Abstract
The epigenome is of fundamental importance for development and ageing. The discovery of 5-hydroxymethylcytosine (5hmC), a further base modification of cytosine beyond 5-methylcytosine, might be of high relevance in understanding the complexity of the human brain, as 5hmC is found in great extent in brain tissue. The aim of this study was to investigate the quantity of 5hmC containing nuclei by immunohistochemistry in human and murine brains at several developmental stages. We performed immunohistochemical stainings on frontal cortex, white matter and cerebellar cortex of 15 healthy controls. Three cases each were assigned to five age groups (foetus, adolescent, adult, elderly, aged). Additionally, cortex and cerebellum of 15 mice sacrificed between day 0 and 120 after birth were investigated. We found marked alterations of 5hmC amount during ageing. In human cortex there was an increase of 5hmC of 50 %, in white matter we found an increase of even 200 % during ageing. In the cerebellum both internal granular cell layer and molecular cell layer showed a significant increase of 5hmC till adulthood. Purkinje cell nuclei showed constantly positive signals for 5hmC. These data were paralleled in murine brains. Co-labelling of 5hmC and markers for mature and immature cells in murine cerebellar cortex at the age of 7 days revealed that 5hmC was found in mature but not in immature cells. In conclusion, the findings described in this study emphasise the importance of 5hmC in brain development and ageing and will help to better understand the complexity and plasticity of the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epigenetics is the study of mechanisms that control gene expression. One of the most common epigenetic events is the modification of cytosine-bases within the promoter region of genes by adding a methyl group to position 5. The resulting 5-methylcytosine (5mC) is strongly associated with the regulation of gene transcription, i.e. the methylation of cytosine residues in the promoter region of genes leads to an inactivation of transcription (Branco et al. 2012; Kraus et al. 2012; Pfaffeneder et al. 2011; Tahiliani et al. 2009).
Kriaucionis and Heintz (2009) described a further modification of cytosine bases in Purkinje cells, a hydroxymethylation, leading to the so-called “sixth base” 5-hydroxymethylcytosine (5hmC). Up to now, the distinct function of 5hmC is still unknown but there is increasing evidence that it is of fundamental importance for active demethylation processes and the regulation of gene transcription (Branco et al. 2012; Mellen et al. 2012; Spruijt et al. 2013). 5hmC is generated by the TET (ten-eleven translocation) enzymes, 2-oxoglutarate/Fe(II)-dependent methylcytosine dioxygenases that convert 5mC to 5hmC by adding a hydroxyl group at position 5 of cytosine (Fig. 1) (Branco et al. 2012; He et al. 2011; Ito et al. 2011; Pfaffeneder et al. 2011). Studies in different organs revealed that 5hmC is unevenly distributed with only very low amounts, e.g. in liver tissue (Munzel et al. 2011) but very high amounts in brain tissue (Jin et al. 2011; Kraus et al. 2012; Munzel et al. 2011). Furthermore, 5hmC is associated with pluripotency and stem cell development (Branco et al. 2012). Ficz et al. (2011) were able to label embryonic stem cells using specific antibodies against 5mC and 5hmC. They found 5hmC to be mostly associated with euchromatin and increased gene transcription levels. Szulwach et al. (2011a, b) described that distinct gene enhancers show enrichments of 5hmC, and thus speculated that 5hmC may be of major importance not only for gene regulation but also for the regulation of neurodevelopment and ageing in general. Detailed analyses in human brain tissue revealed that especially cortical and subcortical regions exhibit considerable amounts of 5hmC (Kraus et al. 2012). Quantifications showed that in cortical cells 1.1 % of all cytosines were hydroxymethylated and 5 % were methylated. In white matter cells 0.6 % of all cytosines were hydroxymethylated and about 4 % were methylated (Kraus et al. 2012). This abundance of 5hmC in brain tissue also suggests probable disturbances of hydroxymethylation levels during the development and progression of neurological and psychiatric disorders. Recently, first evidence for a loss of 5hmC in Huntington’s disease has been found. Villar-Menéndez et al. (2013) report of increased 5-methylcytosine and decreased 5hmC levels in Huntington’s disease, Wang et al. (2013) report a genome-wide loss of 5hmC as novel epigenetic features of Huntington’s disease. In the context of Alzheimer’s disease there is increasing evidence that changes of 5hmC play a crucial role for the progression of the disease (Bradley-Whitman and Lovell 2013; Condliffe et al. 2014; van den Hove et al. 2012). But also in the context of mental disorders, recent studies imply that 5hmC is closely associated with autism (James et al. 2014; Zhubi et al. 2014).
Schematic description of cytosine modification pathways. According to Branco et al. (2012) two major pathways of active 5hmC demethylation are assumed: the oxidative pathway by converting 5hmC to 5fC and 5caC that is followed by decarboxylation reactions, and the deamination pathway by converting 5hmC to 5hmU and a subsequent excision using base excision repair (BER) mechanisms. 5caC 5-carboxycytosine, 5fC 5-formylcytosine, 5hmC 5-hydroxymethylcytosine, 5mC 5-methylcytosine, 5hmU 5-hydroxymethyluracil, AICDA activation-induced cytidine deaminase, APOBEC apolipoprotein B mRNA editing enzyme catalytic polypeptide-like, C cytosine, MBD4 methyl-CpG-binding domain protein 4, DNMT DNA methyltransferase, SMUG1 single-strand-selective monofunction uracil-DNA glycosylase 1, T tymine, TDG tymine-DNA-glycosylase
As there is increasing evidence that disturbances of the hydroxymethylome are associated with neurological diseases, there is the need for a detailed analysis of normal 5hmC quantities during ageing to perform conscious studies on 5hmC in human diseases. Till date, there are only few and unsystematic studies on the 5hmC status in normal brain available, and there is no detailed information on the alterations of 5hmC amounts during brain development and physiological ageing. In this study, we determined the age-dependent quantities of 5hmC by immunohistochemistry in human frontal neocortex, adjacent subcortical white matter and cerebellar cortex as well as in murine frontal cortex and cerebellar cortex as an attempt to determine the significance of 5hmC-changes during ageing. We selected the cortex of the middle frontal gyrus as a representative neocortical region and the cerebellar cortex to get insights into the influence of 5hmC on cognition, neuronal interconnectivity and development. Additionally, we investigated the association of 5hmC with proliferation and maturation of neurons. The data generated in this study will be the basis for further research on the field of neuroepigenomics and will help to better understand the processes of development and physiological ageing.
Materials and methods
Brain samples selection
Formalin-fixed and paraffin-embedded (FFPE) human post-mortem brain tissue samples of 15 cases without neurological or psychiatric disease and without noteworthy neuropathological alterations were selected from anonymized autopsy cases of the Centre for Neuropathology and Prion Research according to the guidelines of the local ethics committee for archived post-mortem material. Samples were assigned to five age groups with three cases per group: foetus (group 1, mean age gestation week 25, range gestation week 17–30), adolescent (group 2, mean age 18 years, range 15–23 years), adult (group 3, mean age 40 years, range 39–42 years), elderly (group 4, mean age 62 years, range 57–68 years), aged (group 5, mean age 79 years, range 78–81 years). To exclude neuropathological alterations, histological sections of the frontal and temporal cortex, basal ganglia, hippocampus, cerebellum, mesencephalon, pons, medulla oblongata and spinal cord were examined. As target regions, frontal neocortex, adjacent subcortical white matter and cerebellar cortex were selected to identify epigenomic changes of cognition and memory as well as motor function. Thereby, we focused on the middle frontal gyrus, adjacent white matter and cerebellar hemisphere cortex to get insights into the importance of 5hmC during development of cortex, white matter and cerebellum. Detailed information on human cases can be found in Table 1. To focus on early brain development, additionally brain tissue samples of 15 mice (strain C57BL/6) were investigated. Both female and male mice were used. Each three mice were sacrificed at day 0, 7, 15, 30 and 120 after birth. These time points correspond with human brains at gestation week 20, gestation week 35, 4 months, 1.5 years and the mature brain. Details on the correspondence of murine and human brain development as well as examples for events occurring at these time points can be found in Supplementary Table 1. Correspondence table was generated using the web-based tool provided by Clancy et al. (2007). Till the age of 3 weeks, mice were housed together with the mother. Afterwards, they were removed from the mother and each three were housed in standard cages (30 × 15 × 20 cm). To identify changes in the amount of 5hmC, each three mice were sacrificed at each time point. The brains were removed, formalin-fixed for 24 h and finally paraffin embedded. Frontal and cerebellar cortex were selected as target regions.
Immunohistochemistry and quantification
Immunohistochemistry was performed following standard protocols. In brief, 4 µm FFPE sections were deparaffinized by subsequent incubation in xylene for 30 min, 100 % ethanol for 10 min, 96 % ethanol for 5 min and 70 % ethanol for 5 min. Then sections were treated with 2 normal HCl for 20 min (at 35 °C) and pre-incubated with blocking solution (3 % I-Block Protein-Based Blocking Reagent; Applied Biosystems, Darmstadt, Germany) for 15 min at room temperature followed by an incubation with a polyclonal primary rabbit antibody against 5hmC diluted 1:1,000 (Active Motif, Rixensart, Belgium) over night at 4 °C. Antibody binding was visualised with a Super Sensitive Polymer-HRP Detection System (BioGenix, San Ramon, USA) according to the instructions of the manufacturer using 3,3′-diaminobenzidine (DAB) as a chromogen. Enzymatic reaction was performed for 5 min. Sections were counterstained in hemalum.
Double immunofluorescence was performed as previously described (Kraus et al. 2012). In brief, 4 µm FFPE sections were deparaffinized as described above. Then sections were treated with 2 N HCl (35 °C, 20 min), pre-incubated with 3 % I-Block Protein-Based Blocking Reagent (Applied Biosystems, Darmstadt, Germany) and incubated with the diluted primary antibodies for 18 h at 4 °C. As primary antibodies, we used a polyclonal rabbit anti-5hmC antibody (1:1,000, Active Motif, Rixensart, Belgium), a monoclonal mouse anti-Ki-67-antibody (1:100, Abcam, Cambridge, UK) and a monoclonal mouse anti-NeuN-antibody (1:300, Millipore, Eschborn, Germany). As secondary antibodies Alexa Fluor 488 goat-anti-rabbit IgG diluted 1:300 (Invitrogen, Darmstadt, Germany) and Alexa Fluor 546 goat-anti-mouse IgG diluted 1:300 (Roth, Karlsruhe, Germany) were used. Nuclear staining was performed with 4′,6-Diamidin-2-phenylindol (DAPI, Roth, Karlsruhe, Germany). Sections were finally coverslipped using Dako fluorescence mounting medium (Dako, Hamburg, Germany).
The quantification of 5hmC positive nuclei was done on digital pictures of the stained regions of interest. Pictures were taken with an Olympus BX50 microscope (Olympus, Tokyo, Japan) and a Color View III camera (Soft Imaging System, Münster, Germany) using 10× and 20× objectives (Olympus, Tokyo, Japan). Immunohistochemical staining intensities were homogenous within each region of interest in each slide. Representative areas without any technical artefacts were selected for quantification. Quantifications of 5hmC positive cell nuclei were done manually. Cells of interest comprised neurons, astrocytes and oligodendrocytes; microglial cells, cells of vessel walls and blood cells were not counted. Cell types were differentiated by morphological criteria (Pelvig et al. 2008). Cells with a nucleus of loose chromatin containing a clearly visible nucleolus free of surrounding heterochromatin and with an apparent cytoplasm were identified as neurons. Cells with a round and pale nucleus, whose heterochromatin was concentrated in granules in a rim beneath the nuclear membrane, and with a relatively translucent cytoplasm were considered as astrocytes. Cells with a small round nucleus of densely packed chromatin and inconspicuous cytoplasm were considered as oligodendrocytes. In this first approach, no quantification of the 5hmC positive fraction of neurons, astrocytes, and oligodendrocytes was performed. In the cerebellum, counting was performed separately for the external granular cell layer (EGCL, only in immature cerebellum), the molecular cell layer (MCL), for Purkinje cells (PC) and for the internal granular cell layer (IGCL). Cells were considered 5hmC positive even when the immunohistochemical signal was weak. Depending on the investigated brain region, layer and age, between 102 cells (in MCL of a foetus) and 619 cells (in IGCL of an adult) were counted in each human case (all counts are listed in Supplementary Table 2). In murine brains between 51 cells (in EGCL of a 15 days old mouse) and 385 cells (in IGCL of a 15 days old mouse) were counted, depending on brain region, layer and age (all counts are listed in Supplementary Table 3). Quantifications were performed using ImageJ software.
Statistical analysis
Statistical evaluations were performed using SPSS Statistics 22 (IBM) and Prism 5 (GraphPad) software. The Kolmogorov–Smirnov test, a visual inspection of the histograms, normal Q–Q plots as well as box plots were applied on previously published data of 22 samples derived from 9 healthy control cases using a similar immunohistochemical quantification protocol for 5hmC (Kraus et al. 2012) to test for normal distribution. These data were also used to determine sex and PMI effects on 5hmC amounts, but indicated no correlation of global 5hmC amount with sex and PMI ranging from 19 to 48 h. As statistical tests ANOVA one-way analysis of variances and Newman–Keuls post hoc test was used. As an explorative study, uncorrected p values were used. Statistical significance was assumed for p values <0.05.
Results
Age-dependant alterations of 5hmC in the brain
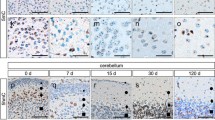
Immunohistochemistry with antibodies against 5hmC in human frontal cortex of foetal (Fig. 2a), adolescent (Fig. 2b), adult (Fig. 2c), elderly (Fig. 2d) and aged brains (Fig. 2e) showed that most but not all cell nuclei were stained. The same was seen in the adjacent white matter of foetal (Fig. 2f), adolescent (Fig. 2g), adult (Fig. 2h), elderly (Fig. 2i) and aged brains (Fig. 2j). Comparing cortex (Fig. 2a–e) with adjacent white matter (Fig. 2f–j) cortical areas showed more 5hmC positive nuclei than white matter.
Immunohistochemical detection of 5hmC in human (a–o) and murine brain (p–y). There are many 5hmC immunopositive nuclei (brown colour) in the human frontal cortex in age group 1 (foetus) (a), group 2 (adolescent) (b), group 3 (adult) (c), group 4 (elderly) (d) and group 5 (aged) (e) as well as in the adjacent white matter in age group 1 (f), group 2 (g), group 3 (h), group 4 (i), group 5 (j), and in the cerebellum in age group 1 (k), group 2 (l), group 3 (m), group 4 (n) and group 5 (o). Equally to the findings in the human brain, the frontal cortex of mice sacrificed at day 0 (p), 7 (q), 15 (r), 30 (s) and 120 (t) after birth as well as the cerebellum of mice sacrificed at day 0 (u), 7 (v), 15 (w), 30 (x) and 120 (y) after birth shows age-dependent amounts of 5hmC positive nuclei. Note the relative lack of 5hmC in the external granular cell layer (small-filled square) in human (k) and mice (u, v, w). High magnification pictures (z–δ) show the distribution of 5hmC in cortical (z) and subcortical (α) cells of the cerebrum as well as in cells of the molecular (β), Purkinje (γ) and granular (δ) cell layer of the cerebellum. Ash indicates case numbers listed in Table 1 and Supplementary Tables 2 and 3. Small-filled square external granular cell layer, filled circle molecular cell layer, filled diamond Purkinje cell layer, filled square internal granular cell layer. Scale bar 100 µm (a–y), 385 µm (z–δ)
In the human cerebellar cortex of foetal (Fig. 2k), adolescent (Fig. 2l), adult (Fig. 2m), elderly (Fig. 2n) and aged brains (Fig. 2o) only subsets of cells were immunopositive for 5hmC in IGCL and MCL. PC nuclei showed constantly positive signals for 5hmC but signal intensities were remarkably weaker compared to nuclei of MCL and IGCL. Interestingly, 5hmC positive nuclei were rare in EGCL of foetal cerebellum (Fig. 2k).
In the frontal cortex of mice results were equal to those in human frontal cortex, with most but not all cells being immunopositive for 5hmC (Fig. 2p–t).
In the murine cerebellar cortex, the number of immunopositive nuclei in IGCL and MCL varied during ageing (Fig. 2u–y). In EGCL, only scattered nuclei were immunopositive for 5hmC, at day of birth, while at age of 15 days, when EGCL fades out, numerous immunopositive nuclei were found in particular in lower layers (Fig. 2w). PC nuclei were constantly immunopositive during ageing with a slight decrease of signal intensities at day 120.
High resolution images of human cortex and subcortical white matter showed that especially neurons of the cortex (Fig. 2z) were 5hmC positive, whereas cortical astrocytes and oligodendrocytes showed only partial 5hmC positivity; in subcortical regions (Fig. 2α) clearly 5hmC positive astrocytes and oligodendrocytes were seen next to clearly 5hmC negative ones. Microglia was predominantly 5hmC negative. In the cerebellar cortex neurons of the molecular cell layer showed only partial 5hmC positivity (Fig. 2β), Purkinje cells stained predominantly intermediate, and partially strong for 5hmC (Fig. 2γ) and neurons of the granular cell layer showed an inhomogeneous 5hmC positivity with 5hmC positive neurons next to 5hmC negative ones (Fig. 2δ).
Statistical evaluations revealed that the number of 5hmC containing nuclei showed a significant increase of 50 % in human frontal cortex during ageing (p = 0.0025, F = 8.911, df = 14 using ANOVA, Fig. 3a). In human subcortical white matter, the increase of 5hmC positive nuclei was even 200 % during ageing (p = 0.0180, F = 4.986, df = 14 using ANOVA, Fig. 3b).
Quantification of 5hmC containing nuclei during ageing in human (a–d) and murine brain (e–h). In human frontal cortex (fctx) (a) and subcortical white matter (wm) (b) the relative number of 5hmC positive cells increases during ageing. In the internal granular cell layer (IGCL) (c) and molecular cell layer (MCL) (d) of human cerebellum, there is an increase of 5hmC positive cells till adulthood (age group 3) followed by a decrease at higher age. In murine frontal cortex (fctx), the number of 5hmC positive cells increases from day 0 to day 120 after birth (e). In murine cerebellum, the external granular cell layer (EGCL) (f) shows a rise of 5hmC positive cells from day 0 till the EGCL fades out at around day 15 after birth. In IGCL (g) and MCL (h) an increase of 5hmC positive cells is seen from day 0 to day 30 followed by a slight decrease till day 120. Age groups: 1 foetal, 2 adolescent, 3 adult, 4 elderly, 5 aged, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
In IGCL and MCL of human cerebellum there was a significant increase of 5hmC positive nuclei till adulthood (p = 0.0364, F = 3.917, df = 14 for IGCL, p = 0.0393, F = 3.809, df = 14 for MCL using ANOVA), followed by a decrease at higher age (Fig. 3c, d).
In murine frontal cortex there was a statistically significant increase of 5hmC positive nuclei from the day of birth (day 0) to day 120 (p = 0.0056, F = 7.095, df = 14 using ANOVA, Fig. 3e).
In murine cerebellar cortex a statistically significant increase of 5hmC positive cells was observed in EGCL from day 0 (with 1.5 % 5hmC positive cells) till day 15, when EGCL fades out (with 51.7 % 5hmC positive cells) (p = 0.0015, F = 23.13, df = 8 using ANOVA, Fig. 3f). In MCL and IGCL the amount of 5hmC positive cells showed a significant increase from day 0 till day 30 and a slight decrease from day 30 to day 120 (p = 0.0022, F = 9.171, df = 14 for MCL, p = 0.0005, F = 13.61, df = 14 for IGCL using ANOVA, Fig. 3g, h).
A regression analysis of 5hmC positive nuclei and age group showed a linear correlation of 5hmC amount and age in human frontal cortex (p = 0.0003, R 2 = 0.6537, 95 % confidence intervals 3.307–8.421, Supplementary Fig. 1a) and white matter (p = 0.0030, R 2 = 0.5055, 95 % confidence intervals 4.450–17.40, Supplementary Fig. 1b). In case of the internal granular cell layer and molecular cell layer regression analysis showed a non-linear correlation with R 2 = 0.5831 and 95 % confidence intervals for SD 1.209–2.220 for IGCL (Supplementary Fig. 1c) and R 2 = 0.5831 and 95 % confidence intervals for SD 1.209–2.220 for GCL (Supplementary Fig. 1d).
In the murine brain, regression analyses revealed a linear correlation of 5hmc amount and age in the case of frontal cortex (p = 0.0091, R 2 = 0.4186, 95 % confidence intervals 0.04252–0.2468, Supplementary Fig. 1e) and cerebellar external granular cell layer (p = 0.0002, R 2 = 0.8770, 95 % confidence intervals 2.232–4.478, Supplementary Fig. 1f). In case of the cerebellar internal granular cell layer and molecular cell layer, regression analysis showed a non-linear correlation with R 2 = 0.8008 and 95 % confidence intervals for SD 49.87–71.76 for IGCL (Supplementary Fig. 1g) and R 2 = 0.7762 and 95 % confidence intervals for SD 40.36–63.70 for MCL (Supplementary Fig. 1h).
5hmC is seen in mature neurons but not in proliferating neuroepithelial cells
In murine cerebellar cortex of three mice sacrificed at day 7 after birth double immunofluorescence for 5hmC and the proliferation-associated antigen Ki67 revealed that the majority of external granular cells was negative for 5hmC but positive for Ki67. Mostly lower cells of the EGCL migrating towards MCL showed 5hmC positivity but were negative for Ki67. In MCL and IGCL numerous 5hmC containing nuclei were found, but no cells expressing Ki67. Purkinje cells as well did not express Ki67 but were positive for 5hmC (Fig. 4a–c). In summary, proliferating cell nuclei did not contain 5hmC.
Immunofluorescence co-labellings of 5hmC with markers for mature neurons and proliferating cells in the cerebellum of a 7-day-old mouse (#M4). 5hmC is not seen in nuclei expressing the proliferation-associated antigen Ki67, a marker for immature proliferating cells (a–c), but is very well co-localised with NeuN, a marker for mature neurons (d–f). Blue colour in c and f represents immunonegative nuclei marked with 4′,6-diamidin-2-phenylindol (DAPI). EGCL external granular cell layer, MCL molecular cell layer, PC Purkinje cells, IGCL internal granular cell layer. Scale bar 100 µm
Immunohistochemistry for NeuN, a marker protein of many mature neurons, revealed no expression in the upper part of the EGCL but positive signals in cells adjacent to the MCL. Co-localisation experiments with antibodies against 5hmC indicated a co-labelling in these cells. IGCL cells showed strong signals for both NeuN and 5hmC. Purkinje cells did not express NeuN but 5hmC (Fig. 4d–f).
Discussion
The data presented in this study reveal that the quantity of 5hmC changes during brain development and ageing. This is the first time that the number of 5hmC containing nuclei has been determined in human post-mortem brains of different age groups focusing on frontal neocortex, subcortical white matter and cerebellum. The data indicate that 5hmC is strongly associated with neurodevelopment as the number of 5hmC positive nuclei increases during ageing in neocortex and subcortical white matter. In cerebellar cortex, we found highest amounts of 5hmC in adulthood with a subsequent age-dependent decrease. This uneven occurrence of 5hmC during brain development and ageing suggests that 5hmC is not only an intermediate in active demethylation processes but also of functional importance.
A regulatory importance of 5hmC has already been described in stem cells. Immunohistochemical analyses in combination with high-throughput sequencing in embryonal stem cells revealed that 5hmC is associated with euchromatin and plays a crucial role in the context of transcriptional activity (Ficz et al. 2011). This is in contrast to 5mC that is associated with transcriptional repression. Thus Ficz et al. (2011) supposed that the balance between 5mC and 5hmC in the genome is directly linked to the balance between pluripotency and lineage commitment. Ito et al. (2010) performed knock-down experiments in mouse embryos to further characterise the role of 5hmC and TET enzymes that are responsible for 5hmC generation. They found that a Tet1 knock-down in murine pre-implantation embryos results in a bias towards trophectoderm differentiation indicating that Tet1 plays an important role in murine embryonic stem cell maintenance and inner cell mass specification. One important step in understanding the biological function of 5hmC has been achieved by Spruijt et al. (2013). They used mass spectrometry to identify dynamic readers for 5mC and 5hmC, i.e. proteins that show high specificity to distinct DNA modifications depending on investigated cell types, e.g. mouse embryonic stem cells, neuronal progenitor cells and adult mouse brain tissue (Spruijt et al. 2013). Depending on the cellular differentiation state, they found distinct readers for 5mC and 5hmC that only partially overlap (Spruijt et al. 2013). These data are in good correlation with our findings.
In murine cerebellum, only 1.5 % of EGCL cells contained 5hmC at day of birth, when they are still immature and in proliferation, while at day 15, when they stop proliferating, differentiate into mature cells expressing NeuN and migrate towards the MCL, 51.7 % of EGCL cells contained 5hmC. New molecular pathways are activated in mature cells, and the readers for 5hmC change (Spruijt et al. 2013).
However, our study has some limitations. First, we investigated only 15 human and 15 murine cases spanning a quite wide age range resulting in only 3 human and 3 murine cases per age group. Due to this small sample size a bias of the results cannot be excluded. Second, there is an imbalance of sex distribution in the age groups. At the early ages, there are more female samples while at the later ages there are more male samples. In a previous study, we investigated 22 tissue samples derived from 9 healthy patients using a similar immunohistochemical quantification protocol (Kraus et al. 2012). An analysis of these data did not show sex-dependent differences of global 5hmC amount. Nevertheless, it cannot be excluded that 5hmC amount is sex dependent in distinct developmental and/or ageing stages. Third, we did not perform absolute quantifications of 5hmC. In our previous study, we correlated immunohistochemical levels of 5hmC to absolute quantifications using mass spectrometry and found a very good correlation of results in multiple cortical and white matter regions of human brains using these two different methods (Kraus et al. 2012). High numbers of 5hmC positive nuclei correspond to a high absolute number of 5hmC base within genomic DNA and lower numbers of 5hmC positive nuclei correspond to lower absolute amounts of 5hmC within genomic DNA. As mass spectrometry requires quite high amounts of DNA extracted from tissue, we did not use absolute quantifications in the current study assuming proportionalities of immunohistochemical 5hmC positive nuclei and absolute 5hmC amounts based on our previous study (Kraus et al. 2012). This approach allows to clearly distinguish between distinct anatomical structures enabling us to accurately determine regional differences of 5hmC expression. As an attempt to validate cell type-specific differences of 5hmC expression, immunofluorescence co-stainings were performed at day 7. Differences of 5hmC distribution were found in different layers and different cell populations. Our results show that there is an uneven distribution of 5hmC depending on age and investigated brain region. Nevertheless, further research will be necessary to specify our findings, including quantifications at multiple time points to identify the effects of 5hmC on adult neurogenesis as well as advanced quantifications by confocal microscopy and cell type-specific analysis of 5hmC using cell separation techniques. Furthermore, we selected the middle frontal cortex, adjacent subcortical white matter and cerebellar cortex in human cases as well as frontal cortex and cerebellar cortex in murine cases. Even though tissue was selected very consciously, a bias based on selecting different subregions cannot be excluded.
Future studies are warranted to confirm our results from this explorative study. With respect to epigenomic effects, larger cohorts need to be investigated allowing more precise age groups. Our data demonstrate an uneven distribution of 5hmC during development and ageing. Thus, further studies are suggested focusing on the embryonic age span including time points close to birth (e.g. gestation week 40) as well as on early ages (e.g. between day 1 and adolescence) in which neurodevelopment is still going in very high levels. Additionally, studies mapping 5hmC positive nuclei in further brain regions (e.g. hippocampus, amygdala, thalamus) are suggested.
In summary, the data presented in this study give first evidence that the amount of 5hmC in human and murine brains is region and age dependent. This underlines that 5hmC is not only an intermediate in the process of demethylation but is also of regulatory importance for stem cell differentiation, brain development and ageing. These data will encourage further research on causal epigenomic changes during ageing.
References
Bradley-Whitman MA, Lovell MA (2013) Epigenetic changes in the progression of Alzheimer’s disease. Mech Ageing Dev 134:486–495. doi:10.1016/j.mad.2013.08.005
Branco MR, Ficz G, Reik W (2012) Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet 13:7–13. doi:10.1038/nrg3080
Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL (2007) Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics 5:79–94
Condliffe D et al (2014) Cross-region reduction in 5-hydroxymethylcytosine in Alzheimer’s disease brain. Neurobiol Aging 35:1850–1854. doi:10.1016/j.neurobiolaging.2014.02.002
Ficz G et al (2011) Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature 473:398–402. doi:10.1038/nature10008
He YF et al (2011) Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 333:1303–1307. doi:10.1126/science.1210944
Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y (2010) Role of Tet proteins in 5mC to 5hmC conversion. ES-cell self-renewal and inner cell mass specification Nature 466:1129–1133. doi:10.1038/nature09303
Ito S et al (2011) Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333:1300–1303. doi:10.1126/science.1210597
James SJ, Shpyleva S, Melnyk S, Pavliv O, Pogribny IP (2014) Elevated 5-hydroxymethylcytosine in the Engrailed-2 (EN-2) promoter is associated with increased gene expression and decreased MeCP2 binding in autism cerebellum. Transl Psychiatry 4:e460. doi:10.1038/tp.2014.87
Jin SG, Wu X, Li AX, Pfeifer GP (2011) Genomic mapping of 5-hydroxymethylcytosine in the human brain. Nucleic Acids Res 39:5015–5024. doi:10.1093/nar/gkr120
Kraus TF et al (2012) Low values of 5-hydroxymethylcytosine (5hmC), the “sixth base,” are associated with anaplasia in human brain tumors. Int J Cancer 131:1577–1590. doi:10.1002/ijc.27429
Kriaucionis S, Heintz N (2009) The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324:929–930. doi:10.1126/science.1169786
Mellen M, Ayata P, Dewell S, Kriaucionis S, Heintz N (2012) MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell 151:1417–1430. doi:10.1016/j.cell.2012.11.022
Munzel M, Globisch D, Carell T (2011) 5-Hydroxymethylcytosine, the sixth base of the genome. Angew Chem 50:6460–6468. doi:10.1002/anie.201101547
Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B (2008) Neocortical glial cell numbers in human brains. Neurobiol Aging 29:1754–1762. doi:10.1016/j.neurobiolaging.2007.04.013
Pfaffeneder T et al (2011) The discovery of 5-formylcytosine in embryonic stem cell DNA. Angew Chem 50:7008–7012. doi:10.1002/anie.201103899
Spruijt CG et al (2013) Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell 152:1146–1159. doi:10.1016/j.cell.2013.02.004
Szulwach KE et al (2011a) Integrating 5-hydroxymethylcytosine into the epigenomic landscape of human embryonic stem cells. PLoS Genet 7:e1002154. doi:10.1371/journal.pgen.1002154
Szulwach KE et al (2011b) 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat Neurosci 14:1607–1616. doi:10.1038/nn.2959
Tahiliani M et al (2009) Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324:930–935. doi:10.1126/science.1170116
van den Hove DL, Chouliaras L, Rutten BP (2012) The role of 5-hydroxymethylcytosine in aging and Alzheimer’s disease: current status and prospects for future studies. Curr Alzheimer Res 9:545–549
Villar-Menendez I et al (2013) Increased 5-methylcytosine and decreased 5-hydroxymethylcytosine levels are associated with reduced striatal A2AR levels in Huntington’s disease. Neuromolecular Med 15:295–309. doi:10.1007/s12017-013-8219-0
Wang F et al (2013) Genome-wide loss of 5-hmC is a novel epigenetic feature of Huntington’s disease. Hum Mol Genet 22:3641–3653. doi:10.1093/hmg/ddt214
Zhubi A, Chen Y, Dong E, Cook EH, Guidotti A, Grayson DR (2014) Increased binding of MeCP2 to the GAD1 and RELN promoters may be mediated by an enrichment of 5-hmC in autism spectrum disorder (ASD) cerebellum. Transl Psychiatry 4:e349. doi:10.1038/tp.2013.123
Acknowledgments
We thank Dr. Thomas Arzberger, Department for Psychiatry and Psychotherapy/Centre for Neuropathology and Prion Research, Ludwig-Maximilians-University Munich, Germany for critically reading the manuscript.
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kraus, T.F.J., Guibourt, V. & Kretzschmar, H.A. 5-Hydroxymethylcytosine, the “Sixth Base”, during brain development and ageing. J Neural Transm 122, 1035–1043 (2015). https://doi.org/10.1007/s00702-014-1346-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-014-1346-4