Abstract
Working on catecholamine systems for years, the neuropharmacologist Arvid Carlsson has made a number of important and pioneering discoveries, which have highlighted the key role of these neuronal and peripheral neurotransmitters in brain functions and adrenal regulations. Since then, major advances have been made concerning the distribution of the catecholaminergic systems in particular by studying their rate-limiting enzyme, tyrosine hydroxylase (TH). Recently new methods of tissue transparency coupled with in toto immununostaining and three-dimensional (3D) imaging technologies allow to precisely map TH immunoreactive pathways in the mouse brain and adrenal glands. High magnification images and movies obtained with combined technologies (iDISCO+ and light-sheet microscopy) are presented in this review dedicated to the pioneer work of Arvid Carlsson and his collaborators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances and ideas in science often emerge from methodological developments capable of breaking a technological lock. In the 1950–1970’s, one of these locks in neuroscience was the distribution and the visualization of neurotransmitter substances which were recently discovered. Thus, extensive studies by the Swedish school of neuroanatomy provided a fantastic evolution and sensitivity improvement of the histofluorescence methods developed by Falck and Hillarp (Falck and Torp 1962; Falck et al. 1982). It made possible for the first time to visualize the distribution of noradrenaline (NA) (Vogt 1954), dopamine (DA) (Carlsson et al. 1958) and the catecholamine neurons and pathways in the rodent brain (Carlsson 1959; Carlsson et al. 1962; Dahlström and Fuxe 1964). It resulted in a series of discoveries on anatomical, pharmacological and mechanistic aspects of monoaminergic systems not only in the central nervous system but also in the diffuse neuroendocrine system (Sladek and Björklund 1982). It similarly allowed to anatomically demonstrate the colocalization of several transmitters (aminoacids, amines and later neuropeptides) in the same neuron (Hökfelt et al. 1986; Seroogy et al. 1988).
Carlsson with Falck and Hillarp (Carlsson et al. 1958) brought the first demonstration of DA production in the brain and provided evidence that the DA distribution was not similar to that of NA or adrenaline, suggesting different roles for these three catecholamines (Carlsson et al. 1961). Thereafter, Dahlström and Fuxe (1964) published a detailed distribution of catecholaminergic containing neurons in the rat brain. Few years later, Nobin and Björklund (1973) reported the presence of such monoamineric neurons in the fetus human brain.
By means of the same histofluorescent methods and more sensitive tracing techniques, it was shown that the DA innervation of the basal ganglia (or striatum) arose from the substantia nigra in the mesencephalon (Anden et al. 1964; Bertler et al. 1964). When later specific antibodies were raised against various neurotransmitters and enzymes involved in the synthesis of these catecholamines such as tyrosine hydroxylase (TH), which existence was first postulated by Blaschko (1939) and isolated by Nagatsu et al. (1964), it was possible, with single and dual immunohistochemical methods, to produce complete maps for monoaminergic systems, confirming and extending various hypotheses on the key roles of such neurotransmitter molecules in the central and peripheral nervous systems (Hökfelt et al. 1976, 1977; Carlsson 2001).
It was demonstrated that the basal ganglia are the major source of DA (Bertler and Rosengren 1966) and led Carlsson to propose that nigrostriatal DA might be involved in the control of motor activity while its decrease could be the cause of the extrapyramidal symptoms in patients with Parkinson’s disease (Carlsson 1959). Such hypothesis was then supported by the observation of a decrease in DA concentrations in preclinical models of Parkinson disease (Sotelo et al. 1973) and in the basal ganglia in patients with Parkinson’s disease. Finally, l-DOPA treatments, by restoring dopamine transmission, had beneficial effects (Birkmayer and Hornykiewicz 1961; Barbeau 1969; Carlsson 1971).
In 1955, coming back from Dr. Brodie’s laboratory at the NIH, A. Carlsson joined Hillarp’s laboratory in Lund where research was dedicated at that time to adrenal glands. Pioneer papers published by Carlsson, Hillarp and coworkers focused on these peripheral endocrine glands. They revealed high amounts of catecholamines in the adrenal medulla in specific granules also containing adenosine triphosphate (Hillarp and Nilson 1954; Carlsson and Hillarp 1956; Carlsson et al. 1957). Thereafter, Carlsson mainly dedicated his studies to the brain, bringing anatomical, biochemical, pharmacological and behavioral evidence for the implication of the different catecholaminergic systems in psychiatric and neurodegenerative disorders (Carlsson 1993, 2001; Tamminga and Carlsson 2002).
TH, the rate-limiting enzyme in catecholamine biosynthesis, has been widely used to study the catecholaminergic systems in particular the DA systems. Since the masterpiece work carried out by Hökfelt and coworkers on the distribution of TH-immunoreactive neurons in the rat brain (Hökfelt et al. 1984), computer science and imaging technologies dramatically improved both the quality and the resolution of images but also the quantitation of catecholaminergic neurons in the central structures and peripheral tissues.
Among such advances, the recently developed clearing methods coupled with light-sheet microscopy allowed to visualize immunolabeled cells in whole 3D organs. Thus, image segmentation procedures and 3D reconstruction at high resolution permit to identify new maps of TH immunoreactive cell bodies as well as TH nerve fiber pathways. The present review extends on mouse brain previous anatomical studies in the rat reported by the Swedish school using TH immunocytochemistry, by means of the iDISCO methodology in a transparent postnatal mouse brain as well as adult adrenals. Such approach enables to obtain a precise 3D distribution of catecholaminergic neuronal cell bodies, nerve pathways and neuroendocrine adrenal cells, that has never been described before.
Methodological considerations
Until recently, immunohistochemical approaches relied on thin tissue sectioning, sometimes followed by serial tissue section reconstitution which yielded incomplete spatial distributions (Simmons and Swanson 2008). New technologies such as light scanning microscopy or two-photon imaging have improved the detection and the imaging of fluorescent immunostaining on thick sections. However, such approaches are still limited by the sample size and by light scattering through thick tissues (Dobosz et al. 2014).
New methods of “clearing samples” recently emerged to avoid in particular light scattering (Richardson and Lichtman 2015). Together with ultramicroscopy, these techniques allow to obtain 3D imaging (Dodt et al. 2007; Erturk et al. 2012). Several chemical improvements of the clearing approaches and the development of various protocols (Belle et al. 2014, 2017; Renier et al. 2014, 2016; Launay et al. 2015; Pan et al. 2016; Klingberg et al. 2017) have been used to carried out this technique on various tissues in different species including human.
Immunohistochemical procedure
All animal procedures were performed in agreement with Institutional guidelines for the care and use of experimental animals (EU Directive 2010/63/UE). Five days-old C57BL/6 (P5) mice were used for the TH immunohistochemical localization in the brain and 14 days-old (P14) and adult mice for the adrenal glands. Mice (Janvier Ltd, Le Genest-Saint Isle, France) were anaesthetized with a mixture of Ketamine (80 mg/kg body weight) and xylazine (8 mg/kg bodyweight, Virbac, France) injected intraperitoneally. Tissues were fixed with an intracardiac perfusion of 4% paraformaldehyde in phosphate buffer saline (0.1 M PBS). Samples were carefully dissected, post-fixed overnight at 4 °C in the same fixative and assigned for TH immunostaining. Tissues were washed in 0.1 M PBS for 1 h twice, then in 50% methanol (in H2O) for 90 min, 80% methanol for 90 min, 100% methanol for 90 min. Tissues were then bleached with 3% H2O2 in 100% methanol overnight at 4 °C in the dark without shaking. Brains and adrenal glands were rehydrated in 100% methanol, 80% methanol, 50% methanol, and 0.1 M PBS, each step for 90 min before in toto staining procedures.
To illustrate the use of the immunohistological approaches in combination with clearing technique, two different immunostaining protocols for TH have been used for postnatal P5 mouse brains and for postnatal P14 and adult mice adrenal glands, respectively.
Pretreated P5 brains were incubated with the blocking and permeabilization PBSG-T solution (0.1 M PBS, 0.2% gelatin, 0.5% Triton-X100, 0.1 g/L thimerosal) for 2 days at 37 °C under agitation. Brains were then incubated with a previously characterized rabbit anti-TH antibody (Millipore, Guyancourt, France, #AB152, dilution 1:300) diluted in PBSG-T containing 0.1% saponin for 7 days at 37 °C with gentle shaking. This primary anti-TH antibody has been previously validated for iDISCO treatment (see the website https://idisco.info/validated-antibodies/which provides the list of antibodies compatible with iDISCO protocols). Brains were then washed in 0.1 M PBS for 1 day and incubated with a secondary antibody (donkey anti-rabbit Cy3, Jackson ImmunoResearch, Newmarket, UK, #705-165-147, 1:500) and TO-PRO 3 iodide to label neuronal cells (Invitrogen, Villebon, France, 1:80) in PBSG-T containing 0.1% saponin for 2 days at 37 °C in a dark room with gentle shaking.
Concerning postnatal P14 and the adult adrenal glands, samples were dehydrated (MeOH 20%, 40%, 60% 80% 100% twice, 1 h each at room temperature), bleached (MeOH 100%/3% H2O2, overnight at 4 °C) and rehydrated (MeOH 80%, 60%, 40%, 20%, 0.1 M PBS 1 h each at room temperature). Tissues were incubated for permeabilization in 0.1 M PBS/0.2% Triton/20% DMSO/23 µg l−1 glycine for 2 days at 37 °C with gentle shaking and for blocking in 0.1 M PBS/0.2% Triton/6% donkey serum/10% DMSO for 2 extra days at 37 °C. Tissues were then incubated with the same primary and secondary antibodies as described above for P5 brains (rabbit TH, Millipore, Guyancourt, France, #AB152, dilution 1:300) in 0.1 M PBS/0.2% Tween 20/0.1 µg ml−1 heparin/5% DMSO/3% donkey serum for 7 days with shaking at 37 °C and with a secondary antibody donkey anti-rabbit Cy3, Jackson ImmunoResearch, Newmarket, UK (#705-165-147, 1:500) in 0.1 M PBS/0.2% donkey serum/3% DMSO for 7 days. Finally, samples were washed in PBS for 36 h before the clearing procedure.
iDISCO+ clearing procedure
P5 mouse brains and P14/adult mouse adrenals were cleared using iDISCO+ protocol (Renier et al. 2016). Briefly, samples were dehydrated in 20%, 40%, 60%, 80% methanol in H2O 1 h each step and 100% methanol for 1 h twice. They were incubated overnight in 1 volume of 100% methanol/2 volumes 100% dichloromethane (DCM) anhydrous (#270997, Sigma-Aldrich, St Quentin-Fallavier, France), and washed twice for 20 min in 100% DCM. Finally, tissues were incubated in 100% dibenzyl ether (DBE) (#108014, Sigma-Aldrich) for at least 3 h before imaging.
Light-sheet imaging
Tissues were imaged with an Ultramicroscope II (LaVision BioTec, Bielefeld, Germany) using the ImspectorPro software (LaVision BioTec). The light sheet was generated by a laser (wavelength 561 nm or 640 nm, Coherent Sapphire Laser, LaVision BioTec) and two cylindrical lenses (Launay et al. 2015; Godefroy et al. 2017). A binocular stereomicroscope (MXV10, Olympus) with a 2× objective (MVPLAPO, Olympus) was used at different magnifications 0.63× for brain and P14 kidneys with adrenals and 3.2× for adult adrenals. Tissues were placed in an imaging reservoir made of 100% quartz (LaVision BioTec) filled with DBE and illuminated from the side by the laser light. Images were acquired with a PCO Edge SCMOS CCD Camera (2560 × 2160 Pixel size, LaVision BioTec). The step size in Z-orientation between each image was fixed at 2 µm resolution for 1.26× magnification and 1 µm for 6.4× magnifications, respectively.
Image processing
2D Images of the brain and adrenal glands were constructed from a Z-series of Ultramicroscope fluorescence images using the Imaris software, version 8.3.0_64 (http://bitplane.com, Zürich, Switzerland). Option tools of the software were used for 3D rendering or segmentation. Three steps were used for this process (Fig. 1): the first step consists in the determination of 2D images from regions of interest (ROI) according, for the brain, to the mouse brain atlas of Franklin and Paxinos (1997) and validation on 3D rendering. The second step aims to create a surface around ROI and in the third step concerning brain structures, false colors were given for brain structures of interest. 3D images and tiff series were obtained using the “animation” tools of the software. Movies were generated using the free software Fiji.
Results
3D localization of TH immunoreactivity in postnatal P5 mouse brain with the iDISCO+ technique
We recently used the iDISCO+ clearing procedure to identify, by means of TH immunostaining, the various catecholaminergic structures and pathways in a whole P5 postnatal mouse brain (Godefroy et al. 2017). This review summarizes the main results obtained in this study.
We took the opportunity of the TH staining to delineate and follow in 3D the original nomenclature A1–A16 of the various catecholamine-containing cell groups that was previously described by several groups (Dahlström and Fuxe 1964; Hökfelt et al. 1984; Lindvall et al. 1984). After two weeks of whole mount labeling procedure with a highly specific anti-TH antibody combined with image processing, a strong TH immunoreactivity corresponding to the various catecholaminergic cell groups was detected in a rostro-caudal localization with different orientations (sagittal, frontal, longitudinal) (Fig. 2). As expected, a strong TH staining corresponding to DA innervation was detected in the striatum, anterior cortical areas and in the olfactory bulbs. In the mesencephalon, a clear distinction was delineated between the well-known A10 DA neurons in the ventral tegmental area (VTA) corresponding to the ventromedial DA pathways projecting rostral to the nucleus accumbens and the frontal cortex, and the nigrostriatal pathway of the A9 DA neurons. Finally, caudal to the A9 dopamine neurons, some cells (A8) dorsal to the medial lemniscus were also detected.
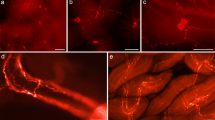
Distribution of TH immunoreactivity in P5 mouse brain. a Sagittal section of the whole P5 mouse brain. Lateral 1.44 mm b Longitudinal section of P5 mouse brain. These different planes are obtained from the 3D reconstruction. c Frontal section at the level of hypothalamus; d Frontal section at the level of mesencephalon; bregma − 3.80 mm, interaural 0.00 mm arc arcuate nucleus (A12), dm dorsomedial hypothalamic nucleus, lc locus coeruleus (A6), me median eminence, mfb medial forebrain bundle, ob olfactory bulb (A16), sn substantia nigra (A9), str striatum, vm ventromedial hypothalamic nucleus, vta ventral tegmental area (A10). Scale bar = 1 mm
In addition, a strong TH immunostaining was observed in the hypothalamus corresponding to the tuberoinfundibular DA neuronal network in the mediobasal hypothalamus, the arcuate nucleus and the median eminence (Fig. 2c) but also to more rostrally located DA cell groups (A11–A15) (Fig. 2c) as previously described by Anden et al. (1965).
Concerning the catecholaminergic neurons located in the pons and in the medulla oblongata, the A1–A6 groups of noradrenergic cell bodies were identified (Fig. 2). More caudally, the two groups of noradrenergic (A cells)/adrenergic (C cells) were found in the nucleus of the solitary tract (A2/C2) and in the medulla oblongata/facial nucleus (A1/C1).
A 3D movie allows to visualize the various catecholaminergic neurons of the mouse P5 brain as well as the ascending and descending catecholaminergic pathways innervating the various parts of the brain obtained with this iDISCO+ technique. It shows for instance on the same brain in 3D imaging the different TH immunoreactive projections from the brainstem to the mesencephalic and the hypothalamic regions via the medial forebrain bundle and the precise localization of the known innervation of the striatum by both nigrostriatal and mesolimbic pathways (Movie 1).
3D TH immunocytochemical localization in postnatal P14 and adult mouse adrenal glands with the iDISCO+ technique
By means of the same experimental approach with slight modifications as described in the methods, a strong TH immunostaining was detected in the developing P14 and in the adult adrenal glands located above the kidneys (Fig. 3). As expected, TH immunoreactivity was found in the chromaffin catecholaminergic cells in the adrenal medulla (Movie 2). 3D images also show the orthosympathic nerves which innervate the adrenal gland. A high TH immunoreactivity was localized in the developing P14 adrenals on the apical side of the kidney, within the inner part of the gland but also in populations of cells scattered in the cortex which will reach the medulla later during postnatal development as previously described (Gallo-Payet et al. 1987; Bornstein et al. 1994) (Fig. 3 and Movie 2).
The physiological catecholaminergic secretory activity of the chromaffin cells is influenced by a wide-range of endocrine (angiotensin II, insulin) (Cavadas et al. 2003; Senthilkumaran et al. 2016) and paracrine (Ehrhart-Bornstein and Bornstein 2008) factors. More recently, a link with the immune system activation has been demonstrated since several cytokines and chemokines can target chromaffin cells (Ait-Ali et al. 2008; Rosmaninho-Salgado et al. 2009; Bunn et al. 2012). The very high concentration of TH in specific granules of the adrenal medulla previously allowed the purification of the enzyme (Markey et al. 1980) and an impressive list of publications related to catecholamine secretion mechanisms, intracellular signaling pathways and regulation of adrenaline and noradrenaline release by various substances which play a major role in physiological adaptation to stress has emerged (Kvetnansky et al. 1970; Waymire et al. 1972; Ehrhart-Bornstein and Bornstein 2008).
Discussion and conclusions
Despite a few limits, the methodology using light-sheet microscopy, as described here, provides a high-resolution 3D imaging of intact tissues. The main drawbacks of the method are related to the penetration of the antibodies or of fluorescent molecules into the cleared tissue. Depending on the composition of the tissue (presence of lipids, myelin, membranes, connective tissues), some well-characterized antibodies used in previous classical immunocytochemical studies may not be able to penetrate the tissue sample. For these reasons, specific protocols have to be tested for each molecule/antibody used (Azaripour et al. 2016; Zhu et al. 2017). The size of the tissue may be also a critical point to obtain high-resolution images. However, iDISCO+ can be used for immunocytochemistry and retrograde transport staining (Launay et al. 2015). Furthermore, Kramer et al. (2018) recently provided the evidence that iDISCO+ enables to visualize mRNAs in cell nuclei of the mouse brain.
Segmentation of the images with false colors may be used to map, distinguish and identify the different catecholaminergic systems present in/or innervating various brain areas and peripheral tissues.
By means of other specific antibodies against catecholamine biosynthesis enzymes or catecholamine antibodies—yet unpublished experiments—it may be possible to independently obtain a precise 3D brain mapping of the DA, NA and adrenaline innervations. This review only focused on the central and peripheral TH immunoreactivity. Further studies using similar combined approaches could be conducted to evaluate the distribution of TH by double labeling with selective enzymes for noradrenaline and adrenaline neurons in healthy and pathological conditions. Under such conditions, the iDISCO+ approach may further provide quantitative information on the number of cells, branching, volume and distances (Soderblom et al. 2015; Pan et al. 2016; Godefroy et al. 2017; Renier et al. 2016; Belle et al. 2017). The robustness of the technique is demonstrated in the present work when comparing the results obtained with the reference works related to the various types of catecholaminergic cell groups (Hökfelt et al. 1984; Balan et al. 2000; Ugrumov et al. 2002; Björklund and Dunnett 2007; Epp et al. 2015; Yeo et al. 2016). Though not analyzed in detail here, 3D determination of cell bodies and tract-tracing may reveal some novel organization of subtle catecholaminergic pathways as already observed in previous classical histochemical methods (Björklund and Dunnett 2007). It may also highlight some differences between species since iDISCO+ has been largely used until now in mice and in human tissues (Belle et al. 2017; Vigouroux et al. 2017; Moore et al. 2018). Concerning the peripheral organs such as the adrenal glands, the iDISCO+ approach can provide an estimation of the volume and number of chromaffin cells expressing TH in various experimental conditions such as stress as previously carried out in the hypothalamus with vasopressin neurons (Godefroy et al. 2017). Finally, the present data also illustrate the possibility to address questions related to the developmental aspects of various organs.
As reported in the introduction, novel technologies may open new ideas and research orientations. The clearing methods may be used to bring new information on the distribution, the development pattern of known or yet undiscovered transmitter substances in the brain and peripheral organs in various animal species and human. iDISCO+ whole-brain imaging of non-sectioned tissues may help to follow the establishment of short and long neuronal projections during development or after injury, analyze the fate of stem cells and stem cell therapies (Qi et al. 2017). Such imaging approach can become a routine microscopic procedure combining high-resolution ultramicroscopic imaging on transparent tissues with computer 3D reconstitution. As it was the case with the development of confocal microscopy, such methodologies will become an essential tool to address new questions in anatomy and cell biology such as neuronal circuitry, migration and developmental processes.
Change history
17 November 2018
Unfortunately, the given name and family name of the fourth author was incorrectly tagged in the xml data, therefore it is abbreviated wrongly as ‘‘Goazigo AR’’ in Pubmed. The correct given name is Annabelle and family name is Reaux‑Le Goazigo.
References
Ait-Ali D, Turquier V, Tanguy Y, Thouennon E, Ghzili H, Mounien L, Derambure C, Jegou S, Salier JP, Vaudry H, Eiden LE, Anouar Y (2008) Tumor necrosis factor (TNF)-alpha persistently activates nuclear factor-kappaB signaling through the type 2 TNF receptor in chromaffin cells: implications for long-term regulation of neuropeptide gene expression in inflammation. Endocrinology 149:2840–2852. https://doi.org/10.1210/en.2007-1192
Anden NE, Carlsson A, Dahlström A, Fuxe K, Hillarp NA, Larsson K (1964) Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci 3:523–530
Anden NE, Dahlström A, Fuxe K, Larsson K (1965) Mapping out of catecholamine and 5-hydroxytryptamine neurons innervating the telencephalon and diencephalon. Life Sci 4:1275–1279
Azaripour A, Lagerweij T, Scharfbillig C, Jadczak AE, Willershausen B, Van Noorden CJ (2016) A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Prog Histochem Cytochem 51:9–23. https://doi.org/10.1016/j.proghi.2016.04.001
Balan IS, Ugrumov MV, Calas A, Mailly P, Krieger M, Thibault J (2000) Tyrosine hydroxylase-expressing and/or aromatic l-amino acid decarboxylase-expressing neurons in the mediobasal hypothalamus of perinatal rats: differentiation and sexual dimorphism. J Comp Neurol 425:167–176
Barbeau A (1969) l-Dopa therapy in Parkinson’s disease: a critical review of nine years’ experience. Can Med Assoc J 101:59–68
Belle M, Godefroy D, Dominici C, Heitz-Marchaland C, Zelina P, Hellal F, Bradke F, Chedotal A (2014) A simple method for 3D analysis of immunolabeled axonal tracts in a transparent nervous system. Cell Rep 9:1191–1201. https://doi.org/10.1016/j.celrep.2014.10.037
Belle M, Godefroy D, Couly G, Malone SA, Collier F, Giacobini P, Chedotal A (2017) Tridimensional visualization and analysis of early human development. Cell 169:161–173. https://doi.org/10.1016/j.cell.2017.03.008
Bertler A, Rosengren E (1966) Possible role of brain dopamine. Pharmacol Rev 18:769–773
Bertler A, Falck B, Gottfries CG, Ljunggren L, Rosengren E (1964) Soeme observations on adrenergic connections between mesencephalon and cerebral hemispheres. Acta Pharmacol Toxicol (Copenh) 21:283–289
Birkmayer W, Hornykiewicz O (1961) The l-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien Klin Wochenschr 73:787–788
Björklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30:194–202. https://doi.org/10.1016/j.tins.2007.03.006
Blaschko H (1939) The specific action of l-dopa decarboxylase. J Physiol (Lond) 96:50–51
Bornstein SR, Gonzalez-Hernandez JA, Ehrhart-Bornstein M, Alder G, Scherbaum WA (1994) Intimate contact of chromaffin and cortical cells within the human adrenal gland forms the cellular basis for important intraadrenal interactions. J Clin Endocrinol Metab 78(1):225–232
Bunn SJ, Ait-Ali D, Eiden LE (2012) Immune-neuroendocrine integration at the adrenal gland: cytokine control of the adrenomedullary transcriptome. J Mol Neurosci 48:413–419. https://doi.org/10.1007/s12031-012-9745-1
Carlsson A (1959) The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev 11:490–493
Carlsson A (1971) Basic concepts underlying recent developments in the field of Parkinson’s disease. Contemp Neurol Ser 8:1–31
Carlsson A (1993) On the neuronal circuitries and neurotransmitters involved in the control of locomotor activity. J Neural Transm Suppl 40:1–12
Carlsson A (2001) A paradigm shift in brain research. Science 294:1021–1024. https://doi.org/10.1126/science.1066969
Carlsson A, Hillarp NA (1956) Release of adenosine triphosphate along with adrenaline and noradrenaline following stimulation of the adrenal medulla. Acta Physiol Scand 37:235–239. https://doi.org/10.1111/j.1748-1716.1956.tb01359.x
Carlsson A, Hillarp NA, Hökfelt B (1957) The concomitant release of adenosine triphosphate and catechol amines from the adrenal medulla. J Biol Chem 227:243–252
Carlsson A, Lindqvist M, Magnusson T, Waldeck B (1958) On the presence of 3-hydroxytyramine in brain. Science 127:471
Carlsson A, Falck B, Hillarp NA, Thieme G, Torp A (1961) A new histochemical method for visualization of tissue catechol amines. Med Exp Int J Exp Med 4:123–125
Carlsson A, Falck B, Hillarp NA (1962) Cellular localization of brain monoamines. Acta Physiol Scand Suppl 56:1–28
Cavadas C, Grand D, Mosimann F, Cotrim MD, Ribeiro F, Brunner CA, Grouzmann HR, E (2003) Angiotensin II mediates catecholamine and neuropeptide Y secretion in human adrenal chromaffin cells through the AT1 receptor. Regul Pept 111:61–65
Dahlström A, Fuxe K (1964) Localization of monoamines in the lower brain stem. Experientia 20:398–399
Dobosz M, Ntziachristos V, Scheuer W, Strobel S (2014) Multispectral fluorescence ultramicroscopy: three-dimensional visualization and automatic quantification of tumor morphology, drug penetration, and antiangiogenic treatment response. Neoplasia 16:1–13
Dodt HU, Leischner U, Schierloh A, Jahrling N, Mauch CP, Deininger K, Deussing JM, Eder M, Zieglgansberger W, Becker K (2007) Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat Methods 4:331–336. https://doi.org/10.1038/nmeth1036
Ehrhart-Bornstein M, Bornstein SR (2008) Cross-talk between adrenal medulla and adrenal cortex in stress. Ann N Y Acad Sci 1148:112–117. https://doi.org/10.1196/annals.1410.053
Epp JR, Niibori Y, Hsiang L, Mercaldo HL, Deisseroth V, Josselyn K, Frankland SA, P.W (2015) Optimization of CLARITY for clearing whole-brain and other intact organs. eNeuro. https://doi.org/10.1523/ENEURO.0022-15.2015
Erturk A, Becker K, Jahrling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, Dodt HU (2012) Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc 7:1983–1995. https://doi.org/10.1038/nprot.2012.119
Falck B, Torp A (1962) New evidence for the localization of noradrenalin in the adrenergic nerve terminals. Med Exp Int J Exp Med 6:169–172
Falck B, Hillarp NA, Thieme G, Torp A (1982) Fluorescence of catechol amines and related compounds condensed with formaldehyde. Brain Res Bull 9:xi–xv
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, New York
Gallo-Payet N, Pothier P, Isler H (1987) On the presence of chromaffin cells in the adrenal cortex: their possible role in adrenocortical function. Biochem Cell Biol 65(6):588–592
Godefroy D, Dominici C, Hardin-Pouzet H, Anouar Y, Melik-Parsadaniantz S, Rostene W, Reaux-Le Goazigo A (2017) Three-dimensional distribution of tyrosine hydroxylase, vasopressin and oxytocin neurones in the transparent postnatal mouse brain. J Neuroendocrinol. https://doi.org/10.1111/jne.12551
Hillarp NA, Nilson B (1954) The structure of the adrenaline and noradrenaline containing granules in the adrenal medullary cells with reference to the storage and release of the sympathomimetic amines. Acta Physiol Scand Suppl 31:79–107
Hökfelt T, Johansson O, Fuxe K, Goldstein M, Park D (1976) Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain. I. Tyrosine hydroxylase in the mes- and diencephalon. Med Biol 54:427–453
Hökfelt T, Johansson O, Fuxe K, Goldstein M, Park D (1977) Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain II. Tyrosine hydroxylase in the telencephalon. Med Biol 55:21–40
Hökfelt T, Everitt B, Meister B, Melander T, Schalling M, Johansson O, Lundberg JM, Hulting AL, Werner S, Cuello C et al (1986) Neurons with multiple messengers with special reference in neuroendocrine systems. Recent Prog Horm Res 42:1–70
Hökfelt T, Martensson R, Björklund A, Kleinau S, Goldstein M (1984) Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Hökfelt T (ed) Handbook of chemical neuroanatomy. Classical transmitters in the CNS, part I. Elsevier, Amsterdam, pp 277–379
Klingberg A, Hasenberg A, Ludwig-Portugall I, Medyukhina A, Mann L, Brenzel A, Engel DR, Figge MT, Kurts C, Gunzer M (2017) Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy. J Am Soc Nephrol 28:452–459. https://doi.org/10.1681/ASN.2016020232
Kramer EE, Steadman PE, Epp JR, Frankland PW, Josselyn SA (2018) Assessing individual neuronal activity across the intact brain: using hybridization chain reaction (HCR) to detect arc mRNA localized to the nucleus in volumes of cleared brain tissue. Curr Protoc Neurosci 84:e49. https://doi.org/10.1002/cpns.49
Kvetnansky R, Weise VK, Kopin IJ (1970) Elevation of adrenal tyrosine hydroxylase and phenylethanolamine-N-methyl transferase by repeated immobilization of rats. Endocrinology 87:744–749. https://doi.org/10.1210/endo-87-4-744
Launay PS, Godefroy D, Khabou H, Rostene W, Sahel JA, Baudouin C, Parsadaniantz M, Goazigo SReaux-Le, A (2015) Combined 3DISCO clearing method, retrograde tracer and ultramicroscopy to map corneal neurons in a whole adult mouse trigeminal ganglion. Exp Eye Res 139:136–143. https://doi.org/10.1016/j.exer.2015.06.008
Lindvall O, Björklund A, Skagerberg G (1984) Selective histochemical demonstration of dopamine terminal systems in rat di- and telencephalon: new evidence for dopaminergic innervation of hypothalamic neurosecretory nuclei. Brain Res 306:19–30
Markey KA, Kondo H, Shenkman L, Goldstein M (1980) Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol 17:79–85
Moore AM, Lucas KA, Goodman RL, Coolen LM, Lehman MN (2018) Three-dimensional imaging of KNDy neurons in the mammalian brain using optical tissue clearing and multiple-label immunocytochemistry. Sci Rep 8:2242. https://doi.org/10.1038/s41598-018-20563-2
Nagatsu T, Levitt M, Udenfriend S (1964) Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem 239:2910–2917
Nobin A, Björklund A (1973) Topography of the monoamine neuron systems in the human brain as revealed in fetuses. Acta Physiol Scand Suppl 388:1–40
Pan C, Cai R, Quacquarelli FP, Ghasemigharagoz A, Lourbopoulos A, Matryba P, Plesnila N, Dichgans M, Hellal F, Erturk A (2016) Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods 13:859–867. https://doi.org/10.1038/nmeth.3964
Qi Y, Zhang XJ, Renier N, Wu Z, Atkin T, Sun Z, Ozair MZ, Tchieu J, Zimmer B, Fattahi F, Ganat Y, Azevedo R, Zeltner N, Brivanlou AH, Karayiorgou M, Gogos J, Tomishima M, Tessier-Lavigne M, Shi SH, Studer L (2017) Combined small-molecule inhibition accelerates the derivation of functional cortical neurons from human pluripotent stem cells. Nat Biotechnol 35:154–163. https://doi.org/10.1038/nbt.3777
Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M (2014) iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159:896–910. https://doi.org/10.1016/j.cell.2014.10.010
Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou Y, Wang VX, Tang CY, Olsen O, Dulac C, Osten P, Tessier-Lavigne M (2016) Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165:1789–1802. https://doi.org/10.1016/j.cell.2016.05.007
Richardson DS, Lichtman JW (2015) Clarifying tissue clearing. Cell 162:246–257. https://doi.org/10.1016/j.cell.2015.06.067
Rosmaninho-Salgado J, Araujo IM, Alvaro AR, Mendes AF, Ferreira L, Grouzmann E, Mota A, Duarte EP, Cavadas C (2009) Regulation of catecholamine release and tyrosine hydroxylase in human adrenal chromaffin cells by interleukin-1beta: role of neuropeptide Y and nitric oxide. J Neurochem 109:911–922. https://doi.org/10.1111/j.1471-4159.2009.06023.x
Senthilkumaran M, Johnson ME, Bobrovskaya L (2016) The effects of insulin-induced hypoglycaemia on tyrosine hydroxylase phosphorylation in rat brain and adrenal gland. Neurochem Res 41:1612–1624. https://doi.org/10.1007/s11064-016-1875-3
Seroogy K, Tsuruo Y, Hökfelt T, Walsh J, Fahrenkrug J, Emson PC, Goldstein M (1988) Further analysis of presence of peptides in dopamine neurons. Cholecystokinin, peptide histidine-isoleucine/vasoactive intestinal polypeptide and substance P in rat supramammillary region and mesencephalon. Exp Brain Res 72:523–534
Simmons DM, Swanson LW (2008) High-resolution paraventricular nucleus serial section model constructed within a traditional rat brain atlas. Neurosci Lett 438:85–89. https://doi.org/10.1016/j.neulet.2008.04.057
Sladek JR, Björklund A (1982) Preface. Brain Res Bull 9:9–10
Soderblom C, Lee DH, Dawood A, Carballosa M, Jimena Santamaria A, Benavides FD, Jergova S, Grumbles RM, Thomas CK, Park KK, Guest JD, Lemmon VP, Lee JK, Tsoulfas P (2015) 3D imaging of axons in transparent spinal cords from rodents and nonhuman primates. eNeuro. https://doi.org/10.1523/ENEURO.0001-15.2015
Sotelo C, Javoy F, Agid Y, Glowinski J (1973) Injection of 6-hydroxydopamine in the substantia nigra of the rat. I. Morphological study. Brain Res 58:269–290
Tamminga CA, Carlsson A (2002) Partial dopamine agonists and dopaminergic stabilizers, in the treatment of psychosis. Curr Drug Targets CNS Neurol Disord 1:141–147
Ugrumov M, Melnikova V, Ershov P, Balan I, Calas A (2002) Tyrosine hydroxylase- and/or aromatic l-amino acid decarboxylase-expressing neurons in the rat arcuate nucleus: ontogenesis and functional significance. Psychoneuroendocrinology 27:533–548
Vigouroux RJ, Belle M, Chedotal A (2017) Neuroscience in the third dimension: shedding new light on the brain with tissue clearing. Mol Brain 10:33. https://doi.org/10.1186/s13041-017-0314-y
Vogt M (1954) The concentration of sympathin in different parts of the central nervous system under normal conditions and after the administration of drugs. J Physiol 123:451–481
Waymire JC, Weiner N, Schneider FH, Goldstein M, Freedman LS (1972) Tyrosine hydroxylase in human adrenal and pheochromocytoma: localization, kinetics, and catecholamine inhibition. J Clin Investig 51:1798–1804. https://doi.org/10.1172/JCI106981
Yeo SH, Kyle V, Morris PG, Jackman S, Sinnett-Smith LC, Schacker M, Chen C, Colledge WH (2016) Visualisation of Kiss1 neurone distribution using a Kiss1-CRE transgenic mouse. J Neuroendocrinol. 28. https://doi.org/10.1111/jne.12435
Zhu X, Xia Y, Wang X, Si K, Gong W (2017) Optical brain imaging: a powerful tool for neuroscience. Neurosci Bull 33:95–102. https://doi.org/10.1007/s12264-016-0053-6
Acknowledgements
The present study was supported by Sorbonne and Normandie Universities, the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Association Française d’Epargne et de Retraite (AFER). Images were obtained on PRIMACEN (http://www.primacen.fr), the Cell Imaging Platform of Normandy, IRIB, Faculty of Sciences, University of Rouen, 76821 Mont-Saint-Aignan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
702_2018_1925_MOESM1_ESM.avi
Supplementary material 1 Movie 1 3D movie of TH distribution in P5 mouse brain. Attribution of false colors and volume “rendering” for dopaminergic (blue, in the striatum and mesencephalic regions), various catecholamines (green, in the hypothalamus), noradrenergic (white, in the olfactory bulbs and yellow, in the pons), noradrenergic/adrenergic neurons (pink, in the brainstem). The cerebellum is in orange (AVI 96719 KB)
Supplementary material 2 Movie 2 3D movie of the localization of TH positive chromaffin cells in the adrenal medulla of adult mice (MP4 28586 KB)
Rights and permissions
About this article
Cite this article
Godefroy, D., Rostène, W., Anouar, Y. et al. Tyrosine-hydroxylase immunoreactivity in the mouse transparent brain and adrenal glands. J Neural Transm 126, 367–375 (2019). https://doi.org/10.1007/s00702-018-1925-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1925-x