Abstract
Methylphenidate (MPD) is used to treat ADHD and as a cognitive enhancement and recreationally. MPD’s effects are not fully understood. One of the sites of psychostimulant action is the ventral tegmental area (VTA). The VTA neuronal activity was recorded from freely behaving rats using a wireless system. 51 animals were divided into groups: saline, 0.6, 2.5, and 10.0 mg/kg MPD. The same repetitive MPD dose can elicit either behavioral sensitization or tolerance; thus the evaluation of the VTA neuronal activity was based on the animals’ behavioral response to chronic MPD exposure: animals exhibiting behavioral tolerance or sensitization. Acute MPD elicits dose-related increases in behavioral activity. About half of the animals exhibited behavioral sensitization or tolerance to each of the MPD doses. 361 units were recorded from the VTA and exhibited similar spike shape on experimental day 1 (ED1) and on ED10. 71, 84, and 79 % of VTA units responded to acute 0.6, 2.5, and 10.0 mg/kg MPD, respectively. The neuronal baseline activity at ED10 was significantly modified in 94, 95, and 100 % of VTA units following 0.6, 2.5 and 10.0 mg/kg MPD, respectively. Following chronic MPD exposure, 91, 98, and 100 % exhibit either electrophysiological tolerance or sensitization of 0.6, 2.6, or 10.0 mg/kg MPD, respectively. In conclusion, the chronic administration of the same dose of MPD caused some animals to exhibit behavioral sensitization and other animals to exhibit tolerance. The VTA units recorded from animals exhibiting behavioral sensitization responded significantly differently to MPD from animals that exhibited behavioral tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methylphenidate (MPD) is the most prescribed drug for treatment of attention deficit hyperactivity disorder (ADHD) in adolescents and adults (Challman and Lipsky 2000; Garland 1998; Lee et al. 2012; Solanto 1998). Chronic exposure to psychostimulants results in the initiation and alteration of biochemical, molecular, and morphological configuration as well as behavioral changes that lead to plasticity in the central nervous system (Chao and Nestler 2004; Dafny and Yang 2006; Dietz et al. 2009; Kim et al. 2009; Nestler 2004; Robison and Nestler 2011). Previous electrophysiological studies investigating the mechanisms of MPD have been done primarily with the use of anesthesia (Lacroix and Ferron 1988; Volz et al. 2009) or in vitro (Gronier 2011; Prieto-Gomez et al. 2004, 2005). It has been shown that anesthesia modulates the central nervous system’s activity; thus the use of anesthesia could potentially interact with MPD and modulate the drug effects on brain activity.
Repetitive psychostimulant exposure can cause either behavioral sensitization and/or tolerance (Askenasy et al. 2007; Robinson and Berridge 1993). Tolerance to a drug refers to drug-induced adaptations that lead to attenuating effects when the same dose of that drug is given again; to get the same effect, a higher dose is required. Alternatively, behavioral sensitization is a progressive amplification of behavioral responses to repeated psychostimulant exposure (Chao and Nestler 2004; Dafny and Yang 2006; Gaytan et al. 1996; Kalivas et al. 1988; Lee et al. 2009; Robinson 1984; Wolf 1998; Yang et al. 2003, 2011). Behavioral tolerance and sensitization are experimental markers used to indicate the potential of a psychostimulant to elicit drug dependence (Kalivas et al. 1998; Kauer 2004; Nestler 2005). It has been shown that stimulants like cocaine, amphetamine, and MPD cause dose-dependent behavioral sensitization in animals (Algahim et al. 2009; Bergheim et al. 2012; Gaytan et al. 1996, 1999; Kalivas et al. 1988; Tang et al. 2009; Yang et al. 2003, 2011). Using behavioral and pharmacological assays, it was proposed that behavioral sensitization exhibits two phases, induction and expression (Kalivas and Stewart 1991). The induction phase is thought to occur at glutamatergic synapses of the dopamine (DA) neurons in the ventral tegmental area (VTA) (Kalivas and Weber 1988; Vezina 1993; Perugini and Vezina 1994; Pert 1998). The expression of behavioral sensitization is suggested to be due to repetitive (chronic) psychostimulant exposure causing increased glutamate transmission and a decrease of D1 DA to GABAergic neurons in the VTA (Bonci and Williams 1996; Pierce and Kalivas 1997; Kalivas and Duffy 1993). In addition, the role of the VTA in the induction of behavioral sensitization was shown by daily local microinjections of amphetamine into the VTA (Papla et al. 2002). Furthermore, it was shown that the mesoaccumbens projection that is formed by the ascending VTA DA neurons to the nucleus accumbens (NAc) has been implicated in the induction of behavioral sensitization (Joyce and Rayport 2000; Kalivas et al. 1993; Wolf 1998). For example, studies using amphetamine as well as DA D1 and NMDA receptor blockers have shown that the VTA is the area responsible for the induction of sensitization following repetitive exposure to psychostimulants and perhaps the control of relapse, dependence, and drug craving (Kalivas and Weber 1988; Kalivas and Stewart 1991).
The VTA is rich in both DA and GABA neurons (Grace and Onn 1989) which project to the NAc and prefrontal cortex (PFC). Both DA and GABA neurons in the VTA get input mediated by GABA and glutamate; those afferents containing glutamate come from the PFC, while those of GABA originate in the NAc (Beckstead et al. 1979; Johnson and North 1992; Kalivas and Duffy 1995). The dopaminergic neurons in the VTA co-express GABA-ergic or glutamatergic markers and it is thought that these DA neurons are activated by the glutamatergic inputs originating in the PFC and are involved with the induction of behavioral sensitization following repetitive exposure of psychostimulants (Scheggi et al. 2002; Tang et al. 2009; Wanchoo et al. 2010). In a morphological study, it has been shown that about two-thirds of the neurons in the VTA are dopaminergic, while the remaining neurons are mostly GABA (Nair-Roberts et al. 2008). The induction phase of behavioral sensitization is suggested to be a result of a sequence of cellular and molecular events caused by exposure to psychostimulants which leads to lasting neuroplasticity changes that are expressed by enhanced drug response to repeated drug exposure and behavioral augmentation (Kalivas and Stewart 1991; Pierce and Kalivas 1997; Prieto-Gomez et al. 2004; White 1998). Therefore, the VTA was chosen as the target of this study.
Different responses to the same dose of MPD across individuals and ADHD patients have been reported (Volkow and Swanson 2003) and behavioral tolerance and sensitization to repetitive MPD exposure was reported (Gaytan et al. 1996; Barron et al. 2009; Yang et al. 2003, 2007, 2011). The hypothesis of this study is that the same chronic exposure of MPD dose will elicit behavioral tolerance in some animals and behavioral sensitization in other animals and that the VTA neuronal population activity recorded from animals expressing behavioral tolerance to chronic MPD exposure will respond differently to MPD compared with VTA neuronal population activity recorded from animals expressing behavioral sensitization. Therefore, the aim of the current study was to examine the dose–response effects of acute and chronic MPD on VTA neurons of non-anesthetized freely moving animals previously implanted with permanent electrodes and to evaluate the VTA neuronal activity based on the animals’ behavioral response to chronic MPD administration, i.e. VTA unit activity recorded from those animals that expressed behavioral sensitization was evaluated separately from those VTA units whose neuronal activities were recorded from animals that exhibited behavioral tolerance.
Methods
Animals
Male Sprague–Dawley rats (N = 51) at post natal of about 50 days were purchased (Harlan, Indianapolis, IN, USA) and were allowed 5–7 days of acclimation in our vivarium room on a 12 h light/dark schedule (lights on 6:00 a.m.). Food and water were given ad libitum. The animals were housed individually in clear acrylic standard cages that served as both home cage and test cage for this study. The experiment was approved by our Animal Welfare Committee and carried out in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals.
Surgeries
Prior to surgery, two Nickel–Chromium Teflon-coated (fully insulated except at tips) 60 μm diameter wires were twisted to make two recording electrodes for each VTA hemisphere. Each of the four wires was secured to a 1.0-cm copper connector pin (A-M systems, INC.). On the day of surgery, the rats were anesthetized with an intraperitoneal (i.p.) injection of 50 mg/kg pentobarbital. The animal’s head was shaved and lidocaine hydrochloride topical gel was applied to the shaved area. The animal was then placed in a stereotaxic apparatus where an incision was made on the scalp and the muscle and connective tissue were removed to expose the skull. Bilateral holes were drilled above the VTA at 6.0 mm posterior to bregma and 0.5 mm lateral from midline, using Paxinos and Watson (1986) brain atlas coordinates. Six anchor screws were inserted in the skull at vacant spots to secure the implanted electrodes and the head plug. Electrodes were then inserted individually into the brain at an initial depth of 8.0 mm from the skull with neuronal activity monitored by Grass emitter Hi Z Probe connected to a Grass P511 series amplifier. When a 3:1 signal to noise ratio spike activity was obtained, the electrode was permanently secured to the skull using web glue cyanoacrylate surgical adhesive. When the neuronal activity exhibited less than a 3:1 signal to noise ratio spike activity, the electrode was lowered in 5–10 μm increments up to a maximum depth of 8.5 mm until a 3:1 ratio activity was observed (Chong et al. 2012; Claussen and Dafny 2012; Dafny 1980, 1982; Dafny and Terkel 1990; Salek et al. 2012). Similar procedures were followed for the second twisted electrode which was implanted into the VTA of the opposite hemisphere. The two copper pins from each twisted electrode from the four recording electrodes were inserted into Amphenol plugs which were secured to the skull using dental acrylic cement creating the skull cap. Animals were allowed 4–7 days’ recovery after electrode implantation during which they were placed daily within their home cage in the experimental apparatus and connected to the wireless (telemetric) head stage transmitter (Triangle BioSystems Intl (TBSI); Durham, NC, USA) for acclimation for at least 2 h/day to the behavioral and electrophysiological recording systems. At the first experimental day, the animal’s weight was between 200 and 220 g and at about post natal 62–64 days.
Drugs
Three methylphenidate hydrochloride (MPD) (obtained from Mallinckrot Hazelwood, MO, USA) doses of 0.6, 2.5, and 10.0 mg/kg, were used; the MPD doses were calculated as a free base and were dissolved in 0.9 % isotonic saline solution. Control injections consisted of 0.8 ml isotonic saline solution (0.9 % NaCl) administered i.p. All injections were equalized to a volume of 0.8 ml with 0.9 % saline to keep injection volumes the same for all of the animals and for all the MPD doses.
Previous MPD dose response experiments, testing behavioral and neurophysiological sensory evoked potential procedures, from 0.1 to 40.0 mg/kg MPD administration, found that behavioral effects of MPD were observed from the 0.6 mg/kg MPD dosage (Algahim et al. 2009; Gaytan et al. 1996, 2000; Lee et al. 2009; Podet et al. 2010; Yang et al. 2003, 2006a, b, c, d, 2007). Therefore, 0.6, 2.5, and 10.0 mg/kg MPD dosages were selected for this study.
There are no universally recognized MPD dosage guidelines or blood levels to achieve optimum dose treatment. A study of 289 patients treated with MPD (White and Yadao 2000) reported that the range of doses ingested was from 0.06 to 29.3 mg/kg. Approximately 2–3 mg/kg i.p. MPD in rodent achieved plasma levels similar to those achieved in clinical use (Gatley et al. 1999; Gerasimov et al. 2000). Drug effects in rodents often require higher doses (on mg/kg basis) than humans because rodents exhibit a more rapid metabolism (Gatley et al. 1999).
Experimental protocol
On experimental day one (ED1), rats with their home cage were placed in a Faraday testing box to reduce noise during the recording session. The wireless (TBSI); (Fan et al. 2011) head stage was connected to the electrode pins of the skull cap and the animals were allowed to acclimate for 30 min prior to the recording session. This time was used to prepare the recording software parameters and the injections of both saline and MPD. After acclimation, the animals received a saline injection of 0.8 ml (standardized for all injections) and the neuronal and behavioral activity was recorded to obtain the baseline activity for 1 h followed by either saline, or 0.6, 2.5 or 10.0 mg/kg MPD injection, and the behavioral and the neuronal activity recordings were resumed for an additional hour post injection (Table 1). The wireless TBSI head stage sent neuronal activity signals from the four recording electrodes to a receiver that was connected to a Cambridge Electronic Design (CED) Cambridge England analog-to-digital converter (Micro1401-3; CED) which collected and stored the recorded data on a PC using Spike 2.7 CED software. On ED2 through ED6 animals received either saline or daily MPD injections similar to ED1 injection in their home cage without recording. On ED7 through ED9 the animals underwent washout in which no injections were given proceeded by ED10 in which a saline injection was given and the neuronal and behavioral activity was recorded for an hour post saline injection followed by a rechallenge administration of either saline or MPD similar to ED1 and recordings were resumed for an additional 1 h just as on ED1 (Table 1).
Behavior apparatus
Locomotor activity was recorded concomitantly with neuronal activity, using an open field computerized animal activity system (Opto-M3, Columbus Instruments, Columbus, OH, USA). The animal’s home cage (40 cm in length, 20 cm in width) fit into the recording apparatus allowing the recording of the animals in his home cage. The cage and the recording system were located inside a Faraday box to reduce noise and any outside interference. The open field system 16 and 8 infrared beams and their sensors on the opposite side were set at 5 cm above the floor of the cage. The open field assay has been previously described in detail (Gaytan et al. 1996, 1997a, b, 2000; Yang et al. 2006a, 2007, 2011). In short, the activity monitoring system checked each of the sensor beams at a 100-Hz frequency to determine whether beams were interrupted. The interrupted beams were compiled by the software and downloaded to a PC every 10 min (i.e. 6 bins/h). The program organized the beam interruption into different locomotor movement indices, such as horizontal activity (HA) which records the overall locomotor activity that used to assess the overall amount of locomotor activity and the number of stereotypic activity (NOS) which counts the number of repetitive movement episodes with at least an one-second interval before the beginning of another episode of movements. The six bin counts following each hour were used for the statistical analysis and to produce temporal graphs and histograms for total activity/h for both the saline (baseline activity) and the activity after MPD administration for ED1 and ED10.
Electrophysiological apparatus
A Triangle BioSystems International (TBSI) telemetric head stage weighing 4.5 g was attached to the electrode pins of the skull cap of the rat. The head stage sent neuronal activity signals (sampling rates up to 200 kHz) to a receiver connected to the Cambridge Electronic Design (CED) analog-to-digital converter (Micro 1401-3; CED, Cambridge, UK) which stored the data on a PC using the Spike 2 version 7 software for offline analysis.
Data analysis
Behavioral analysis
The locomotor activity was recorded and summed in 10 min bins for 60 min after saline injection proceeding another 60 min of behavioral activity counts recorded following MPD injection (12 bins total) on ED1 and again on ED10 following saline and MPD exposure (Table 1). (1) Behavioral analysis of HA and NOS of activity following acute MPD was compared with the activity after saline (control) at ED1; (2) the activity post saline at ED10 was compared with the activity after saline on ED1, and (3) the chronic MPD effect was determined by comparing the activity following MPD on ED10 to the activity after MPD at ED1 using the Critical Ratio CR Test; (CR) = E − C/\(\sqrt {E + C}\) ± 1.96 = P < 0.05 and the student paired t test (Chong et al. 2012; Claussen and Dafny 2012; Salek et al. 2012; Yang et al. 2006a, b, d, 2007), Where, for example, E represents the HA after MPD administration at ED1, and C represents the HA after saline injection of ED1. When a CR value more than 1.96 was obtained it indicated that the drug elicited significantly increased activity, i.e. behavioral sensitization and when a CR value more than −1.96 was obtained it indicated that the drug elicited significant attenuation as compared with the initial drug effects, i.e. behavioral tolerance.
Based on the above analysis each rat was individually classified as expressing either behavioral sensitization or tolerance and thus two sub groups were created: sensitized and tolerant/non-sensitized category. These rat subgroups were analyzed again as a group using analysis of variance (ANOVA: treatment days and drug doses). Any statistical significance was determined with the post hoc Fischer’s LSD method. Statistical significance was set at P < 0.05 for all comparisons.
Spike sorting
The Spike 2 version 7 software (CED) was used for spike sorting. The data were captured by the program (sampling rates up to 200 kHz) and processed using low- and high-pass filters (0.3–3 kHz). There were two window discriminator levels, one for positive-going spikes and one for negative-going spikes. The spikes with peak amplitudes within the window were used to create templates using 1,000 waveform data points. The parameters that were used to capture a spike pattern allows the extraction of templates that provide high-dimensional reference points which can be used to perform accurate spike sorting, despite some movement artifacts noise, false threshold crossing, and waveform overlap. All temporally displaced templates are compared with the incoming spike event to find the best fitting to the selected template amplitude that yields the minimum residue variance. When the distance between the template and waveform exceeds some threshold (80 %), the waveforms are rejected. This means that the spike sorting accuracy in the reconstructed data is about 95 %. All of the parameters of spike sorting for each electrode at ED1 were stored and reused for the activity sorting at ED10 aiming to count the same spike amplitude and pattern at ED1 and ED10 following saline and MPD administration from the same electrode. Spikes with peak amplitudes outside these limits and spikes that did not fit the template were rejected.
Electrophysiological data evaluation
Once the neuronal activity was sorted and counted, the activity post saline and MPD exposure was exported to a spreadsheet to produce sequential firing rate graph spikes/s and counting the total neuronal activity. The initial 60 min following saline injection at ED1 was used as the ED1 baseline activity. Similar calculations were done for ED10, i.e. 60 min post ED10 saline for the ED10 baseline activity and 60 min post MPD at ED10, the effect of MPD rechallenge. The activity post MPD at ED1 compared to ED1 baseline activity was used to determine the MPD effect. The baseline activity on ED10 was compared with the baseline activity on ED1 to find out whether the six consecutive daily injection and the three washout days resulted in the baseline alteration, and last, the effect of drug administration on ED10 was compared with the effects of drug administration on ED1 to find out whether sensitization or tolerance was expressed.
Multiple methods were employed to find out whether MPD elicits significant effect on VTA neuronal activity as follows: the mean firing rate after MPD treatment needs to be at least two standard errors (SE) difference from the control mean firing rate. Firing rates were evaluated also for normality assumptions to determine parametric or non parametric methods. To evaluate differences between the above comparisons, the firing rates were determined to not hold normality assumptions, so we assessed also differences in firing rates using the critical ratio test. Critical ratio test (CR = \(\frac{E - C}{{\sqrt {E + C} }}\) = ±1.96 = P < 0.05) (C = control, E = activity after drug exposure) for the first comparison. For the second comparison, E was the baseline at ED10 and C is the baseline at ED1, while for the third comparison E was the data post MPD exposure at ED10 and C the data post MPD exposure at ED1 (Chong et al. 2012; Claussen and Dafny 2012; Dafny 1975, 1982; Salek et al. 2012; Yang et al. 2006a, b, c).
In addition, the natural log ratio and the log linear model statistical tests were used: the natural logs ratio was utilized to determine the likelihood that VTA neuronal populations recorded from animals expressing behavioral sensitization are the same or different from the VTA neuronal populations recorded from animals expressing behavioral tolerance to repetitive MPD exposure. This was done for the data obtained following the initial (acute) MPD injection compared with ED1 baseline, the baseline neuronal activity at ED10 compared with the baseline activity at ED1, and the recording following rechallenge of MPD at ED10 compared with the effect of initial MPD injection at ED1. To compensate for the smaller values observed on certain days for varying doses, 0.5 was added to all numbers for computation of the odds ratio. A number of 1 and higher in the odds ratio test indicates a significantly higher likelihood of different responses to MPD exposure (increases or decreases in the neuronal activity) in the neuronal populations recorded from behaviorally sensitized animals compared with recordings from animals expressing behavioral tolerance. Conversely, a number smaller than 1 represents a less likely chance of differences between the two VTA neuronal populations (Morris and Gardner 1988) in response to MPD exposure.
A log linear analysis with a chi square distribution was used (Waterson et al. 2012) with a likelihood ratio statistic value (p < 0.05) to compare the differences in the response to MPD. The exposures between the neuronal populations recorded from animals expressing behavioral sensitization was compared to the neuronal population recorded from animals expressing behavioral tolerance in response to both acute and chronic MPD exposure.
Histological verification of electrode placement
Upon completion of the recording at ED10, the animals were overdosed with sodium pentobarbital. The rat was then perfused intracardially with 10 % formaldehyde solution containing 3 % potassium ferrocyanide. A 2-mA DC current was passed through the tip of each electrode for 40 s to create a small lesion to identify the electrode location; the brain was extracted from the skull and placed in 10 % formaldehyde for several days. The brains were sliced into 40- to 60-μm sections and histologically stained with Cresyl Violet. The position of the electrode tip was identified by the location of the lesion and the Prussian blue spot using the Rat Brain atlas (Paxinos and Watson 1986).
Results
Only data from electrodes confirmed to be within the VTA and exhibiting similar spike amplitude and pattern at the ED1 and ED10 recording session were included. A total of 51 rats with 111 electrodes confirmed to be in the VTA were used in this experiment. Twenty, 14, 7, and 10 rats were treated with saline, 0.6, 2.5, and 10.0 mg/kg MPD, respectively, and 361 units were recorded: 76, 132, 62, and 91 after saline, 0.6, 2.5, and 10.0 mg/kg MPD, respectively.
Behavior
Control Twenty animals were treated only with saline (Table 1). Following acute and repetitive saline injection, all animals expressed similar locomotor activity following the 2nd saline injection compared with the first on ED1. This showed that saline and handling had no effect on the rats’ behavioral activity. Therefore, the activity after saline injection at ED1 can be used and was used as the control for the MPD effects, i.e. any significant changes due to drug exposure were due to the effects of MPD.
Fourteen animals were treated with 0.6 mg/kg MPD; the ANOVA test showed no significant changes in locomotor activity after acute or chronic 0.6 mg/kg MPD or when the baseline activity at ED10 (after 6 daily 0.6 mg/kg MPD and 3 washout days) was compared with ED1 baseline (Figs. 1, 2Aa). When these 14 animals were divided based on their individual behavioral responses to chronic MPD exposure, 3 animals at ED10 following MPD exposure exhibited significant (P < 0.05) increases in their locomotion compared with the activity following MPD injection on ED1 (Fig. 2Ab). These three animals at ED1 responded to MPD by significant (P < 0.05) decreases by 52 % in their locomotor activity compared with their ED1 baseline (Fig. 2Ab). The baseline locomotor activity at ED10 after six daily injections of 0.6 mg/kg MPD and three washout days was significantly (P < 0.05) elevated by 38 % compared with the baseline at ED1 (Fig. 2Ab). The remaining 11 animals treated with 0.6 mg/kg MPD showed reduction in their locomotor activity at ED10 post MPD compared with ED1 post MPD exposure, but this attenuation appears to be non-significant as a group. These animals did not respond significantly to acute 0.6 mg/kg MPD and their ED10 baseline behavioral activity compared with ED1 baseline behavioral activity was relatively unchanged (Fig. 2Ac).
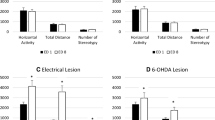
Picture represents the horizontal activity (HA) data recorded from the animals following each MPD dose (0.6, 2.5 and 10.0 mg/kg). The three figures in the left column summarize the activity of all the animals when grouped together for each dose (Aa, Bd, Cg). In addition, data were broken down for each MPD dose into those animals that exhibited behavioral sensitization (Ab, Be, Ch) and those animals that exhibited behavioral tolerance (Ac, Bf, Ci) to chronic MPD exposure, respectively. For each group, the HA of ED1 after MPD exposure is compared with ED1 Baseline activity; the ED10 Baseline HA is compared with ED1 Baseline HA; and the HA activity on ED10 following MPD exposure is compared with the ED1 HA post MPD exposure. ED experimental day, BL baseline, MPD methylphenidate; *P < 0.05
Seven animals were treated with acute and chronic 2.5 mg/kg MPD, and when grouped together for analysis of their behavioral activity, it was observed (ANOVA test) that all the animals exhibited significant (P < 0.05) increases in their locomotion following the initial (acute) 2.5 mg/kg MPD exposure at ED1 (Fig. 2Bd). However, the activity post MPD exposure at ED10 compared with activity post MPD at ED1, as well as the baseline activity at ED10 compared with the baseline at ED1, was about the same, i.e. was not significantly different (Fig. 2Bd). When these seven rats were evaluated individually using the C.R. test and then grouped based on their individual responses to chronic MPD exposure, three animals exhibited behavioral sensitization (using the ANOVA test, P < 0.05). The acute 2.5 mg/kg MPD to these three animals elicited non-statistically significant increases in locomotion (Fig. 2Be). The baseline activity at ED10 of these three animals compared with baseline at ED1 exhibited non-significant attenuation, and there were increases in their locomotor activity at ED10 compared with ED1 post MPD exposure, but due to a large standard error and small N, it was not significant (Fig. 2Be). The remaining 4 rats that individually exhibited a decrease in their locomotor activity (P < 0.05) following chronic MPD exposure, i.e. exhibited behavioral tolerance. Their behavioral response to the initial (acute) MPD was robustly (P < 0.05) increased (Fig. 2Bf) and the baseline activity of this group at ED10 compared with ED1 baseline exhibited a non-significant difference. The activity following chronic MPD exposure at ED10 compared with the effect of MPD on ED1 (Fig. 2Bf) after 2.5 mg/kg MPD exposure as a group exhibited a non-significant decrease in their behavioral activity.
The ten animals treated with 10.0 mg/kg MPD exhibited a significant (P < 0.001) increase in locomotor activity by 580 % compared with their baseline arbitrarily set at 100 % (Fig. 2Cg). The ED10 baseline activity remained the same compared with the initial baseline activity at ED1. MPD exposure at ED10 compared with the initial MPD exposure at ED1 elicits similar effects (Fig. 2Cg). When the animals were analyzed individually based on their chronic response to the drug to the acute effect, five of the animals exhibited significant (P < 0.05) behavioral sensitization (Fig. 2Ch), and five animals exhibited a significant (P < 0.05) reduction in their locomotor activity (Fig. 2Ch, Ci). Those individual animals that exhibit behavioral sensitization exhibited significantly (P < 0.05) increased responses to MPD in response to the acute dose of 10.0 mg/kg by 487 % (Fig. 2Ch). The baseline activity at ED10 compared with ED1 baseline was increased, but non-significantly. The chronic effect of the drug at ED10 as compared with the initial MPD exposure at ED1 showed a significant (P < 0.05) increase in activity by 177 % (Fig. 2Ch). The five animals that individually exhibited behavioral tolerance in response to 10.0 mg/kg MPD exhibited a significant (P < 0.05) increase in response to acute drug on ED1 by 665 %. Their acute response to MPD was significantly (P < 0.05) higher compared with those animals in the sensitized group (Fig. 2Ci). The baseline activity of these five tolerant rats at ED10 was similar to ED1 baseline. In response to chronic MPD on ED10 compared with ED1 post MPD, the animals exhibited a decrease in activity, but this reduction in activity was not significant due to a large SE (Fig. 2Ci). The number of stereotypic movements (NOS) was analyzed as well as a second measure to verify that the animals exhibiting behavioral tolerance or no change were not exhibiting an increased stereotyped behavior (a form of behavioral sensitization). The HA results show that all animals expressing behavioral tolerance also exhibited significant (P < 0.05) decreases in NOS (not shown).
Electrophysiology
A total of 321 units were histologically confirmed to be recorded from the VTA and exhibited similar amplitude and wave form at ED1 and ED10. Thirty-six VTA units were recorded following acute and repetitive saline injection. Two units showed decreased activity at ED1; the baseline activity of one VTA unit at ED10 compared with ED1 baseline exhibited a decrease in activity, and one VTA unit exhibited an increase in activity at ED10 following saline injection compared to ED1 baseline activity. This observation revealed that the saline injections and animal handling did not alter the neuronal activity of VTA neurons.
Two hundred and eighty-five VTA units were recorded following acute and chronic MPD exposure. Seventy-six percent (217/285) of the VTA units exposed to acute (0.6, 2.5 or 10.0 mg/kg) MPD responded significantly (P < 0.05) to MPD at ED1 and the majority (61 %; 133/217) of these responding units exhibited significant (P < 0.05) increases in their neuronal firing rates. Ninety-six percent (274/285) of the total units expressed significant changes in their baseline activity at ED10 compared with ED1 with 65 % (178/274) exhibiting a decrease in their baseline activity. At ED10 95 % (272/285) of the VTA units responded significantly (P < 0.05) to MPD rechallenge compared with activity post MPD exposure on ED1 where the majority, 60 % (163/272), exhibited a significant (P < 0.05) decrease in their neuronal activity (Table 3).
Effect of 0.6 mg/kg MPD in all VTA units
The neuronal activity of 132 VTA units were recorded after acute and chronic administration of 0.6 mg/kg MPD; 71 % (93/132) of the VTA units responded significantly (P < 0.05) to the initial exposure of the drug (Table 2A, Acute) by changing their firing rates compared with their baseline firing rate. Most of the units, 94 % (124/132), exhibited significant (P < 0.05) changes in their ED10 baseline activity after six daily MPD injections and three washout days compared with their ED1 baseline (Table 2A, Saline). At ED10, upon MPD rechallenge compared with the initial MPD exposure at ED1, 91 % (120/132) of the VTA units exhibited significant (P < 0.05) changes in their neuronal activity (Table 2A, Rechallenge).
Of the 93 responsive VTA units to the initial (acute) MPD exposure on ED1 (Table 3A), the majority, 57 % (53/93), exhibited a significant (P < 0.05) decrease in firing rate in response to acute MPD exposure (Table 3A, Acute). Of the VTA units that exhibited significant change at ED10 baseline compared with ED1 baseline activity, the majority 60 % (75/124) showed a significant (P < 0.05) decrease in baseline activity (Table 3A, Saline). Following rechallenge of 0.6 mg/kg MPD at ED10 compared with the initial MPD injection at ED1, the majority of the responding units, 52 % (62/120), responded to the drug by attenuating their neuronal activity (Table 3A, Rechallenge). The 27 VTA units that did not respond to MPD exposure on ED1 exhibited a significant (P < 0.05) change in their ED10 baseline as well as in response to MPD rechallenge at ED10 after the six daily injections and three washout days (Table 2A, All).
Effect of 2.5 mg/kg MPD in all VTA units
The neuronal activity of 62 VTA units were recorded after acute and chronic administration of 2.5 mg/kg MPD; 84 % (52/62) of the VTA units responded significantly (P < 0.05) to the initial exposure of the drug (Table 2B, Acute) and of these, the majority 79 % (41/52) exhibited a significant (P < 0.05) increase in firing rate in response to the initial MPD exposure (Table 3B, Acute; Fig. 3b). The baseline activity of ED10 was altered significantly (P < 0.05) in 95 % (59/62) of the VTA units compared with ED1 baseline (Table 2B, Saline) activity as a result of six daily MPD exposures and three washout days (Fig. 4). Of the above VTA units, the majority 53 % (31/59), showed a significant (P < 0.05) decrease in their neuronal activity (Table 3B, Saline). At ED10, upon MPD rechallenge compared with the initial MPD exposure at ED1, 98 % (61/62) of the VTA units exhibited significant (P < 0.05) changes in their neuronal activity (Table 2B, Rechallenge), of which the majority 52 % (32/61) of the VTA units responded to the drug by significantly (P < 0.05) attenuating their neuronal activity (Table 3B, Rechallenge). The nine VTA units that did not respond to MPD exposure on ED1 exhibited a significant (P < 0.05) change in their ED10 baseline as well as in response to MPD rechallenge at ED10 after the six daily MPD injections and three washout days (Table 2B, All).
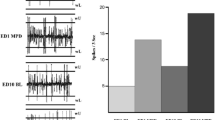
A representation of sequential frequency firing rates of two VTA units following acute 2.5 mg/kg MPD. Both histograms show the neuronal unit activity post saline for 60 min, followed by the neuronal unit activity after MPD on ED1 for the following 60 min. The left histogram (a) shows the VTA unit exhibited a decrease in firing rate activity to acute MPD administration compared with the baseline activity, while the right histogram (b) shows the VTA unit exhibited an increase in firing rate activity to acute MPD administration compared with the baseline activity
A representation of sequential frequency firing rates of the baseline of two VTA units on experimental day 1 (ED1) compared with the baseline at ED10 following six daily 2.5 mg/kg MPD injections and three washout days. The left histogram (a) shows the VTA unit that exhibited a decrease in its baseline firing rate activity at ED10 following six daily 2.5 mg/kg MPD injections and three washout days compared with the baseline activity on ED1, while the right histogram (b) shows the VTA unit exhibited an increase in its baseline firing rate activity at ED10 following six daily 2.5 mg/kg MPD injections and three washout days compared with the baseline activity on ED1
Effect of 10.0 mg/kg MPD in all VTA units
The neuronal activity of 91 VTA units were recorded after acute and chronic administration of 10.0 mg/kg MPD; 79 % (72/91) of the VTA units responded significantly (P < 0.05) to the initial exposure of the drug (Table 2C, Acute) and of these, the majority, 72 % (52/72), exhibited a significant (P < 0.05) increase in firing rate in response to MPD exposure (Table 3C, Acute). All of the units (91/91) exhibited significant (P < 0.05) changes in their ED10 baseline compared with their ED1 baseline (Fig. 2C, Saline) of which, the majority 79 % (72/91) showed a significant (P < 0.05) decrease in their baseline activity (Table 3C, Saline). At ED10, upon MPD rechallenge compared with the initial MPD exposure at ED1, 100 % (91/91) of the VTA units exhibited significant (P < 0.05) changes in their neuronal activity (Table 2C, Rechallenge), of which the majority 76 % (69/91) of the VTA units responded to the drug by attenuating their neuronal activity (Table 3C, Rechallenge). The 19 VTA units that did not respond to MPD exposure on ED1 exhibited a significant (P < 0.05) change in their ED10 baseline as well as in response to MPD rechallenge at ED10 after the six daily MPD injections and three washout days (Table 2C, All).
VTA units recorded from animals that exhibited behavioral sensitization in response to chronic 0.6 mg/kg MPD
The neuronal activity of 22 VTA units was recorded from animals that exhibited behavioral sensitization following acute and chronic 0.6 mg/kg MPD administration. The majority of them, 86 % (19/22), responded significantly (P < 0.05) to acute administration of MPD and all 19 responsive VTA units exhibited a decrease in their neuronal activity following 0.6 mg/kg MPD exposure (Table 2A, Sensitization: Acute). Most of these sensitized VTA neuronal units, 95 % (21/22) exhibited significant (P < 0.05) changes in their ED10 baseline compared with ED1 baseline, and the majority of them, 67 % (14/21) exhibited attenuation in their ED10 baseline compared with ED1 baseline (Table 2A, Sensitization: Saline). At ED10 upon rechallenge with MPD compared with post MPD given at ED1, all of the VTA units (22/22) exhibited significant (P < 0.05) changes in their neuronal activity (Table 2A, Sensitization: Rechallenge), and 64 % (14/22) of these responding VTA units exhibited a significant (P < 0.05) decrease in their activity following 0.6 mg/kg MPD exposure on ED10 compared with 0.6 mg/kg MPD exposure on ED1.
The three VTA units that did not respond to 0.6 mg/kg MPD dose on ED1 (Table 2A, Sensitization: Acute) exhibited a significant (P < 0.05) change in their ED10 baseline activity as well as to MPD rechallenge at ED10 after six daily MPD injections and three washout days.
VTA units recorded from animals that exhibited behavioral sensitization in response to chronic 2.5 mg/kg MPD
The neuronal activity of 24 VTA units was recorded from animals that exhibited behavioral sensitization following acute and chronic 2.5 mg/kg MPD administration. The majority, 75 % (18/24), responded to acute administration of MPD (Table 2B, Sensitization: Acute) and of these 18 responding VTA units, 56 % (10/18) exhibited a significant (P < 0.05) increase in neuronal firing rate in response to MPD (Table 3B, Sensitization: Acute). The ED10 baseline activity compared with ED1 baseline was changed significantly (P < 0.05) by 96 % (23/24) of the VTA units (Table 2B, Sensitization: Saline) of which the majority, 78 % (18/23), showed a significant (P < 0.05) decrease in their neuronal activity (Table 3B, 2.5 mg/kg Sensitization: Saline). At ED10 upon rechallenge with MPD compared with post MPD given at ED1, the majority of the VTA units, 96 % (23/24), exhibited significant (P < 0.05) changes in their neuronal activity (Table 2B, Sensitization: Rechallenge), of which the majority of these responsive units, 78 % (18/23), exhibited a significant (P < 0.05) decrease in their neuronal activity. The five units that did not respond to the 2.5 mg/kg MPD dose on ED1 (Table 3B, Sensitization: Acute) exhibited a significant (P < 0.05) change in their ED10 baseline activity as well as MPD rechallenge at ED10 after six daily MPD injections and three washout days.
VTA units recorded from animals that exhibited behavioral sensitization in response to chronic 10.0 mg/kg MPD
The neuronal activity of 40 VTA units was recorded from animals that exhibited behavioral sensitization following acute and chronic 10.0 mg/kg MPD administration. The majority, 93 % (37/40), responded significantly (P < 0.05) to acute administration of MPD (Table 2C, Sensitization: Acute) and of these, 54 % (20/37) exhibited a significant (P < 0.05) increase in neuronal firing rate in response to MPD (Table 3C, Sensitization: Acute). All of the units (40/40) exhibited significant (P < 0.05) changes in their ED10 baseline firing rate compared with ED1 baseline firing rate (Table 2C, Sensitization: Saline), of which the majority, 67 % (27/40), showed a significant (P < 0.05) decrease in neuronal activity (Table 3C, Sensitization: Saline). At ED10 upon rechallenge with MPD compared with post MPD given at ED1, all of the VTA units (40/40) exhibited significant (P < 0.05) changes in their neuronal activity (Table 2C, Sensitization: Rechallenge) of which 65 % (26/40) exhibited a significant (P < 0.05) decrease in their neuronal activity (Table 3C, Sensitization: Rechallenge). The 3 units that did not respond to the 10.0 mg/kg MPD dose on ED1 (Table 2C, Sensitization: Acute), exhibited a significant (P < 0.05) change in their ED10 baseline activity as well as following MPD rechallenge at ED10 after six daily MPD injections and three washout days.
VTA units recorded from animals that exhibited behavioral tolerance in response to chronic 0.6 mg/kg MPD
The neuronal activity of 110 VTA units was recorded from animals that exhibited behavioral tolerance following acute and chronic 0.6 mg/kg MPD administration. The majority, 67 % (74/110), of them responded to acute administration of MPD (Table 2A, Tolerance: Acute), while 94 % (103/110) exhibited significant (P < 0.05) changes in their ED10 baseline compared with their ED1 baseline (Table 2A, Tolerance: Saline). At ED10 upon rechallenge with 0.6 mg/kg MPD compared with post MPD given at ED1, the majority 89 % (98/110) of these VTA units exhibited significant (P < 0.05) changes in their neuronal activity (Table 2A, Tolerance: Rechallenge).
Of the responding 74 VTA units to acute MPD on ED1, the majority, 54 % (40/74), exhibited a significant (P < 0.05) increase in neuronal firing rate in response to MPD (Table 3A, Tolerance: Acute). Of the 103 VTA units that exhibited significant change at ED10 baseline compared with ED1 baseline activity, the majority, 59 % (61/103), showed a significant (P < 0.05) decrease in their neuronal activity (Table 3A, Tolerance: Saline). Upon rechallenge of 0.6 mg/kg MPD on ED10 compared with the initial effect of MPD at ED1, 51 % (50/98) of the responsive VTA units exhibited a significant (P < 0.05) increase in their neuronal activity (Table 3A, Rechallenge). The 24 units that did not respond to 0.6 mg/kg MPD on ED1 (Table 2A, Tolerance: Acute) exhibited a significant (P < 0.05) change in their ED10 baseline activity compared with ED1 baseline activity as well as to MPD rechallenge at ED10 after six daily MPD injections and three washout days compared with ED1 post MPD administration.
VTA units recorded from animals that exhibited behavioral tolerance in response to chronic 2.5 mg/kg MPD
The neuronal activity of 38 VTA units was recorded from animals that exhibited behavioral tolerance following acute and chronic 2.5 mg/kg MPD administration. The majority, 90 % (34/38), responded to acute administration of MPD (Table 2B, Tolerance: Acute), of which 91 % (31/34) exhibited a significant (P < 0.05) increase in neuronal firing rate in response to MPD exposure (Table 3B, Tolerance: Acute). The ED10 baseline activity compared with ED1 baseline activity was significantly (P < 0.05) changed for 95 % (36/38) of the VTA units (Table 2B, Tolerance: Saline), and of these units, 64 % (23/36) showed a significant (P < 0.05) increase in their neuronal baseline activity (Table 3B, Tolerance: Saline). At ED10 upon rechallenge with MPD compared with post MPD given at ED1, all of the VTA units (38/38) exhibited significant (P < 0.05) changes in their neuronal activity (Table 2B, Tolerance: Rechallenge) of which 63 % (24/38) exhibited a significant (P < 0.05) increase in their neuronal activity. The 4 units that did not respond to the 2.5 mg/kg MPD dose on ED1 (Table 2B, Tolerance: Acute), exhibited a significant (P < 0.05) change in their ED10 baseline activity as well as MPD rechallenge at ED10 after six daily MPD injections and three washout days.
VTA units recorded from animals that exhibited behavioral tolerance to chronic 10.0 mg/kg MPD
The neuronal activity of 51 VTA units was recorded from animals that exhibited behavioral tolerance following acute and chronic 10.0 mg/kg MPD administration. The majority, 69 % (35/51), of them responded to acute administration of MPD (Table 2C, Tolerance: Acute), of which 91 % (32/35) exhibited a significant (P < 0.05) increase in their neuronal firing rate in response to MPD exposure (Table 3C, Tolerance: Acute). All of the units (51/51) exhibited significant (P < 0.05) changes in their ED10 baseline activity compared with ED1 baseline (Table 2C, Tolerance: Saline) of which the majority, 88 % (45/51), showed a significant (P < 0.05) decrease in their neuronal activity (Table 3C, Tolerance: Saline). At ED10 upon rechallenge with MPD compared with post MPD given at ED1, all of the VTA units (51/51) exhibited significant (P < 0.05) changes in their neuronal activity (Table 2C, Tolerance: Rechallenge) and 84 % (43/51) of these exhibited a significant (P < 0.05) decrease in their neuronal activity. The 16 units that did not respond to the 10.0 mg/kg MPD dose on ED1 (Table 2C, Tolerance: Acute), exhibited a significant (P < 0.05) change in their ED10 baseline activity compared with their ED1 baseline neuronal firing rate as well as following MPD rechallenge at ED10 after six daily MPD injections and three washout days, compared with the initial MPD exposure.
Statistical comparison between the VTA unit population recorded from animals that exhibited behavioral tolerance to VTA unit population recorded from animals exhibiting behavioral sensitization following 0.6 mg/kg administration
The natural log odds ratio statistical test shows that for 0.6 mg/kg the neuronal recordings obtained from animals expressing behavioral tolerance were more likely (3.82) to show an increase in neuronal activity during initial (acute) MPD exposure than those animals expressing behavioral sensitization. In addition, for both the baseline neuronal activity of ED10 compared with baseline neuronal activity recorded at ED1 and the neuronal activity recorded on ED10 following rechallenge with MPD administration compared with the neuronal activity on ED1 following MPD exposure, the odds ratio revealed that both the behaviorally sensitized and behaviorally tolerant animals were about as likely to show increased neuronal activity.
Statistical comparison between the VTA unit population recorded from animals that exhibited behavioral tolerance to VTA units population recorded from animals exhibiting behavioral sensitization following 2.5 mg/kg administration
The natural log odds ratio statistical test shows that for 2.5 mg/kg the neuronal recordings obtained from animals expressing behavioral tolerance were more likely (1.99, 1.77 and 1.74) to show an increase in neuronal activity for all three comparisons, i.e. the initial (acute) MPD exposure, the baseline neuronal activity at ED10 compared with baseline activity at ED1 and the neuronal activity recorded at ED10 following MPD rechallenge compared with the neuronal activity on ED1 in response to the initial MPD exposure.
Statistical comparison between the VTA units population recorded from animals that exhibited behavioral tolerance to VTA unit population recorded from animals exhibiting behavioral sensitization following 10.0 mg/kg administration
The natural log odds ratio statistical test shows that for 10.0 mg/kg the neuronal recording obtained from animals expressing behavioral tolerance were more likely (2.07) to show an increase in neuronal activity during the initial (acute) MPD exposure phase compared with the VTA units recorded from animals expressing behavioral sensitization to chronic MPD exposure. In addition, the odds ratio statistical test of the VTA units recorded from animals exhibiting behavioral tolerance showed that they are more likely (1.23, 1.03) than the behaviorally sensitized animals to exhibit a decrease in their baseline neuronal activity at ED10 compared with their ED1 baseline activity as well as for ED10 neuronal activity after MPD rechallenge compared with the neuronal activity on ED1 following MPD administration, respectively.
Statistical comparison between the groups comparing for dose
The statistical log linear model test with significance set at (P < 0.05) summarizes the statistical comparison of the relationship between dose, behavior, and firing patterns for each MPD group (0.6, 2.5 and 10.0 mg/kg). The results show that the response of VTA units to MPD recorded from animals that exhibited behavioral sensitization following 0.6 mg/kg MPD exhibited a significant (df 2; χ 2 6.02; P < 0.05) difference from the VTA units which were recorded from animals exhibiting behavioral tolerance, while the animals exhibiting behavioral sensitization following 2.5 mg/kg MPD were not significantly (df 2; χ 2 0.08; P = 0.9614) different from animals exhibiting behavioral tolerance. The response pattern of VTA units recorded from animals that exhibited behavioral sensitization to 10.0 mg/kg MPD dose are significantly (df 2; χ 2 18.47; P < 0.001) different from the VTA units firing patterns recorded from animals that exhibited behavioral tolerance, respectively.
The above statistical comparisons (odds ratio and log linear statistical test) show the importance of evaluating the effect of chronic drug exposure on neurophysiological events based on animal behavioral response to repetitive drug exposure.
Discussion
Methylphenidate (MPD) is one of the most prescribed medications for ADHD, yet its mechanism of action is still under investigation (Seeman and Madras 1998; Solanto 1998; Vanderschuren et al. 2008). MPD has pharmacological similarity to several drugs of abuse such as cocaine and amphetamine, and a chemical structure similar to amphetamine and methamphetamine (Kallman and Isaac 1975; Patrick and Markowitz 1997; Teo et al. 2003). Dopamine imbalance is thought to be one of the main causes underlying ADHD and explains why MPD is an effective therapeutic remedy (Izenwasser et al. 1999; Massello and Carpenter 1999; Patrick and Markowitz 1997; Volkow and Swanson 2008). MPD binds to the dopamine transporter (DAT), thus preventing the reuptake of DA from the synaptic cleft to the presynaptic terminals (Volkow et al. 2002). The consequence of this binding is that DA stays longer in the synaptic cleft. Most addictive drugs elicit tolerance, sensitization, withdrawal and dependence, by action on the CNS neuronal circuit which includes the VTA (Pierce and Kalivas 1995; Kalivas et al. 1993; Nestler 2008).
In the present study, the VTA neuronal unit activity was simultaneously recorded with the animal’s behavioral locomotion before and after the administration of different acute and chronic MPD doses. To eliminate the contribution of the environment to the drug effect such as novel condition, all the recordings and MPD exposures were carried out in the animal’s home cages. This arrangement warrants that any change from baseline activity is due to the drug’s (MPD) effect. The main findings of the study are that all of the repetitive MPD exposure doses (0.6, 2.5, and 10.0 mg/kg) caused some animals to express behavioral sensitization while causing other animals to express behavioral tolerance which confirm our hypothesis that the same repetitive MPD dose can elicit in some animals behavioral sensitization and in other animals behavioral tolerance. Due to this dual effect of each MPD dose, we evaluated the neuronal unit activity for all the animals with no correlation to their behavioral responses to chronic MPD exposure, as well as based on their behavioral response to chronic MPD exposure to determine whether the VTA neurons recorded from animals expressing behavioral sensitization will respond to acute or chronic MPD differently from those recorded from animals expressing behavioral tolerance. Indeed, this evaluation showed that in general, the VTA neuronal population recorded from animals expressing behavioral sensitization responded differently to MPD compared with those VTA neuronal populations recorded from animals expressing behavioral tolerance, thus confirming our hypothesis.
Is it possible that the same dose elicits two opposing phenomena? Castellanos et al. (1996) and Arnsten and Dudley (2005) reported individual differences due to differences in phenotype and drug metabolism rate between the animals in response to MPD exposure. Volkow and Swanson (2003) using MRI scans in humans reported different responses to MPD in normal and ADHD subjects due to differences in the basal activity of DA in each individual. In the current study, about four animals came from one shipment, which makes it possible that each shipment had animals with a different phenotype and metabolism rate of MPD, which may explain the different responses observed. This observation stresses the importance to evaluate each animal individually.
Overall, the three MPD doses (0.6, 2.5 and 10.0 mg/kg) activated most of the VTA neuronal activities. The lowest number of VTA units responded to acute MPD was seen following 0.6 mg/kg, while the dose of 2.5 mg/kg had the most responsiveness, followed by 10.0 mg/kg. Following 0.6 mg/kg MPD exposure, the majority of the VTA units responded to acute MPD by attenuating their firing rate, while following 2.5 and 10.0 mg/kg MPD, the majority of the VTA units responded by increasing their firing rate. The overall baseline activity at ED10 compared with the baseline at ED1 showed dose–response increases in the total number of units whose baseline was changed, i.e. increasing MPD dose from 0.6 to 10.0 mg/kg MPD, more units expressed changes in their ED10 baseline after six daily MPD exposures and three washout days compared with ED1 baseline. This change in baseline activity may express withdrawal or expectation (Algahim et al. 2009; Bergheim et al. 2012; Lee et al. 2011). Upon rechallenge with MPD on ED10 compared with the effects of MPD at ED1, the majority of the VTA units responded to MPD exposure by a decrease in their neuronal activity. The acute and chronic MPD exposure to VTA units recorded from animals that expressed behavioral sensitization exhibits a “U shape” in the number of units that responded to the MPD dose response experiment and reverse “U shape” from the units recorded from animals expressing behavioral tolerance. Moreover, the responses to MPD exposure recorded from animals expressing behavioral sensitization compared with those VTA units recorded from animals expressing behavioral tolerance were significantly different.
Molecular studies (Chao and Nestler 2004; Kim et al. 2009; Nestler 2004) have in essence shown similar observations to this study using dose–response protocol of cocaine, morphine, and amphetamine, i.e. showing dual observations, and this may provide possible explanations as to why the same dose of MPD elicited in some VTA units excitation and in others attenuation as well as behavioral sensitization in some animals while causing behavioral tolerance in others. In their study with cocaine and methamphetamine, it was noted that some animals express upregulation of either CREB or the transcription factor ΔFosB levels during behavioral tolerance and/or behavioral sensitization, respectively. Upregulation of CREB in the NAc as a result of chronic drug administration decreases the rewarding effects of cocaine and morphine (Chao and Nestler 2004). A target of CREB is dynorphin, an opioid peptide expressed in NAc medium spiny neurons (MSN). Dynorphin release is known to contribute to dysphoria through what is thought to be a negative-feedback loop to VTA DA neurons and thus inhibits their activity (Hyman and Malenka 2001; Spanagel et al. 1992). Thus, for the animals exhibiting behavioral tolerance to repetitive MPD exposures, it can be surmised that upregulation of CREB is partly responsible for attenuating the effect of the drug, i.e. upregulation of CREB induces behavioral tolerance as well as a decrease in neuropil density (Chao and Nestler 2004; Dietz et al. 2009; Kim et al. 2009; Nestler 2004, 2008). This in turn causes a decrease in the rewarding aspects of MPD and other psychostimulants (Chao and Nestler 2004).
It was reported also that acute exposure to psychostimulants induces upregulation of ΔFosB, and with repeated drug exposure, ΔFosB is accumulated (Moratalla et al. 1996; Chao and Nestler 2004). Upregulation of ΔFosB following cocaine exposure result in increased locomotion and may be involved in eliciting behavioral sensitization in mice and responsible for the motivational effects of cocaine (Kelz et al. 1999; Chao and Nestler 2004). Blocking ΔFosB is shown to have opposite effects (Peakman et al. 2003). Such dual changes in activity could possibly be attributed to molecular and morphological plasticity in the MSN density and an upregulation of ΔFosB. Thus, animals that exhibit higher MSN density or express an upregulation of ΔFosB as a result of MPD exposure would show a further increase in activity to rechallenge MPD. Therefore, it is possible that the animals expressing behavioral sensitization or tolerance could be experiencing these dual molecular transcription factor plasticity in the mesolimbic circuitry and provide further explanation to the present observation as to why the same dose of MPD can cause both behavioral sensitization and tolerance or increase activity in some VTA units and decreases in their activity in response to MPD exposure in other VTA units.
The VTA neurons contain both D1 and D2 DA receptors, both of which are affected by MPD and glutamatergic afferent inputs from the PFC as well as fellow neurons in the VTA. DA D1 receptors when activated have an excitatory effect, while the DA D2 receptors have an inhibitory effect on other cells. Therefore, it is possible that the VTA neurons that exhibit mainly a decrease in activity are being recorded from an area that contains mostly DA D2 receptors whose activation give a decrease in activity. The cells that exhibited mainly an increase in activity are most likely to have been recorded from an area of the VTA containing mostly DA D1 or glutamatergic receptors. In addition to these units, the afferent input of glutamatergic neurons from the PFC to DA neurons in the VTA can be stimulated by MPD and could also be partially responsible to the different responses to MPD and affect how a single dose could cause both behavioral sensitization and tolerance.
It was reported that low doses of MPD activate mainly D2-like DA autoreceptors which leads to the attenuation of DA release in response to a stimulus (Seeman and Madras, 1998, 2002). These autoreceptors counter regulate extracellular DA levels and are likely the reason that 0.6 mg/kg MPD elicits a decrease in their locomotor activity in some animals (Fig. 2b). Exposure to higher doses of MPD (2.5 and 10.0 mg/kg) modulates the inhibitory effects of the presynaptic autoreceptors with post synaptic activation that causes subsequent amplification of DA signals (Volkow et al. 2005) as observed in this study. The sentence should be: In those animals that exhibited behavioral sensitization, the majority of their neuronal responses to MPD exhibited a decrease in activity on ED10 regardless of whether there was an increase or decrease in activity on ED1 to MPD. Regarding those animals that expressed behavioral tolerance, different patterns were observed.
MPD is thought to work in a biphasic action, including “spontaneous” phasic and tonic release of DA. Phasic releases of DA from terminals are large, but brief and activate postsynaptic DA receptors and evoke DA-dependent behavioral responses (Seeman and Madras 1998, 2002). On the other hand, tonic DA release from the VTA is regulated by presynaptic NMDA receptors by glutamatergic afferents from the PFC (Grace 1991). Basal levels of DA rise about 60-fold during normal nerve impulses and quickly diminish back to normal. With low-dose MPD (0.6 mg/kg), the resting level of DA rises about sixfold and is thought to act mainly on the DA D2 autoreceptors which reduces the impulse that triggered DA release. At higher MPD dose exposure (2.5 and 10.0 mg/kg), both the resting DA levels and the triggered DA output are markedly increased causing wide-spread stimulation of postsynaptic DA receptors (Seeman and Madras 1998, 2002) and can explain why a low dose of MPD could cause a lower level of neuronal activation and the 2.5 and 10.0 mg/kg MPD doses provide increased levels of activity upon acute administration.
Several studies reported that psychostimulants in some animals elicit increases in their neuropil (the density and the dendritic branching of the MSN) in the NAc and PFC, while in other animals, the same dose exposure elicits a decrease in the neuropil (Kim et al. 2009; Nestler 2008; Robinson and Kolb 1997, 1999). We posit that those animals that express increases in their neuropil as a result of chronic psychostimulant exposure will respond to rechallenge drug administration by increasing their firing rate and those animals that elicit decreases in their neuropil to chronic psychostimulant exposure will respond to the drug by decreasing their firing rate.
Based on the responses to acute and chronic MPD, it is possible to classify that there are two types of neuronal activity: one type expresses neurophysiological sensitization (Fig. 5b) and the other type expresses neurophysiological tolerance (Fig. 5a). Those units that express both an increase in neuronal activity on ED1 following MPD exposure and a further increase in their neuronal activity on ED10 to MPD rechallenge and those VTA units that exhibit a decrease in neuronal activity to MPD on ED1 and a further decrease in activity on ED10 to MPD rechallenge are said to show neurophysiological sensitization. Some units that did not respond significantly to MPD on ED1, but did respond significantly on ED10, can also be said to exhibit neurophysiological sensitization. Those VTA units that at ED1 responded to the drug by either increase or attenuation in their neuronal activity and at ED10 failed to respond to MPD rechallenge, or those VTA units that show the opposite effects at ED1 compared with ED10 are said to show neurophysiological tolerance.
A representation of sequential frequency firing rates of two VTA units following acute and chronic MPD administration. Both histograms show the neuronal unit activity after 60 min of saline, i.e. baseline activity, followed by an additional 60 min post 2.5 mg/kg MPD on ED1, and finally by another 60 min post 2.5 mg/kg MPD administration on ED10. In a the acute effect of MPD on ED1 elicits an increase in VTA neuronal unit firing rate compared with the baseline activity. The effect of MPD rechallenge on ED10, after six daily MPD injections and three washout day of no drug, results in a lower response to the drug when compared with the effect of the drug on ED1. This neuronal unit activity was classified as neurophysiological tolerance. In b, the acute effect of 2.5 mg/kg MPD on ED1 results in an increase in neuronal unit activity compared with the ED1 baseline activity. The effect of MPD administration on ED10 after six daily MPD injections and three washout days results in a further increase in neuronal unit activity compared with the initial MPD effects on ED1. This type of response was classified as neurophysiological sensitization
The log linear statistical calculation and the odds ratio test were used to compare whether the MPD effect on VTA neuronal units recorded from animals expressing behavioral sensitization were responding differently to MPD exposure than those units recorded from animals expressing behavioral tolerance for each dose. It was found that the neuronal populations recorded in the VTA from animals that express behavioral sensitization to repetitive MPD exposure do in fact respond differently to MPD compared to those VTA units recorded in animals expressing behavioral tolerance. These two tests show the importance of evaluating the neuronal firing rates following psychostimulant exposure based on the animal’s behavior to find any correlations between drug dose and firing rates with behavioral activity.
In conclusion, the current study showed that the VTA neuronal population activity recorded from animals expressing behavioral sensitization to chronic MPD, respond differently to MPD exposure than those VTA units recorded from animals expressing behavioral tolerance. Thus, the effect of MPD on VTA neurons is different from other psychostimulants, i.e. MPD has its own unique effect that needs to be elucidated. Moreover, the observation that the same repetitive MPD dose elicited behavioral sensitization in some animals while causing behavioral tolerance in others is essential information that the professional practitioners and the MPD users need to consider.
References
Algahim MF, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N (2009) Prolonged methylphenidate treatment alters the behavioral diurnal activity pattern of adult male Sprague–Dawley rats. Pharmacol Biochem Behav 92:93–99
Arnsten AF, Dudley AG (2005) Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav Brain Funct 1:2
Askenasy EP, Taber KH, Yang PB, Dafny N (2007) Methylphenidate (Ritalin): behavioral studies in the rat. Int J Neurosci 117:757–794
Barron E, Yang PB, Swann AC, Dafny N (2009) Adolescent and adult male spontaneous hyperactive rats (SHR) respond differently to acute and chronic methylphenidate (Ritalin). Int J Neurosci 119:40–58
Beckstead RM, Domesick VB, Nauta WJ (1979) Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res 175:191–217
Bergheim M, Yang PB, Burau KD, Dafny N (2012) Adolescent rat circadian activity is modulated by psychostimulants. Brain Res 1431:35–45
Bonci A, Williams JT (1996) A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron 16:631–639
Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL (1996) Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry 53:607–616
Challman TD, Lipsky JJ (2000) Methylphenidate: its pharmacology and uses. Mayo Clin Proc 75:711–721
Chao J, Nestler EJ (2004) Molecular neurobiology of drug addiction. Annu Rev Med 55:113–132
Chong SL, Claussen CM, Dafny N (2012) Nucleus accumbens neuronal activity in freely behaving rats is modulated following acute and chronic methylphenidate administration. Brain Res Bull 87:445–456
Claussen CM, Dafny N (2012) Acute and chronic methylphenidate modulates the neuronal activity of the caudate nucleus recorded from freely behaving rats. Brain Res Bull 87:387–396
Dafny N (1975) Electrophysiological properties of caudate neurons following substantia nigra, motor cortex, and amygdaloid nuclear complex stimulation of the rat. Appl Neurophysiol 38:259–272
Dafny N (1980) Multiunit recording from medial basal hypothalamus following acute and chronic morphine treatment. Brain Res 190:584–592
Dafny N (1982) The hypothalamus exhibits electrophysiologic evidence for morphine tolerance and dependence. Exp Neurol 77:66–77
Dafny N, Terkel J (1990) Hypothalamic neuronal activity associated with onset of pseudopregnancy in the rat. Neuroendocrinology 51:459–467
Dafny N, Yang PB (2006) The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull 68:393–405
Dietz DM, Dietz KC, Nestler EJ, Russo SJ (2009) Molecular mechanisms of psychostimulant-induced structural plasticity. Pharmacopsychiatry 42(Suppl 1):S69–S78
Fan D, Rich D, Holtzman T, Ruther P, Dalley JW, Lopez A, Rossi MA, Barter JW, Salas-Meza D, Herwik S, Holzhammer T, Morizio J, Yin HH (2011) A wireless multi-channel recording system for freely behaving mice and rats. PLoS ONE 6:e22033
Garland EJ (1998) Intranasal abuse of prescribed methylphenidate. J Am Acad Child Adolesc Psychiatry 37:1242–1243
Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, Logan J (1999) Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology 146:93–100
Gaytan O, Ghelani D, Martin S, Swann A, Dafny N (1996) Dose response characteristics of methylphenidate on different indices of rats’ locomotor activity at the beginning of the dark cycle. Brain Res 727:13–21
Gaytan O, Ghelani D, Martin S, Swann A, Dafny N (1997a) Methylphenidate: diurnal effects on locomotor and stereotypic behavior in the rat. Brain Res 777:1–12
Gaytan O, Al-rahim S, Swann A, Dafny N (1997b) Sensitization to locomotor effects of methylphenidate in the rat. Life Sci 61:L101–L107
Gaytan O, Lewis C, Swann A, Dafny N (1999) Diurnal differences in amphetamine sensitization. Eur J Pharmacol 374:1–9
Gaytan O, Nason R, Alagugurusamy R, Swann A, Dafny N (2000) MK-801 blocks the development of sensitization to the locomotor effects of methylphenidate. Brain Res Bull 51:485–492
Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL (2000) Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J Pharmacol Exp Ther 295:51–57
Grace AA (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41:1–24
Grace AA, Onn SP (1989) Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci 9:3463–3481
Gronier B (2011) In vivo electrophysiological effects of methylphenidate in the prefrontal cortex: involvement of dopamine D1 and alpha 2 adrenergic receptors. Eur Neuropsychopharmacol 21:192–204
Hyman SE, Malenka RC (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2:695–703
Izenwasser S, Coy AE, Ladenheim B, Loeloff RJ, Cadet JL, French D (1999) Chronic methylphenidate alters locomotor activity and dopamine transporters differently from cocaine. Eur J Pharmacol 373:187–193
Johnson SW, North RA (1992) Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol 450:455–468
Joyce MP, Rayport S (2000) Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience 99:445–456
Kalivas PW, Duffy P (1993) Time course of extracellular dopamine and behavioral sensitization to cocaine, II. Dopamine perikarya. J Neurosci 13:276–284
Kalivas PW, Duffy P (1995) D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci 15:5379–5388
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244
Kalivas PW, Weber B (1988) Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther 245:1095–1102
Kalivas PW, Duffy P, DuMars LA, Skinner C (1988) Behavioral and neurochemical effects of acute and daily cocaine administration in rats. J Pharmacol Exp Ther 245:485–492
Kalivas PW, Sorg BA, Hooks MS (1993) The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmacol 4:315–334
Kalivas PW, Pierce RC, Cornish J, Sorg BA (1998) A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol 12:49–53
Kallman WM, Isaac W (1975) The effects of age and illumination on the dose-response curves for three stimulants. Psychopharmacologia 40:313–318
Kauer JA (2004) Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol 66:447–475
Kelz MB, Chen J, Carlezon WA Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ (1999) Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature 401:272–276
Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P (2009) Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc Natl Acad Sci USA 106:2915–2920
Lacroix D, Ferron A (1988) Electrophysiological effects of methylphenidate on the coeruleo-cortical noradrenergic system in the rat. Eur J Pharmacol 149:277–285
Lee MJ, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N (2009) Does repetitive Ritalin injection produce long-term effects on SD female adolescent rats? Neuropharmacology 57:201–207
Lee SS, Humphreys KL, Flory K, Liu R, Glass K (2011) Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev 31:328–341
Lee SH, Seo WS, Sung HM, Choi TY, Kim SY, Choi SJ, Koo BH, Lee JH (2012) Effect of methylphenidate on sleep parameters in children with ADHD. Psychiatry Investig 9:384–390
Massello W III, Carpenter DA (1999) A fatality due to the intranasal abuse of methylphenidate (Ritalin). J Forensic Sci 44:220–221
Moratalla R, Vallejo M, Elibol B, Graybiel AM (1996) D1-class dopamine receptors influence cocaine-induced persistent expression of Fos-related proteins in striatum. NeuroReport 8:1–5
Morris JA, Gardner MJ (1988) Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J (Clin Res Ed) 296:1313–1316
Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA (2008) Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152:1024–1031
Nestler EJ (2004) Molecular mechanisms of drug addiction. Neuropharmacology 47(Suppl 1):24–32
Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8:1445–1449
Nestler EJ (2008) Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos. Trans R Soc Lond B Biol Sci 363:3245–3255
Papla I, Filip M, Przegalinski E (2002) Effect of intra-tegmental microinjections of 5-HT1B receptor ligands on the amphetamine-induced locomotor hyperactivity in rats. Pol J Pharmacol 54:351–357
Patrick KS, Markowitz JS (1997) Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder. Hum Psychopharmacol Clin Exp 12:527–546
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Peakman MC, Colby C, Perrotti LI, Tekumalla P, Carle T, Ulery P, Chao J, Duman C, Steffen C, Monteggia L, Allen MR, Stock JL, Duman RS, McNeish JD, Barrot M, Self DW, Nestler EJ, Schaeffer E (2003) Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res 970:73–86
Pert A (1998) Neurobiological substrates underlying conditioned effects of cocaine. Adv Pharmacol 42:991–995
Perugini M, Vezina P (1994) Amphetamine administered to the ventral tegmental area sensitizes rats to the locomotor effects of nucleus accumbens amphetamine. J Pharmacol Exp Ther 270:690–696
Pierce RC, Kalivas PW (1995) Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther 275:1019–1029
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216
Podet A, Lee MJ, Swann AC, Dafny N (2010) Nucleus accumbens lesions modulate the effects of methylphenidate. Brain Res Bull 82:293–301
Prieto-Gomez B, Benitez MT, Vazquez-Alvarez AM, Yang PB, Reyes VC, Dafny N (2004) Dopaminergic ventral tegmental neurons modulated by methylphenidate. Life Sci 74:1581–1592
Prieto-Gomez B, Vazquez-Alvarez AM, Martinez-Pena JL, Reyes-Vazquez C, Yang PB, Dafny N (2005) Methylphenidate and amphetamine modulate differently the NMDA and AMPA glutamatergic transmission of dopaminergic neurons in the ventral tegmental area. Life Sci 77:635–649
Robinson TE (1984) Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology 84:466–475
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Robinson TE, Kolb B (1997) Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 17:8491–8497
Robinson TE, Kolb B (1999) Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11:1598–1604
Robison AJ, Nestler EJ (2011) Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12:623–637
Salek RL, Claussen CM, Perez A, Dafny N (2012) Acute and chronic methylphenidate alters prefrontal cortex neuronal activity recorded from freely behaving rats. Eur J Pharmacol 679:60–67
Scheggi S, Leggio B, Masi F, Grappi S, Gambarana C, Nanni G, Rauggi R, De Montis MG (2002) Selective modifications in the nucleus accumbens of dopamine synaptic transmission in rats exposed to chronic stress. J Neurochem 83:895–903
Seeman P, Madras BK (1998) Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry 3:386–396
Seeman P, Madras B (2002) Methylphenidate elevates resting dopamine which lowers the impulse-triggered release of dopamine: a hypothesis. Behav Brain Res 130:79–83
Solanto MV (1998) Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res 94:127–152
Spanagel R, Herz A, Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA 89:2046–2050
Tang A, Wanchoo SJ, Swann AC, Dafny N (2009) Psychostimulant treatment for ADHD is modulated by prefrontal cortex manipulation. Brain Res Bull 80:353–358
Teo SK, Stirling DI, Thomas SD, Khetani VD (2003) Neurobehavioral effects of racemic threo-methylphenidate and its d and l enantiomers in rats. Pharmacol Biochem Behav 74:747–754
Vanderschuren LJ, Trezza V, Griffioen-Roose S, Schiepers OJ, Van LN, De Vries TJ, Schoffelmeer AN (2008) Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology 33:2946–2956
Vezina P (1993) Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: an in vivo microdialysis study in the rat. Brain Res 605:332–337
Volkow ND, Swanson JM (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160:1909–1918
Volkow ND, Swanson JM (2008) Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry 165:553–555
Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ (2002) Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord 6(Suppl 1):S31–S43
Volkow ND, Wang GJ, Fowler JS, Ding YS (2005) Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1410–1415
Volz TJ, Farnsworth SJ, Hanson GR, Fleckenstein AE (2009) Method development and validation of an in vitro model of the effects of methylphenidate on membrane-associated synaptic vesicles. J Neurosci Methods 177:177–182
Wanchoo SJ, Lee MJ, Swann AC, Dafny N (2010) Bilateral six-hydroxydopamine administration to PFC prevents the expression of behavioral sensitization to methylphenidate. Brain Res 1312:89–100
Waterson P, Eason K, Tutt D, Dent M (2012) Using HIT to deliver integrated care for the frail elderly in the UK: current barriers and future challenges. Work 41(Suppl 1):4490–4493
White FJ, Kalivas PW (1998) Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend 51:141–153
White SR, Yadao CM (2000) Characterization of methylphenidate exposures reported to a regional poison control center. Arch Pediatr Adolesc Med 154:1199–1203
Wolf ME (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720
Yang PB, Amini B, Swann AC, Dafny N (2003) Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res 971:139–152
Yang PB, Swann AC, Dafny N (2006a) Sensory-evoked potentials recordings from the ventral tegmental area, nucleus accumbens, prefrontal cortex, and caudate nucleus and locomotor activity are modulated in dose-response characteristics by methylphenidate. Brain Res 1073–1074:164–174
Yang PB, Swann AC, Dafny N (2006b) Chronic methylphenidate modulates locomotor activity and sensory evoked responses in the VTA and NAc of freely behaving rats. Neuropharmacology 51:546–556
Yang PB, Swann AC, Dafny N (2006c) Dose-response characteristics of methylphenidate on locomotor behavior and on sensory evoked potentials recorded from the VTA, NAc, and PFC in freely behaving rats. Behav Brain Funct 2:3
Yang PB, Swann AC, Dafny N (2006d) Acute and chronic methylphenidate dose-response assessment on three adolescent male rat strains. Brain Res Bull 71:301–310
Yang PB, Swann AC, Dafny N (2007) Chronic administration of methylphenidate produces neurophysiological and behavioral sensitization. Brain Res 1145:66–80
Yang PB, Atkins KD, Dafny N (2011) Behavioral sensitization and cross-sensitization between methylphenidate amphetamine, and 3,4-methylenedioxymethamphetamine (MDMA) in female SD rats. Eur J Pharmacol 661:72–85
Acknowledgments
Supported by NIH DA RO1 027222 grant. We would also like to thank Catherine Claussen and Dr. Bin Tang for their help in carrying out this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, Z., Dafny, N. Acute and chronic dose–response effect of methylphenidate on ventral tegmental area neurons correlated with animal behavior. J Neural Transm 121, 327–345 (2014). https://doi.org/10.1007/s00702-013-1101-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-1101-2