Abstract
Alzheimer’s disease (AD) is the most prevalent and age-related dementia accompanied by neurodegenerative disorder, memory loss, and abnormal behaviors. Recent studies have shown an increasing interest in studying the role of microRNAs (miRNAs) and their potential values in the early diagnostics of AD. MiR-425-5p has extensively expression within various tissues and organs, acting as an important regulator in many pathological procedures. The functions of miR-425-5p involved in AD were investigated in the present study. The results showed that miR-425-5p was upregulated in patients with AD and HEK293/tau cells. Transfections with miR-425-5p overexpression vector significantly enhanced cell apoptosis, activated glycogen synthase kinase-3β (GSK-3β), and increased tau phosphorylation in HEK293/tau cells. Heat shock protein B8 (HSPB8) was directly targeted by miR-425-5p. Upregulation of miR-425-5p induced cell apoptosis and promoted tau phosphorylation partially via targeting HSPB8 in AD. Therefore, miR-425-5p might act as a new therapeutic target for AD treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease (AD) is the most prevalent dementia accompanied by neurodegenerative disorder, memory loss, and abnormal behavioral (Hardy and Higgins 1992; Khachaturian 1985). The development and progression of AD involve complex mechanisms such as neuronal dysplasia, angiogenic changes, and production of inflammatory factors (Geekiyanage et al. 2012; Nunez-Iglesias et al. 2010). Despite the advances from clinicians, it is still quite hard to diagnose AD at its early stage. Recent studies have shown an increasing interest in the role of microRNAs (miRNAs) regarding their potential values in the early diagnostics for AD (Kumar et al. 2017).
MiRNAs are endogenous small RNAs with a length of ~ 22 nucleotides (Lu et al. 2008). They can regulate target gene expression by binding to their 3′ untranslated regions (3′-UTR) and suppressing translation (Delay et al. 2012; Schonrock et al. 2010). MiRNAs are abundant in the brains and play essential roles in neurodevelopment and synaptic plasticity (Nelson et al. 2008). Dysregulations of miRNA expression in AD have been reported in previous studies. It suggested that miRNAs significantly contribute to the pathogenesis of AD (Geekiyanage and Chan 2011). For instance, Zhu et al. (2012) demonstrated that miR-195 down-regulated AD amyloid-β production by targeting BACE1. Another study also demonstrated that miR-125b induced tau hyperphosphorylation and cognitive deficits in AD (Banzhaf-Strathmann et al. 2014). MiR-425-5p has been demonstrated as a potential prognostic biomarker for cervical cancer (Sun et al. 2017). It has also been reported to have regulatory functions in multiple types of human cancers such as colorectal cancer (Zhang et al. 2016), hepatocellular carcinoma (Fang et al. 2017), and gastric cancer (Zhang et al. 2015). This study aimed to investigate the regulatory roles of miR-425-5p in the development of AD.
Heat shock protein B8 (HspB8) is a molecular chaperone for misfolded proteins which could stimulate autophagy with Bag3 (Crippa et al. 2010b; Hamouda et al. 2014). HspB8 is involved in the regulation of neurodegenerative diseases (Crippa et al. 2010a; Fontaine et al. 2006). For example, HspB8 was shown to participate in the autophagic removal of misfolded proteins related to neurodegenerative diseases (Crippa et al. 2010b). And the mutation of HspB8 could result in motor neuron-specific neurite degeneration (Irobi et al. 2010). In this study, we investigated whether HspB8 is associated with miR-425-5p and AD. Herein, we conducted a series of characterizations to study the expressions of miR-425-5p and HspB8 in AD patient samples. We also investigated the roles of miR-425-5p in regulating cell apoptosis of HEK293/tau cells.
Materials and methods
Sample information
Human postmortem brain samples were obtained from the Qingdao Mental Health Center. Experiments using human materials were performed in strict accordance with the Declaration of Helsinki. Brains had dissections under the supervision of neuroanatomists. We had precise identifications of various brain regions. Brain tissue samples (50 mg) were sliced from seven brain regions and stored at − 80 °C prior to use. Supplementary Table 1 provides all the information regarding the patients’ age, gender, postmortem delay, and clinical and neuropathological diagnosis. This study has been approved by the ethics committee of the Qingdao Mental Health Center (No. QMHC2015021156673).
Cell line and cell culture
HEK293/tau (HEK293 cells stably transfected with human tau) cell line was established in our laboratory following the approach from previous report (Yu et al. 2011). N2a cells stably expressing human APP695 (N2a/APP cells) and the wild-type N2a cells were also established in our laboratory. HEK293/tau cells or N2a/APP cells were cultured in DMEM containing 10% FBS and 0.2 g/L G418 (Invitrogen, USA) at 37 °C with 5% CO2. Transfection was conducted using Lipofectamine 2000 (Invitrogen, USA).
Plasmid information
The overexpression vector of miR-425-5p was provided by GeneCopoeia, USA. The overexpression vector of HSPB8 was produced by cloning the coding sequence of HSPB8 into pcDNA4 (Invitrogen, USA). The 3′-UTR of HSPB8 with putative sequences of miR-425-5p was made from Tsingke, China, and inserted to psi-CHECK2 (Promega, USA). Mutations were carried out by mutation kits from Agilent (Brighton, USA). Primers used for luciferase reporter gene vector are listed in Table 1.
qRT-PCR
Total RNAs were isolated using Trizol (Invitrogen, USA). MiRNAs and cDNAs were obtained using the miRNA extraction and cDNA synthesis kits (Tiangen, China). qRT-PCR was conducted on ABI Plus system (Invitrogen, USA) using the SYBR Green Mix (Takara, Japan). The expression was calculated by the 2−△△CT method. GAPDH or U6 was used as endogenous control. Primers were purchased from GeneCopoeia (Rockville, MD, USA), and the sequences were listed below. HSPB8, forward 5′-TCTCCAGAGGGTCTGCTCAT-3′, reverse 5′-GCAGGTGACTTCCTGGTTGT-3′; Bax, forward 5′GGCCCACCAGCTCTGAGCAGA-3′, reverse 5′-GCCACGTGGGCGTCCCAAAGT-3′; Bcl-2, forward 5′GTGGAGGAGCTCTTCAGGGA-3′, reverse 5′-AGGCACCCAGGGTGATG-3′; GAPDH, forward 5′-ATTCAACGGCACAGTCAA-3′, reverse 5′-CTCGCTCCTGGAAGATGG-3′; MiR-425-5p, forward 5′-TGCGGAATGACACGATCACTCCCG-3′, reverse 5′-CCAGTGCAGGGTCCGAGGT-3′; U6, forward 5′-CTCGCTTCGGCAGCACA-3′, reverse 5′-AACGCTTCACGAATTTGCGT-3′.
Dual-luciferase reporter assay
HEK293 cells had co-transfection with miR-425-5p or control, and HSPB8-WT (wild type) or HSPB8-MT (mutant). Cells were harvested after 2 days, and dual-luciferase reporter assay was conducted using the luciferase reporter assay system (Promega, USA).
TUNEL assay
Cells were seeded in a 24-well plate for overnight and were stained by TUNEL (Roche, Germany). The rates of positive staining were measured by a Nikon fluorescence microscope.
Western blot
Total proteins were extracted using RIPA (Beyotime, China). Protein samples were loaded to 10% SDS-PAGE for electrophoresis. NC membranes were used to perform gel transfer. The membrane was then incubated with anti-Bax (1:1000, Abcam, ab32503), anti-Bcl-2 (1:1000, Abcam, ab32124), anti-pS396 (1:1000, Abcam, ab109390), anti-pS404 (1:1000, Abcam, ab92676), anti-pT231 (1:1000, Abcam, ab151559), anti-tau5, (1:1000, Abcam, ab80579), anti-HSPB8 (1:1000, Abcam, ab151552), anti-S9 (1:5000, Abcam, ab75814), anti-GSK-3β (1:500, Abcam, ab93926), anti-Y307 (1:500, Santa Cruz Biotechnology, sc-24620), anti-PP2A (1:500, Santa Cruz Biotechnology, sc-80989), or anti-GAPDH (1:3000, Abcam, ab181602) for at 4 °C for overnight. After washing, the membrane was incubated with a secondary antibody (LI-COR, Lincoln, USA) 37 °C for 1 h. The signals were measured by Odyssey Imaging (LI-COR, USA).
Statistical analysis
SPSS 19.0 software was used to perform all data analyses. The results were presented as mean ± standard deviation (SD). The difference was analyzed by t test (between two groups) or one-way ANOVA (among multiple groups). P < 0.05 was statistically significant.
Results
Expression of miR-425-5p was upregulated in patients with mild cognitive impairment (MCI) and AD model
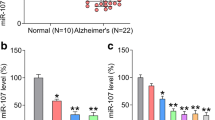
The results revealed that the expression of miR-425-5p was significantly upregulated in MCI and AD patients compared to that in the healthy samples (P < 0.01). And the expression of miR-425-5p was even higher in the AD group compared with the MCI group (P < 0.01) (Fig. 1a). As shown in Fig. 1b, the expression of miR-425-5p was markedly elevated in HEK293/tau cells (P < 0.01). The expression of miR-425-5p was significantly upregulated in N2a/APP compared to that in N2a/WT (P < 0.01) (Fig. 1c). Our results indicated that miR-425-5p was upregulated in AD patient samples.
Expression of miR-425-5p was elevated in patients with MCI and AD models. a qRT-PCR evaluating the expression of miR-425-5p in normal, MCI, and AD tissues. b qRT-PCR evaluating the expression of miR-425-5p in HEK293/WT and HEK293/tau cells. c Expression of miR-425-5p in cells transfected with N2a/APP or N2a/WT. **P < 0.01
Overexpression of miR-425-5p resulted in cell apoptosis
The expression level of miR-425-5p was significantly increased in cells transfected with miR-425-5p overexpression vector (P < 0.01) (Fig. 2a). TUNEL assay showed that cell apoptosis was considerably promoted in cells transfected with miR-425-5p, which greatly increased the rate of cell death (P < 0.01) (Fig. 2b). As shown in Fig. 2c and e, the expression levels of Bax (pro-apoptotic factor) mRNA and protein were significantly increased compared to the control (P < 0.01), while the expression levels of Bcl-2 (anti-apoptotic factor) were significantly decreased compared to that in the control (P < 0.01) (Fig. 2d and e). These results indicated that miR-425-5p could induce cell apoptosis.
Overexpression of miR-425-5p resulted in cell apoptosis. a Expression of miR-425-5p of cells in transfection with miR-425-5p overexpression vector or the scramble control by qRT-PCR. b TUNEL assay to detect cell death. c qRT-PCR and d Western blot showing expression of Bax and Bcl-2 after transfections with miR-425-5p in HEK293/tau cells. e Western blotting bands of protein expression of Bax, Bcl-2, and GAPDH in cells transfected by control or miR-425-5p. **P < 0.01
Overexpression of miR-425-5p enhanced tau phosphorylation
Overexpression of miR-425-5p significantly enhanced phosphorylating of tau at Ser396, S404, and Thr231 (P < 0.01) (Fig. 3a). As shown in Fig. 3b, activities of glycogen synthase kinase-3β (GSK-3β) were induced, along with reduced phosphorylation of Ser9 and GSK-3β (P < 0.05). However, there was no change in the activities of phosphatase and protein phosphate 2A (PP2A) along with the phosphorylation at Tyr307 of PP2A, which was inversely related to PP2A activities (P > 0.05). Our results suggested that miR-425-5p enhanced tau phosphorylation and activated GSK-3β.
HSPB8 was a direct target of miR-425-5p
TargetScan 7.2 and miRanda revealed that HSPB8 might be a target of miR-425-5p (Fig. 4a). Luciferase reporter assay verified that miR-425-5p significantly suppressed the luciferase activities of HSPB8-WT, but didn’t affect that of HSPB8-MT (Fig. 4b). Overexpression of miR-425-5p markedly inhibited the expression levels of HSPB8 mRNA and protein (P < 0.01) (Fig. 4c and d). In addition, the expression of miR-425-5p was significantly suppressed in the AD group compared to the normal group and MCI group (Fig. 4e). These results indicated that HSPB8 was targeted by miR-425-5p.
HSPB8 was directly targeted by miR-425-5p. a Shared binding sites between miR-425-5p and HSPB8. b Dual-luciferase activities for cell in co-transfection with miR-425-5p or control and HSPB8-WT or HSPB8-MT. c and d Expression of HSPB8 mRNA and protein after transfections with miR-425-5p. e HSPB8 expression in the normal group, MCI group, and AD group **P < 0.01
MiR-425-5p resulted in cell apoptosis and enhanced tau phosphorylation by targeting HSPB8
Within the regular cells, tau could stabilize axonal microtubules, which are the tracks for intracellular traffic. In AD, tau is abnormally phosphorylated, which could aggregate to helical filaments and lose the abilities in maintaining the microtubule tracks. Overexpression of miR-425-6p+HSPB8 significantly decreased the expression levels of Bax mRNA and protein, which were significantly increased by miR-425-5p alone (P < 0.01) (Fig. 5a–c). In addition, miR-425-6p+HSPB8 greatly relieved the phosphorylating of tau (Fig. 5d) and activation of GSK-3β (Fig. 5e), which were induced by miR-425-5p (P < 0.05, P < 0.01). It indicated that miR-425-5p induced cell apoptosis and promoted tau phosphorylation partially via targeting HSPB8.
MiR-425-5p resulted in cell death and enhanced tau phosphorylation by targeting HSPB8. For cells transfection with control, miR-425-5p, or miR-425-5p + HSPB8: (a–c) mRNA and protein expression of Bax and Bcl-2 after transfections. d Phosphorylation level at several sites of tau by Western blot. e Phosphorylation of GSK-3β and HSPB8 via Western blot. *P < 0.05, **P < 0.01
Discussion
It has been reported that the expression of miR-34a was upregulated in a double transgenic mouse model of AD via inhibition of Bcl2 translation (Wang et al. 2009). The functions of miR-425-5p were widely reported in tumorigenesis, but less is known in the field of neurodiseases, in particular AD. To the best of our knowledge, this is the first report investigating the roles miR-425-5p play in AD. Our results showed that the expression level of miR-425-5p was significantly higher in MCI and AD patients. And the expression level of miR-425-5p was even higher in the AD group compared to that in the MCI group. The expression of miR-425-5p was markedly elevated in HEK293/tau cells. Our results demonstrated that expression of miR-425-5p was upregulated in patients with mild cognitive impairment and in the AD model.
Previous studies have reported that miR-26b was upregulated in AD, activated cell cycle entry, tau phosphorylation, and apoptosis in postmitotic neurons (Absalon et al. 2013). MiR-125b was shown to promote neurons cell death and tau phosphorylation in AD (Ma et al. 2017). In the present study, the TUNEL assay revealed that the cell apoptosis rate was considerably elevated in cells transfected with miR-425-5p. The expression levels of the pro-apoptotic protein BAX mRNA and protein were both significantly enhanced, and the expression levels of the anti-apoptotic protein Bcl-2 were significantly decreased with the overexpression of miR-425-5p.
Previous studies have discussed that many miRNAs could induce tau phosphorylation and cell apoptosis in neurons. For instance, miR-125b was reported to induce tau hyperphosphorylation in AD (Banzhaf-Strathmann et al. 2014). Our results demonstrated that miR-425-5p enhanced the tau phosphorylation at Ser396 and Thr231. The activities of glycogen synthase kinase-3β were enhanced, along with reduced phosphorylation of Ser9 and GSK-3β. However, the activities of PP2A had no change, along with phosphorylation at Tyr307 of PP2A, which was appositively related to PP2A activities. In consistence with previous findings, we also found that overexpression of miR-425-5p enhanced tau phosphorylation.
HspB8 has been reported to be targeted by some specific miRNAs, such as miR-126a-5p (Jiang et al. 2017). Our results showed that HSPB8 was a direct target of miR-425-5p, and the luciferase reporter assay revealed that miR-425-5p greatly suppressed the luciferase activities of the HSPB8-WT, but did not affect HSPB8-MT. Overexpression of miR-425-5p markedly suppressed expression of HSPB8 mRNA and protein. Moreover, miR-425-6p+HSPB8 decreased the expression of Bax, which was significantly elevated by miR-425-5p alone. MiR-425-5p+HSPB8 greatly relieved tau phosphorylation and GSK-3β activation that was induced by miR-425-5p. Based on all these results and previous findings, we demonstrated that miR-425-5p induced cell death and promoted tau phosphorylation partially via targeting HSPB8.
Conclusions
In conclusion, upregulation of miR-425-5p induced cell apoptosis and promoted tau phosphorylation partially by targeting HSPB8 in AD. MiR-425-5p might act as a new therapeutic target for AD treatment.
References
Absalon S, Kochanek DM, Raghavan V, Krichevsky AM (2013) MiR-26b, upregulated in Alzheimer's disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J Neurosci 33:14645–14659
Banzhaf-Strathmann J et al (2014) MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer's disease. EMBO J. 33:1667–1680
Crippa V et al (2010a) A role of small heat shock protein B8 (HspB8) in the autophagic removal of misfolded proteins responsible for neurodegenerative diseases. Autophagy 6:958–960. https://doi.org/10.4161/auto.6.7.13042
Crippa V et al (2010b) The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet 19:3440–3456. https://doi.org/10.1093/hmg/ddq257
Delay C, Mandemakers W, Hébert SS (2012) MicroRNAs in Alzheimer's disease. Neurobiol Dis 46:285–290
Fang F, Song T, Zhang T, Cui Y, Zhang G, Xiong Q (2017) MiR-425-5p promotes invasion and metastasis of hepatocellular carcinoma cells through SCAI-mediated dysregulation of multiple signaling pathways. Oncotarget 8:31745
Fontaine JM, Sun X, Hoppe AD, Simon S, Vicart P, Welsh MJ, Benndorf R (2006) Abnormal small heat shock protein interactions involving neuropathy-associated HSP22 (HSPB8) mutants. FASEB J 20:2168–2170. https://doi.org/10.1096/fj.06-5911fje
Geekiyanage H, Chan C (2011) MicroRNA-137/181c regulates serine palmitoyltransferase and in turn amyloid β, novel targets in sporadic Alzheimer's disease. J Neurosci 31:14820–14830
Geekiyanage H, Jicha GA, Nelson PT, Chan C (2012) Blood serum miRNA: non-invasive biomarkers for Alzheimer's disease. Exp Neurol 235:491–496
Hamouda MA et al (2014) The small heat shock protein B8 (HSPB8) confers resistance to bortezomib by promoting autophagic removal of misfolded proteins in multiple myeloma cells. Oncotarget 5:6252–6266. https://doi.org/10.18632/oncotarget.2193
Hardy JA, Higgins GA (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256:184–186
Irobi J et al (2010) Mutant HSPB8 causes motor neuron-specific neurite degeneration. Hum Mol Genet 19:3254–3265
Jiang B et al (2017) MicroRNA-126a-5p enhances myocardial ischemia-reperfusion injury through suppressing Hspb8 expression. Oncotarget 8:94172
Khachaturian ZS (1985) Diagnosis of Alzheimer's disease. Arch Neurol 42:1097–1105
Kumar S, Vijayan M, Bhatti JS, Reddy PH (2017) Chapter three-microRNAs as peripheral biomarkers in aging and age-related diseases. Prog Mol Biol Transl Sci 146:47–94
Lu M, Zhang Q, Deng M, Miao J, Guo Y, Gao W, Cui Q (2008) An analysis of human microRNA and disease associations. PLoS ONE 3:e3420
Ma X, Liu L, Meng J (2017) MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci Lett 661:57–62
Nelson PT, Wang WX, Rajeev BW (2008) MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol 18:130–138
Nunez-Iglesias J, Liu C-C, Morgan TE, Finch CE, Zhou XJ (2010) Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer's disease cortex reveals altered miRNA regulation. PLoS ONE 5:e8898
Schonrock N, Ke YD, Humphreys D, Staufenbiel M, Ittner LM, Preiss T, Götz J (2010) Neuronal microRNA deregulation in response to Alzheimer's disease amyloid-β. PLoS ONE 5:e11070
Sun L, Jiang R, Li J, Wang B, Ma C, Lv Y, Mu N (2017) MicoRNA-425-5p is a potential prognostic biomarker for cervical cancer. Ann Clin Biochem 54:127–133
Wang X et al (2009) miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer's disease, inhibits bcl2 translation. Brain Res Bull 80:268–273
Yu G, Li Y, Tian Q, Liu R, Wang Q, Wang J-Z, Wang X (2011) Berberine attenuates calyculin A-induced cytotoxicity and Tau hyperphosphorylation in HEK293 cells. J Alzheimer's Dis 24:525–535
Zhang Z, Li Y, Fan L, Zhao Q, Tan B, Li Z, Zang A (2015) microRNA-425-5p is upregulated in human gastric cancer and contributes to invasion and metastasis in vitro and in vivo. Exp Therap Med 9:1617–1622
Zhang Y et al (2016) Micro RNA-425-5p regulates chemoresistance in colorectal cancer cells via regulation of Programmed Cell Death 10. J Cell Mol Med 20:360–369
Zhu H-C, Wang L-M, Wang M, Song B, Tan S, Teng J-F, Duan D-X (2012) MicroRNA-195 downregulates Alzheimer's disease amyloid-β production by targeting BACE1. Brain Res Bull 88:596–601
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by JY, YW, LL, and CL. The first draft of the manuscript was written by CL, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was obtained from the Ethics Committee of Qingdao Mental Health Center (No. QMHC2015021156673). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, J., Wu, Y., Li, L. et al. MicroRNA-425-5p promotes tau phosphorylation and cell apoptosis in Alzheimer’s disease by targeting heat shock protein B8. J Neural Transm 127, 339–346 (2020). https://doi.org/10.1007/s00702-019-02134-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02134-5