the method of Fu et al. [17]. Briefly, cultures (50 ml) of transgenic D. salina cells, including Ds-VP28 and Ds-empty (1 × 108 cells ml−1; Table 1) were centrifuged, and cell pellets were resuspended in 15 ml of supernatant at 4 °C. Resuspended cells were subjected to two cycles of freeze-thawing for initial lysis of cells. Cells were then sonicated (250 W, 25 kHz, 7 s on/10 s off, 50 cycles) on ice, and lysates were observed with a microscope to ensure that D. salina cells were fully inactivated. Lysed cells were spread over 50 g of commercial crayfish feed, incubated on ice for 30 min to allow the feed to absorb lysed cellular material, and the feed was coated with fish oil. The negative and positive control pellets were coated with phosphate-buffered saline (PBS). Four groups of 20 WSSV-free crayfish were established. Each group was subjected to the vaccination experiment three times. The negative and positive control crayfish were orally administered PBS-coated feed, whereas the crayfish in groups 3 and 4 were orally administered Ds-VP28 and Ds-empty coated feed, respectively, for 10 consecutive days. Experimental crayfish were fed with coated food pellets to 5 % of their body weight. After the final vaccination, the crayfish in groups 2–4 were immediately challenged by immersing them in seawater containing WSSV stock solution (1 × 10−4 dilution) for 3 h. Negative control crayfish were challenged with PBS. All crayfish were then transferred to fresh WSSV-free seawater, and mortality at 20 days post-challenge was recorded. Dead crayfish were tested by one-step PCR to determine the presence of WSSV.
Statistical analysis
Data from all experiments were analyzed using a paired-samples t-test with SPSS (version16.0, Chicago, IL, USA). A P-value less than 0.05 was considered statistically significant.
Results
Gene cloning and vector construction
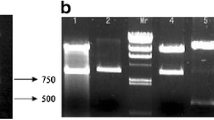
The PCR results revealed a specific fragment of 615 bp in lane 1 (Fig. 3A), consistent with expectations. Gene sequencing results showed that the sequence of the amplified DNA fragment was consistent with those in GenBank. Our findings demonstrate that the WSSV VP28 gene was successfully cloned and had the correct sequence, and that it could be liberated from pUΩ-VP28 (Fig. 3B). The bar box within pUΩ-VP28-bar was successfully excised (Fig. 3C).
Cloning the WSSV VP28 gene. (A) PCR amplification products of the WSSV VP28 gene. M, DNA marker; lane 1, amplification products. (B) Restriction enzyme analysis of pUΩ-VP28. Lane 1, pUΩ-VP28 digested with SmaI and SacI; 2, pUΩ-VP28; M, DNA marker. (C) Restriction enzyme analysis of pUΩ-VP28-bar. Lane 1, pUΩ-VP28-bar digested with HindIII; 2, pUΩ-VP28-bar; M, DNA marker
Transformation of D. salina
Positive D. salina transformants appeared as uniform greenish algal colonies with diameters of about 2–3 mm (Fig. 4A and B). Three colonies were selected and inoculated in selective liquid selective containing 3 μg ml−1 PPT. After incubation for 7 days, all colonies exhibited cell growth with uniform size and movement. The recombinant eukaryotic expression vector was successfully introduced into the D. salina cells.
RT-PCR analysis of transformants
RT-PCR results revealed that the predicted size of the VP28 gene, a 615-bp PCR fragment, was detected in all transformants (Fig. 5) but not in the negative controls. Sequence results indicated that the amplified DNA fragment was consistent with the coding region of the VP28 gene. PPT resistance tests demonstrated that all transformants survived repeated PPT selection and were stable for 3 months, indicating that the recombinant vector was successfully integrated into the genome of D. salina cells and stably expressed in progeny cells.
ELISA and western blot analysis of transformants
Total protein concentrations were 16.7, 16.9, and 17.1 μg μl−1 for each D. salina transformant, and 16.6 μg μl−1 for the negative control. ELISA results show that the levels of VP28 protein in transformants 1–3 were 3.04 ± 0.26, 0.99 ± 0.08, and 2.13 ± 0.17 ng mg−1, respectively (Fig. 6). No protein was detected in the negative control (Fig. 6). Approximately 78 μg of recombinant VP28 protein was obtained from 100 ml cultures of D. salina.
Recombinant VP28 proteins appeared to be approximately 28 kDa in all three transformants and were lacking in the negative control (Fig. 7A). Our western blot results show that protein extracts from the transformant with maximal expression levels contained a band corresponding to 28 kDa (Fig. 7B). These results confirm that the recombinant VP28 protein was successfully expressed in D. salina cells and that it had specific immunologic activity.
SDS-PAGE and western blot analysis of D. salina transformants. (A) SDS-PAGE analysis of different D. salina transformants. M, protein marker; NC, negative control; T1–3, D. salina transformants. (B) Western blot analysis of the transformant with highest protein expression levels. Lane 1, recombinant VP28 protein; M, protein marker
Vaccination experiment and WSSV challenge
After vaccination, experimental crayfish were observed twice a day. Ds-VP28-vaccinated crayfish had significantly higher survival rates (59 % mortality) than the Ds-empty control and the positive control groups (100 % mortality; Fig. 8). No deaths occurred among the crayfish in the negative control group throughout the experimental period. Using PCR, dead crayfish were found to be positive for the presence of WSSV, and all randomly selected survivors were found to be negative for virus.
The temporal-mortality relationship in orally vaccinated crayfish. Cumulative mortality rates of crayfish from the experimental groups vaccinated with Ds-VP28 (●), Ds-empty (♦), positive control (▲), and negative control (■), as indicated in Table 1
Discussion
No adequate method or approach is currently available for controlling white spot disease. Initial treatments, such as reducing densities and decreasing water carrying capacities, are often infeasible because of their inefficiency and high cost [11]. Therefore, studies have focused on alternative methods of disease control, in particular vaccines against WSSV [14–17]. However, given the drawbacks of most expression systems, vaccines have not been able to be suitably applied. Thus, we attempted to produce a novel subunit vaccine against WSSV using D. salina cells.
In the present study, D. salina was used as a novel expression system for producing the WSSV subunit vaccine. After transformation, PPT-resistant D. salina transformants were obtained. The WSSV VP28 gene was successfully introduced into the genome of D. salina cells and expressed, resulting in a protein with natural immunological activity. The Ds-VP28 vaccinated crayfish showed an approximate 59 % survival rate, whereas Ds-empty and positive control crayfish exhibited 100 % mortality. This result shows that protection against WSSV can be conferred in crayfish using transgenic D. salina as an oral vaccine.
The immune effects of the recombinant WSSV subunit vaccine have a direct relationship to the expression level of the target protein and its bioactivity [12]. In our previous study, the expression levels of the VP28 protein varied among different transformants. This may be attributed to the random integration of the VP28 gene into the D. salina genome at different sites. Therefore, more-detailed work involving optimization of codon dependence [28], transformation-associated genotypic modifications [29], reduction of sensitivity to proteases [30], and fusion of recombinant products with native proteins [31] should be conducted to obtain higher VP28 expression levels. Our next study will focus on the selection and effects of adjuvants, the optimal immunization procedure, the best formulation for the specific immunogen, immunization time, and dose of transgenic D. salina for shrimps.
In summary, transgenic D. salina strains successfully expressed the WSSV VP28 gene, and induced protection against WSSV in crayfish through an oral delivery approach. Our results further imply that producing an oral vaccine by D. salina is feasible for the control of WSSV infections and will also provide a new method for controlling other viral diseases in crustacean species.
References
Namikoshi A, Wu JL, Yamashita T, Nishizawa T, Nishioka T, Arimoto M (2004) Vaccination trials with Penaeus japonicus to induce resistance to white spot syndrome virus. Aquaculture 229:25–35
Zhu F, Du HH, Miao ZG, Quan HZ, Xu ZR (2009) Protection of Procambarus clarkii against white spot syndrome virus using inactivated WSSV. Fish Shellfish Immunol 26:685–690
Witteveldt J, Cifuents CC, Vlak JM, Van Hulten MCW (2004) Protection of Penaeus monodon against white spot syndrome virus by oral vaccination. J Virol 78:2057–2061
Rajeshkumar S, Venkatesan C, Sarathi M, Sarathbabu V, Thomas J, Anver Basha K, Sahul Hameed AS (2009) Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV). Fish Shellfish Immunol 26:429–437
Mejía-Ruíz CH, Vega-Peña S, Alvarez-Ruiz P, Escobedo-Bonilla CM (2011) Double-stranded RNA against white spot syndrome virus (WSSV) vp28 or vp26 reduced susceptibility of Litopenaeus vannamei to WSSV, and survivors exhibited decreased susceptibility in subsequent re-infections. J Invertebr Pathol 107:65–68
Zhu F, Zhang X (2012) Protection of shrimp against white spot syndrome virus (WSSV) with β-1, 3-d-glucan-encapsulated vp28-siRNA particles. Mar Biotechnol (NY) 14:63–68
Tsai JM, Wang HC, Leu JH, Hsiao HH, Wang AH, Kou GH, Lo CF (2004) Genomic and proteomic analysis of thirty-nine structural proteins of shrimp white spot syndrome virus. J Virol 78:11360–11370
Tsai JM, Wang HC, Leu JH, Wang AH, Zhung Y, Walker PJ, Kou GH, Lo CF (2006) Identification of the nucleocapsid, tegument, and envelope proteins of the shrimp white spot syndrome virus virion. J Virol 80:3021–3029
Van Hulten MCW, Witteveldt J, Snippe M, Vlak JM (2001) White spot syndrome virus envelope protein VP28 is involved in the systemic infection of shrimp. Virology 285:228–233
Yi GH, Wang ZM, Qi YP, Yao LG, Qian J, Hu LB (2004) Vp28 of shrimp white spot syndrome virus is involved in the attachment and penetration into shrimp cells. J Biochem Mol Biol 37:726–734
Jha RK, Xu ZR, Bai SJ, Sun JY, Li WF, Shen J (2007) Protection of Procambarus clarkii against white spot syndrome virus using recombinant oral vaccine expressed in Pichia pastoris. Fish Shellfish Immunol 22:295–307
Caipang CM, Verjan N, Ooi EL, Kondo H, Hirono I, Aoki T, Kiyono H, Yuki Y (2008) Enhanced survival of shrimp, Penaeus (Marsupenaeus) japonicus from white spot syndrome disease after oral administration of recombinant VP28 expressed in Brevibacillus brevis. Fish Shellfish Immunol 25:315–320
Tang X, Hew CL (2007) Expression, purification and crystallization of two major envelope proteins from white spot syndrome virus. Acta Crystallogr Sect F Struct Biol Cryst Commun 63:624–626
Hou CL, Cao Y, Xie RH, Wang YZ, Du HH (2011) Characterization and diagnostic use of a monoclonal antibody for VP28 envelope protein of white spot syndrome virus. Virol Sin 26:260–266
Syed SM, Kwang J (2011) Oral vaccination of baculovirus-expressed VP28 displays enhanced protection against white spot syndrome virus in Penaeus monodon. PLoS One 6(11):e26428
Wei KQ, Yang JX (2011) Histological alterations and immune response in the crayfish Procambarus clarkii given rVP28-incorporated diets. Fish Shellfish Immunol 31:1122–1128
Fu LL, Shuai JB, Xu ZR, Li JR, Li WF (2010) Immune responses of Fenneropenaeus chinensis against white spot syndrome virus after oral delivery of VP28 using Bacillus subtilis as vehicles. Fish Shellfish Immunol 28:49–55
Ning D, Leng X, Li Q, Xu W (2011) Surface-displayed VP28 on Bacillus subtilis spores induce protection against white spot syndrome virus in crayfish by oral administration. J Appl Microbiol 111:1327–1336
Xue LX, Pan WD, Jiang GZ, Wang JR (2006) Patent No: US 7081567 B2: transgenic Dunuliella salina as a bioreactor
Walker TL, Purton S, Becker DK, Collet C (2005) Mocroalgae as bioreactors. Plant Cell Rep 24:629–641
Wang HH, Yin WB, Hu ZM (2009) Advances in chloroplast engineering. J Genet Genomics 36:387–398
Barzegari A, Hejazi MA, Hosseinzadeh N, Eslami S, Mehdizadeh Aghdam E, Hejazi MS (2010) Dunaliella as an attractive candidate for molecular farming. Mol Biol Rep 37:3427–3430
Hosseini Tafreshi A, Shariati M (2009) Dunaliella biotechnology: methods and applications. J Appl Microbiol 107:14–35
Feng SY, Xue LX, Liu HT, Lu PJ (2009) Improvement of efficiency of genetic transformation for Dunaliella salina by glass beads method. Mol Biol Rep 36:1433–1439
Du HH, Fu LL, Xu YX, Kil ZS, Xu ZR (2007) Improvement in a simple method for isolating white spot syndrome virus (WSSV) from the crayfish Procambarus clarkii. Aquaculture 262:532–534
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Yoganandhan K, Syed SM, Narayanan RB, Sahul Hameed AS (2004) Production of polyclonal antiserum against recombinant VP28 protein and its application for the detection of white spot syndrome virus in crustaceans. J Fish Dis 27:517–522
Rosales-Mendoza S, Paz-Maldonado LM, Soria-Guerra RE (2012) Chlamydomonas reinhardtii as a viable platform for the production of recombinant proteins: current status and perspectives. Plant Cell Rep 31:479–494
Surzycki R, Greenham K, Kitayama K, Dibal F, Wagner R, Rochaix JD, Ajam T, Surzycki S (2009) Factors effecting expression of vaccines in microalgae. Biologicals 37:133–138
Doran PM (2006) Foreign protein degradation and instability in plants and plant tissue cultures. Trends Biotechnol 24:426–432
Muto M, Henry RE, Mayfield SP (2009) Accumulation and processing of a recombinant protein designed as a cleavable fusion to the endogenous Rubisco LSU protein in Chlamydomonas chloroplast. BMC Biotechnol 9:1–11
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30900796) and the Doctor’s Research Initial Fund of Henan University of Science and Technology (No. 09001413). Special thanks are owed to David Michael Grobe from Indiana University-Purdue University Indianapolis for his proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, S., Feng, W., Zhao, L. et al. Preparation of transgenic Dunaliella salina for immunization against white spot syndrome virus in crayfish. Arch Virol 159, 519–525 (2014). https://doi.org/10.1007/s00705-013-1856-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-013-1856-7