Abstract
Cognitive impairment is frequent in progressive supranuclear palsy (PSP) and less common in multiple system atrophy (MSA), but characteristics and progression compared with Parkinson’s disease (PD) need to be properly defined. We evaluated 35 PSP with Richardson’s syndrome (PSP-RS), 30 MSA as well as 65 age-, sex-, and education-matched PD with an extensive clinical and neuropsychological assessment, allowing Level II cognitive diagnosis. Eighteen PSP, 12 MSA and 30 PD had a second evaluation between 12 and 18 months (mean 15 months) after the first assessment. PSP performance at Montreal Cognitive Assessment (MoCA), verbal fluencies (phonemic and semantic tasks), Stroop test (Error and Time), Digit Span Sequencing (DSS), incomplete letters of Visual Object and Space Perception (VOSP) and Benton’s Judgment of Line Orientation (JLO) performance were significantly poorer at baseline compared to PD and MSA. Executive, language and visuospatial abilities declined longitudinally in PSP, but not in PD and MSA. After 1.5 year, 16% of PSP converted to dementia. Our study provides evidence that cognitive progression is more severe and rapid in PSP-RS than PD and MSA. Further, we observed that MoCA, verbal fluency (particularly semantic), DSS and Benton’s JLO are valuable tests to detect cognitive progression in PSP-RS and may be proposed as possible biomarker to assess efficacy of disease modification strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive supranuclear palsy (PSP) is a rapidly progressive neurodegenerative diseases characterized by abnormal cerebral tau-protein aggregations (Williams and Lees 2009). The most common clinical presentation of PSP is Richardson’s syndrome (PSP-RS), in which patients have early and prominent gait and postural instability, frequent falls and abnormal vertical eye movements (supranuclear gaze palsy) (Ali et al. 2019; Litvan et al. 1996; Höglinger et al. 2017). In addition to motor deficits, PSP patients present pronounced cognitive and neuropsychiatric changes (Gerstenecker 2017). There is general consensus that fronto-executive deficits dominate the neuropsychological profile of PSP (Gerstenecker et al. 2013). In this regard, verbal fluency dysfunctions, particularly phonemic, have been reported as distinct cognitive deficits vs. Parkinson’s disease (PD) and multiple system atrophy (MSA) (Fiorenzato et al. 2016; Rittman et al. 2013). Recently, we reported that the phonemic fluency subitem included in the Montreal Cognitive Assessment (MoCA) is sensitive in detecting cognitive deficits in PSP, while the Mini-Mental State Examination (MMSE) is less helpful (Fiorenzato et al. 2016). Further, previous studies reported deficits in visuospatial processing, memory and language in PSP (Bak et al. 2006; Burrell et al. 2014). However, the exact nature of cognitive dysfunctions in this pathology and its clinical relevance still need to be explored. Indeed, it is worth to investigate whether PD clinical criteria for mild cognitive impairment (MCI) and dementia (Dubois et al. 2007; Litvan et al. 2012) can be applied to PSP-RS. Using these criteria, an exploratory study found dementia in about the 10% of MSA patients (Auzou et al. 2015).
Moreover, tests that can best detect cognitive progression are still not defined (Soliveri et al. 2000). This is important given the ongoing attempts of disease modification by monoclonal antibodies and the need to define not only motor but also cognitive biomarkers of progression. In addition, definition of impaired cognitive domains can be important in differential diagnoses among different PSP variants and versus PD and MSA.
Only few longitudinal studies evaluated cognitive progression in PSP (Rittman et al. 2013; Soliveri et al. 2000). However, they were based on unclassified PSP cases and used mostly screening tools or brief neuropsychological assessments, without exploring the full spectrum of cognitive functions (attention/working memory, executive, memory, visuospatial and language domains).
Based on these considerations, we have now expanded our previous work (Fiorenzato et al. 2016), and used a comprehensive battery to identify tests that best characterize PSP and could be predictive of cognitive evolution. We also applied PD-cognitive criteria to evaluate the severity of cognitive deficits in PSP.
Materials and methods
Patients
This study included 35 PSP and 30 MSA patients referred consecutively, and 65 PD matched for age, education and sex. All patients were evaluated at baseline with neuropsychological, neuropsychiatric and motor assessment. Among these patients, 18 PSP, 12 MSA and 30 matched PD underwent a second evaluation after a mean of 15-month follow-up (range 12–18 months). Patients were recruited at the Parkinson’s Disease and Movement Disorders Unit, Neurology Clinic, University of Padua, Italy; and at the San Camillo Hospital in Venice, Italy, between June 2012 and August 2017. Our PSP patients were all PSP-RS by 1996 criteria (Litvan et al. 1996) and also met the newer diagnostic criteria for probable PSP-RS (Höglinger et al. 2017). Probable MSA was diagnosed according to the established criteria (Gilman et al. 2008) and PD according to UK Parkinson’s Disease Society Brain Bank diagnostic criteria (Gelb et al. 1999). This present study was approved by the Venice Research Ethics Committee of Venice, Italy. Written informed consent was obtained from all study subjects after full explanation of the procedure involved, according to the 1964 Declaration of Helsinki and its later amendments.
Clinical and neuropsychological assessment
Patients’ motor symptom severity and their impact on daily functioning were assessed with Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) parts III and II, respectively. Levodopa (LEDD) and dopamine agonist equivalent daily (DAED) doses were calculated (Tomlinson et al. 2010), as well as the presence of ongoing anticholinergic treatments.
Our neuropsychological battery was developed to include at least two tests for each cognitive domain (i.e., attention/working memory, executive, memory, language and visuospatial/visuoperceptive functions), to diagnose dementia and MCI according to Level II PD criteria (Dubois et al. 2007; Litvan et al. 2012), namely attention/working memory domain were tested with the Trail Making Test (TMT B-A) (Giovagnoli et al. 1996) and Digit Span Sequencing (DSS) of Wechsler Adult Intelligence Scale-Fourth Edition (Wechsler 2008). Executive functions were evaluated with the Stroop Color/Word test (Caffarra et al. 2002) and phonemic fluency (Novelli et al. 1986a). Memory was assessed with word-paired-associated task (WPAT) and prose memory tests (Novelli et al. 1986b). Language was tested with the semantic fluency task and Novelli’s Naming Test (Novelli et al. 1986a). Visuospatial and visuoperceptive functions were assessed by the Benton’s Judgment of Line Orientation (JLO) test (Gullett et al. 2013), and Visual Object and Space Perception (VOSP) battery incomplete letters subtask (Warrington and James 1991). MMSE and MoCA were used to assess general cognitive functions. We assessed the presence of depression, anxiety, apathy and quality of life using the Beck Depression Scale (BDI-II), State–Trait Anxiety Inventory (STAI Y-1, Y-2), Starkstein’s Apathy Scale and 8-item version of Parkinson’s disease quality of life (PDQ-8), respectively (Yamanishi et al. 2013). In this study, we have applied these criteria to PSP and MSA patients, as currently there are no available cognitive guidelines for atypical parkinsonisms. Further, subjective cognitive complaints and their impact on daily functioning were assessed during the clinical interview using the Parkinson’s Disease Cognitive Functional Rating Scale (PD-CFRS) (Kulisevsky et al. 2013), while functional autonomy was evaluated with activities of daily living (ADL) and instrumental ADL (IADL) scales. Patients underwent clinical and neuropsychological assessment in the morning after the intake of medications.
MMSE and MoCA total scores were adjusted for age and education, and z scores were calculated for all the cognitive tests according to the published Italian normative data. Further, we reverse z scores of those tests assessing reaction time (Trail Making Test and Stroop Color/Word test) to have consistent z scores, namely a positive z score indicates a performance above the average, while a negative z score below the normative population average. We classified patients as MCI if the z score for a given test was at least 1.5 standard deviation (SD) below appropriate norms in two tests (e.g., within a single cognitive domain or at least one test in two or more cognitive domains) (Litvan et al. 2012). Presence of dementia was assessed based on cognitive examination, functional autonomy and neuropsychiatric assessment (Dubois et al. 2007). Subjects without cognitive deficits were defined as cognitively normal (NC).
Statistical analyses
Three-level one-way analysis of variance (ANOVA) was used to compare sociodemographic and clinical data between PSP, MSA and PD groups at baseline. Between-group differences in neuropsychological measures were investigated using a three-level one-way analysis of covariance including disease duration, as this variable presented intergroup differences. Pearson’s Chi-squared test was run to compare categorical variables (sex and MSA subtypes). Distribution normality was checked with Kolmogorov–Smirnov tests and homogeneity of variance with Levene’s test. Further, to verify if the whole sample (n = 130) and the followed-up subsample (n = 60), differed in sociodemographic and clinical variables, a one-way ANOVAs was used.
Due to the small sample size that remained at follow-up, a non-parametric method was adopted to run longitudinal analyses. Within-group comparisons (follow-up vs. baseline) were analyzed with Wilcoxon signed-rank test. One-year rate change was calculated for the performance at each cognitive test, whose score changed significantly. Spearman’s rank correlations between cognitive tests and relevant clinical variables, such as motor symptoms (as assessed by MDS-UPDRS-III) or dopaminergic medications, were sought when useful to clarify the results. Finally, frequencies across cognitive states (NC, MCI and dementia) were calculated.
All statistics were performed using SPSS version 24 (IBM SPSS, Chicago, IL) and statistical significance threshold was set at p ≤ 0.05. Post hoc analyses, followed by Bonferroni correction for multiple comparisons, were applied when appropriate.
Results
Demographic, clinical and cognitive characteristics at baseline
As shown in Table 1, PSP were older than MSA patients (p = 0.009), while PD patients were in the same range for age, sex and education with the other groups. PSP and MSA had shorter disease duration than PD patients (p = 0.002 and p = 0.003, respectively) and more severe motor symptoms (p < 0.0001). Of note, motor deficits in PSP and MSA had higher impact on daily functioning compared to PD (p < 0.0001). PSP patients were on lower LEDD and DAED doses than PD (p = 0.007 and p = 0.004, respectively). In addition, PSP and MSA patients were characterized by reduced functional autonomy (as assessed by ADL) (p < 0.001), which was linked also to cognitive dysfunctions (using the PD-CFRS) only in PSP patients (p < 0.001).
PSP had the worst performance on global cognitive scales (MMSE and MoCA) (see Table 1). Further, PSP showed more severe executive (Stroop test Time and Errors, DSS, phonemic and semantic fluencies) and visuospatial deficits (VOSP and Benton’s JLO) than the other groups. Of note, PSP impairment was clinically meaningful in MMSE, MoCA, Stroop test, and Benton’s JLO, as average scores were below cut-off or 1.5SD.
From a behavioral standpoint, PSP patients showed more severe apathy than PD (p < 0.001). Moreover, PSP and MSA were more depressed (p < 0.004) and reported reduced quality of life (p < 0.001) compared to PD.
The whole sample (n = 130) and the subsample (n = 60) that was followed prospectively had similar demographic and clinical variables (Online Resource 1).
Clinical and cognitive follow-up
Clinical, neuropsychological and functional autonomy progression data at follow-up are reported in Table 2.
Mean follow-up interval was 15.25 months (12–18 min–max values). Motor severity and its impact on functional autonomy decreased significantly in PSP and MSA groups. This is in line also with ADL and IADL scores that decreased significantly in each group. Moreover, in PD and PSP groups, cognitive dysfunctions affected also functional autonomy (PD-CFRS), although only seen as a trend for the latter (p = 0.022 and p = 0.052, respectively).
At mean 15-month follow-up, PSP was the only group, whose performance worsened in MoCA, semantic fluency, DSS and Benton’s JLO (p = 0.029; p = 0.033; p = 0.023 and p = 0.035, respectively). MoCA total score in PSP decreased 1.60 points in a year. MSA and PD did not show any significant poorer performance in any cognitive tests compared to the baseline, except for MMSE, whose score was significantly lower in PD (p = 0.042). In addition, no significant correlation was found between poor performance in MoCA, DSS, Benton’s JLO and semantic fluency with motor severity (MDS-UPDRS-III) or LEDD and DAED in PSP group.
Cognitive state change
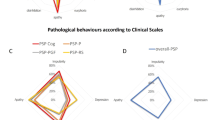
MSA and PSP patients, despite similar disease duration, showed different distribution of cognitive states at baseline (Fig. 1). In PSP, 22% (4/18) was classified as cognitively normal, 61% (11/18) MCI and 17% (3/18) as dementia. At follow-up (approximately at 6-year disease duration in both disorders), two PSP patients (11%) with MCI converted to cognitive normal state, and 17% (3/18) to dementia as opposed to 25% (3/12) of MSA patients, who had converted to MCI, but none to dementia. Specifically, the two early diagnosed PSP patients who reverted back from MCI to normal cognition presented improved performance at one test belonging to the attentive or executive domain (i.e., Stroop test or TMT B-A). No significant correlation was found between Stroop test or TMT B-A performance and motor severity (UPDRS-III) or dopaminergic medications (LEDD, DAED) in PSP patients.
In PD, although the disease duration was longer compared to PSP and MSA, at baseline, 40% (12/30) was classified as PD-NC, 57% (17/30) as PD-MCI (mainly with multidomain deficits) and 3% (1/30) as having dementia. At follow-up, 10% (3/30) of PD-NC converted to MCI and 7% (2/30) of PD-MCI to dementia.
Discussion
To our knowledge, this is the first study to investigate longitudinal cognitive changes using an extensive neuropsychological battery in PSP-RS classified according the new criteria vs. MSA and PD patients. Our main finding is that PSP-RS patients have similar but more severe deficits in executive and visuospatial functions (as assessed by verbal fluencies, Stroop test, DSS, VOSP and Benton’s JLO) than PD, whilst MSA did not show any significant cognitive alterations or changes during the observation period. Most importantly, we found that some of these tests (semantic fluency, DSS and Benton’s JLO tests) are particularly suited to monitor cognitive alterations over time in PSP, as their scores significantly changed within the 15-month observation time.
Assessment at baseline showed that PSP have consistently worse performance than PD and MSA patients in MoCA, verbal fluencies (both in phonemic and semantic tasks), Stroop test (Time and Errors), DSS, VOSP and Benton’s JLO. These cognitive tests were the most sensitive in showing differences between these parkinsonian syndromes. In addition, some tests (MoCA, semantic fluency, DSS and Benton’s JLO) showed also good sensitivity in monitoring PSP cognitive progression.
These results are aligned with previous studies showing fronto-executive deficits, as the core features of PSP cognitive profile (Gerstenecker et al. 2013; Gerstenecker 2017). We extended this evidence by demonstrating that visuospatial dysfunctions can also be observed in PSP patients with cognitive decline (Bak et al. 2006). Our findings showed that visuospatial/perceptive abilities are differently affected in atypical parkinsonian syndromes with MSA showing preserved functions at each time point (T0 and T1) vs. worsening visuospatial performance in PSP-RS.
VOSP incomplete letters subtask is an ‘object decision’ tasks, which requires visual recognition and recall of degraded letters abilities. It has been related to ventral visual stream (‘what’), involving the inferior temporal lobe (Bak et al. 2006; Goodale and Milner 1992) and has been already observed in PSP patients (Bak et al. 2006; Rittman et al. 2013). Indeed, atrophy of temporal areas is one of the most frequent pathological findings of PSP, suggesting greater decline in visuospatial tasks is presumably due to the pathological involvement of this region (Massey et al. 2012).
However, PSP-RS patients presented clinically relevant deficits at first evaluation as well as at follow-up, in the Benton’s JLO, a ‘spatial location’ task, mainly related to the parietal alterations of the dorsal stream of visuospatial processing (‘where’). This observation adds to the view that parietal abnormality can also play a part in cognitive alterations in the visuospatial domain (Massey et al. 2012). These findings corroborate previous evidence of Soliveri et al. (2000), which showed PSP poorer performance at Benton’s JLO than MSA and PD, although they did not explore the within changes overtime. Of note, we are aware that Benton’s JLO test requires vertical shift of attention, which is more difficult for PSP patients because of the vertical gaze palsy, slow horizontal saccades and visual fixation difficulties. Indeed, we cannot exclude that their performance may have been degraded by these symptoms.
These findings highlight the importance of a visuospatial functions comprehensive assessment, involving both the dorsal and ventral visual processing streams, as both pathways can be impaired in PSP patients.
We also found that cognitive flexibility, inhibition, verbal recall and working memory abilities were impaired in PSP compared to MSA and PD patients. These results corroborate previous findings suggesting that different aspects of executive functions (such as complex problem solving, planning, shifting, behavioral sets maintaining, verbal fluency, and working memory) are altered in PSP (Gerstenecker et al. 2013; Lee et al. 2012).
Specifically, our study shows that at baseline PSP-RS patients were significantly slower and presented poorer inhibition abilities, recalled fewer words and had difficulties in reorganizing number of digits (as assessed by Stroop test, verbal fluencies and DSS task, respectively). Evidence from functional imaging suggests the involvement of mainly frontal areas and striatal regions in Stroop test, DSS and verbal fluencies task performance (Heyder et al. 2004; Shedlack et al. 2009) and are in line with the neuropathological changes in PSP, which tend to be most pronounced in subcortical regions (i.e., subthalamic nucleus, substantia nigra, superior colliculi and internal pallidum) and frontal lobes (Hauw et al. 1994). Overall, these results underline the importance of frontal–striatal-based tests and particularly of verbal fluency assessment as screening instruments for PSP patients vs. PD and MSA.
Verbal fluency is a quick and easy-to-administer test and most importantly for our purpose, it requires minimal motor function making it only marginally affected by motor deficits and dysarthria (Rittman et al. 2013). Cross-sectionally, our results of PSP-RS show deficits in the semantic but even more in the phonemic fluency task, whose performance at both time points (about 19 words in 3 min) confirms this last subtest as a distinctive and psychometrically reliable disease-specific feature, suggesting the use of seven words per minute cut-off (Fiorenzato et al. 2016; Rittman et al. 2013).
Moreover, we observed that semantic fluency performance, showed also high sensitivity in detecting cognitive worsening at follow-up despite preserved naming abilities. This is also in line with evidence of higher level language deficits (namely dynamic aphasia) in PSP which goes behind dysarthric speech. Overall, it seems that PSP-RS patients show impairment in tasks which requires active initiation (letter and category completion) despite preserved abilities in naming, comprehension and repetition (Madden et al. 2019; Robinson et al. 2015).
Although previous functional MRI studies demonstrated a key role of both frontal and temporal areas in the execution of semantic verbal fluency tasks, evidence suggests this task has a stronger association with language rather than the executive domain (Whiteside et al. 2016).
Semantic recall alterations have been reported to be sensitive in differentiating performance across cognitive states and in predicting cognitive decline and dementia also in PD patients (Biundo et al. 2014; Kehagia et al. 2010; Williams-Gray et al. 2007). Within the context of more rapid progression of cognitive abilities in PSP (Soliveri et al. 2000), this task may detect changes in this pathology earlier than in PD. However, additional longitudinal studies are warranted to clarify whether semantic fluency alterations may be a biomarker of cognitive decline in PSP-RS.
Our results indicate that MMSE and MoCA scores are both significantly altered in each cohort, but PSP-RS patients showed the lowest scores as well as greater MoCA progression than PD.
Finally, we observed a difference in cognitive states distribution between PSP, PD and MSA. Specifically, PSP and MSA had similar disease duration, but the percentage of patients with dementia was higher in PSP compared to MSA (33% vs. no patients with dementia). PSP and MSA patients with MCI at baseline had all multidomain deficits, but only patients with PSP-MCI converted to dementia.
Although disease duration was longer in PD than in PSP, and at baseline both groups showed similar proportion of MCI, the percentage of patients who converted to dementia was lower in PD (7% in PD vs. 16% in PSP). Overall, these findings suggest a different pattern of cognitive progression, wherein PSP has the most severe and rapid cognitive decline.
Of note, we did not find MSA patients with dementia—while in a previous study, we had reported a prevalence of dementia between 8 and 11% (Auzou et al. 2015). According to published criteria (Dubois et al. 2007), dementia can be diagnosed if cognitive deficits are severe enough to impact daily functioning. However, in atypical parkinsonisms, this is challenging as functional autonomy is usually impaired due to motor dysfunctions and isolating the cognitive component of impaired functional tasks can be difficult. For this reason, we used for the first time in these populations, the PD-CFRS scale to minimize the influence of motor component in assessing the functional autonomy due to cognitive impairments (Kulisevsky et al. 2013). In this regard, differences in dementia prevalence may be due to different cognitive tools adopted.
Overall, the different characteristics and severity of cognitive alterations in PSP, MSA and PD may also be related to the discrete neuroanatomical process of these pathologies with the involvement of different brain regions.
MSA is characterized by degeneration involving primarily subcortical structures, and cortical pathology is not considered a predominant feature (Papp and Lantos 1994). A recent neuropathological study did not identify neuroanatomical regions associated with cognitive impairment in MSA (Koga et al. 2016). This is aligned with our previously published volumetric study findings, showing in MSA only an association between frontal focal atrophy and cognitive deficits, and suggesting a marginal contribution of cortical pathology to deficits in cognition (Fiorenzato et al. 2017). By contrast, PSP tau pathology extends from the frontal cortex to the dentate nucleus of the cerebellum, and numerous studies report an association between cortical tau burden and cognitive/behavioral severity (Cordato et al. 2002).
Finally, our findings confirm that apathy and depression are more severe and common in PSP than PD (Gerstenecker 2017). Particularly, apathy seems to be a distinctive feature of PSP-RS, and this can be possibly associated with the distribution of pathology involving frontal regions (Cordato et al. 2002). MSA patients showed depression and reduced quality of life, which were worse than in the other groups; indeed, as previously reported, neuropsychiatric symptoms in MSA strongly correlate with autonomic and motor dysfunctions (Lee et al. 2013).
Our study has important caveats. First, we lack pathological confirmation of clinical diagnoses. However, we applied the most recent clinical consensus criteria (Gilman et al. 2008; Höglinger et al. 2017) and patients were followed up prospectively. Second, we focused only on PSP-RS phenotype and our findings may not apply to other PSP clinical variants. Third, PD patients’ disease duration did not match PSP and MSA, but given the different disease course, a match for severity and progression would have been impossible. Therefore, we chose to consider primarily PD patients with similar sex, age and education.
To conclude, we have shown that semantic fluency, DSS and Benton’s tests are sensitive tasks to monitor cognitive progression in PSP and may be considered as possible biomarkers of cognitive decline in PSP-RS. This is relevant in light of the ongoing disease clinical trials targeting disease modification.
We have also shown that a detailed cognitive assessment is useful in conjunction with other clinical evaluations—disease progression, response to medication, motor and clinical features—to differentiate PSP from the other parkinsonisms and to support clinical diagnosis.
Taken together, our findings show that cognition in PSP-RS patients is more impaired than MSA. Compared to PD, PSP-RS involves similar cognitive domains but with greater severity and faster decline which can be detected by specific cognitive tests. Additional studies are needed to define the patterns of cognitive and clinical deterioration in other PSP subtypes.
References
Ali F, Martin PR, Botha H, Ahlskog JE, Bower JH, Masumoto JY, Maraganore D, Hassan A, Eggers S, Boeve BF, Knopman DS, Drubach D, Petersen RC, Dunkley ED, van Gerpen J, Uitti R, Whitwell JL, Dickson DW, Josephs KA (2019) Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov Disord. https://doi.org/10.1002/mds.27619
Auzou N, Dujardin K, Biundo R, Foubert-Samier A, Barth C, Duval F, Tison F, Defebvre L, Antonini A, Meissner WG (2015) Diagnosing dementia in multiple system atrophy by applying Movement Disorder Society diagnostic criteria for Parkinson’s disease dementia. Parkinsonism Relat Disord 21:1273–1277
Bak TH, Caine D, Hearn VC, Hodges JR (2006) Visuospatial functions in atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry 77:454–456
Biundo R, Weis L, Facchini S, Formento-Dojot P, Vallelunga A, Pilleri M, Antonini A (2014) Cognitive profiling of Parkinson disease patients with mild cognitive impairment and dementia. Parkinsonism Relat Disord 20:394–399
Burrell JR, Hodges JR, Rowe JB (2014) Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov Disord 29:684–693
Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A (2002) Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci 22:443–447
Cordato NJ, Pantelis C, Halliday GM, Velakoulis D, Wood SJ, Stuart GW, Currie J, Soo M, Olivieri G, Broe GA, Morris JGL (2002) Frontal atrophy correlates with behavioural changes in progressive supranuclear palsy. Brain 125:789–800
Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, Emre M (2007) Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord 22:2314–2324
Fiorenzato E, Weis L, Seppi K, Onofrj M, Cortelli P, Zanigni S, Tonon C, Kaufmann H, Shepherd TM, Poewe W, Krismer F, Wenning G, Antonini A, Biundo R, Movement Disorders Society MSAN, Imaging Study G (2017) Brain structural profile of multiple system atrophy patients with cognitive impairment. J Neural Transm (Vienna) 124:293–302
Fiorenzato E, Weis L, Falup-Pecurariu C, Diaconu S, Siri C, Reali E, Pezzoli G, Bisiacchi P, Antonini A, Biundo R (2016) Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) performance in progressive supranuclear palsy and multiple system atrophy. J Neural Transm (Vienna) 123:1435–1442
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39
Gerstenecker A (2017) The neuropsychology (broadly conceived) of multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Arch Clin Neuropsychol 32:861–875
Gerstenecker A, Mast B, Duff K, Ferman TJ, Litvan I, Group EPS (2013) Executive dysfunction is the primary cognitive impairment in progressive supranuclear palsy. Arch Clin Neuropsychol 28:104–113
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17:305–309
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25
Gullett JM, Price CC, Nguyen P, Okun MS, Bauer RM, Bowers D (2013) Reliability of three Benton judgment of line orientation short forms in idiopathic Parkinson’s disease. Clin Neuropsychol 27:1167–1178
Hauw J-J, Daniel S, Dickson D, Horoupian D, Jellinger K, Lantos P, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015
Heyder K, Suchan B, Daum I (2004) Cortico-subcortical contributions to executive control. Acta Psychol (Amst) 115:271–289
Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I, Movement Disorder Society-endorsed PSPSG (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864
Kehagia AA, Barker RA, Robbins TW (2010) Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol 9:1200–1213
Koga S, Parks A, Uitti RJ, van Gerpen JA, Cheshire WP, Wszolek ZK, Dickson DW (2016) Profile of cognitive impairment and underlying pathology in multiple system atrophy. Mov Disord 32(3):405–413
Kulisevsky J, Fernandez de Bobadilla R, Pagonabarraga J, Martinez-Horta S, Campolongo A, Garcia-Sanchez C, Pascual-Sedano B, Ribosa-Nogue R, Villa-Bonomo C (2013) Measuring functional impact of cognitive impairment: validation of the Parkinson’s disease cognitive functional rating scale. Parkinson Relat Disord 19:812–817
Lee W, Williams DR, Storey E (2012) Cognitive testing in the diagnosis of parkinsonian disorders: a critical appraisal of the literature. Mov Disord 27:1243–1254
Lee CN, Kim M, Lee HM, Jang JW, Lee SM, Kwon DY, Park KW, Koh SB (2013) The interrelationship between non-motor symptoms in Atypical Parkinsonism. J Neurol Sci 327:15–21
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47:1–9
Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27:349–356
Madden DL, Sale MV, O’Sullivan J, Robinson GA (2019) Improved language production with transcranial direct current stimulation in progressive supranuclear palsy. Neuropsychologia 127:148–157
Massey LA, Micallef C, Paviour DC, O’Sullivan SS, Ling H, Williams DR, Kallis C, Holton JL, Revesz T, Burn DJ, Yousry T, Lees AJ, Fox NC, Jäger HR (2012) Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord 27:1754–1762
Novelli G, Papagno C, Capitani E, Laiacona M (1986a) Three clinical tests to research and rate the lexical performance of normal subjects. Arch Psicol Neurol Psichiatr 47(4):477–506
Novelli G, Papagno C, Capitani E, Laiacona M (1986b) Three clinical tests for the assessment of verbal long-term memory function: Norms from 320 normal subjects. Arch Psicol Neurol Psichiatr 47(2):278–296
Papp MI, Lantos PL (1994) The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain 117:235–243
Rittman T, Ghosh BC, McColgan P, Breen DP, Evans J, Williams-Gray CH, Barker RA, Rowe JB (2013) The Addenbrooke’s cognitive examination for the differential diagnosis and longitudinal assessment of patients with parkinsonian disorders. J Neurol Neurosurg Psychiatry 84:544–551
Robinson GA, Spooner D, Harrison WJ (2015) Frontal dynamic aphasia in progressive supranuclear palsy: distinguishing between generation and fluent sequencing of novel thoughts. Neuropsychologia 77:62–75
Shedlack KJ, Hunter R, Wyper D, McLuskie R, Fink G, Goodwin GM (2009) The pattern of cerebral activity underlying verbal fluency shown by split-dose single photon emission tomography (SPET or SPECT) in normal volunteers. Psychol Med 21:687
Soliveri P, Monza D, Paridi D, Carella F, Genitrini S, Testa D, Girotti F (2000) Neuropsychological follow up in patients with Parkinson’s disease, striatonigral degeneration-type multisystem atrophy, and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 69:313–318
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653
Warrington EK, James M (1991) The visual object and space perception battery. Thames Valley Test Company, Bury St Edmunds
Wechsler D (2008) Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). NCS Pearson, San Antonio
Whiteside DM, Kealey T, Semla M, Luu H, Rice L, Basso MR, Roper B (2016) Verbal Fluency: language or Executive Function Measure? Appl Neuropsychol Adult 23:29–34
Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8:270–279
Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA (2007) Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 130:1787–1798
Yamanishi T, Tachibana H, Oguru M, Matsui K, Toda K, Okuda B, Oka N (2013) Anxiety and depression in patients with Parkinson’s disease. Intern Med 52:539–545
Acknowledgements
We would like to thank the patients for participating in this study.
Funding
The work of the authors was supported by the Ministry of Health under Grant number GR-2016-02361986.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This study was approved by the Venice Research Ethics Committee of Venice, Italy.
Informed consent
Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fiorenzato, E., Antonini, A., Camparini, V. et al. Characteristics and progression of cognitive deficits in progressive supranuclear palsy vs. multiple system atrophy and Parkinson’s disease. J Neural Transm 126, 1437–1445 (2019). https://doi.org/10.1007/s00702-019-02065-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02065-1