Abstract
Since Toxoplasma gondii can establish a persistent infection in the central nervous system in humans, we studied its effects on a host’s neurotransmitter and neuropeptide systems (NNS). Using microarray technology, we have screened the expression of genes coding for NNS in human neuroepithelioma cells in response to representative strains of Toxoplasma to identify potential target genes. Transcripts that displayed expression levels distinct from uninfected controls were examined by RT-PCR and Western blot. Our results indicate the presence of disturbed NNS upon Toxoplasma infection and the extent of this disturbance varies considerably among the three strains. In cells infected by type I strain, three neurotransmitter systems (dopamine, glutamate and serotonin) and two neuropeptides (PROK2 and TAC1) displayed abnormalities relative to controls. Type III infection led to the change of a critical enzyme, TDO2, in the kynurenine pathway. No significant effects of type II infection were found in the NNS. These data may have implications for understanding the pathogenesis and heterogeneity of neurologic disturbances in toxoplasmosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii is a ubiquitous protozoan that can infect humans and other animals. Most Toxoplasma isolates that have been identified in Europe and North America belong to three distinct clonal lineages (Howe and Sibley 1995), referred to as types I, II and III. This parasite preferentially encysts within the tissues of the brain after a short phase of systemic replication thereby establishing a lifelong infection. Notably, Toxoplasma is one of the few pathogens that regularly cross the placenta. Congenital toxoplasmosis is linked to severe neuropsychiatric and ophthalmological symptoms (Carruthers and Suzuki 2007). In immunocompromised patient, severe neurological disease such as toxoplasmic encephalitis can occur due to either acute infection or reactivation of chronic infection. While infection of healthy adults is usually relatively mild, the tropism of Toxoplasma for brain tissue has been linked with specific behavioral changes in humans and in animals (Havlícek et al. 2001; Flegr et al. 2002; Vyas et al. 2007; Kannan et al. 2010). Taken together, these lines of evidence document the specific effects that Toxoplasma has on the brain.
Several studies reported that changes in neurotransmitter levels result from Toxoplasma infection. In a post-natal infection model employing the intraperitoneal infection of Swiss-Webster mice, Stibbs (1985) found changes in several neurochemicals using a type III strain (C56) during early acute and chronic phases. Gatkowska et al. (2013) reported changes in monoamine systems with regard to mouse gender and time after a Toxoplasma type II (ME49) infection. However, Goodwin et al. (2012) recently found no change in monoamine systems in mice congenitally infected with a type III strain (VEG). These discrepancies might be related to differences in infection model, route of infection, challenge dose, and mouse or parasite genotypes. Since studies have documented distinct differences in cellular tropism and virulence among the three Toxoplasma types (Saeij et al. 2005), it becomes important to compare changes in neurotransmitter levels in concurrent experiments using the three main Toxoplasma strains.
We have recently shown that infection of a human neural cell line with Toxoplasma results in transcriptional alteration of a number of genes associated with different neurological functions, with the extent of the changes varying considerably among the three strains (Xiao et al. 2011). These findings prompted us to look for abnormal expression of genes coding for neurotransmitter and neuropeptide systems (NNS) and furthermore how the three different Toxoplasma genotypes might differ in this regard. Because tachyzoites are the first developmental stage of Toxoplasma to reach the brain, such data may have implications for understanding the pathogenesis of neurologic disturbances in Toxoplasma infection. Herein, we report on our further analysis of gene expression data from human neuroepithelioma cells infected with three clonal Toxoplasma strains in an effort to identify such altered gene expression. As mRNAs are generally translated into proteins, we also attempted to determine how these mRNA changes manifest at the level of the proteome. Because some biological processes may be transcriptionally regulated and thus potentially revealed by microarray analysis, the true effectors are proteins, the activities of which are governed by both their absolute levels and their “activation” statuses. The measurements taken from mRNA and protein levels are complementary and both are necessary for a comprehensive understanding of how the gene modulates in the presence of the parasite.
We therefore not only identified the altered genes coding for NNS at the transcriptional level, but also examined their expression at the protein level. Although we found variable correlations between protein abundances and mRNA expression, our current study revealed the presence of disturbed NNS upon Toxoplasma infection with the extent of disturbance varying considerably among the different strains.
Experimental procedures
Study design and analysis
The details of this study are described in the original publication (Xiao et al. 2011). The human neuroepithelioma cell line SK-N-MC was chosen for study in light of the ability to propagate it under defined and reproducible conditions and to generate sufficient amounts of standardized target material (Barnes et al. 1981). In brief, the SK-N-MC cells were infected at a multiplicity of infection (MOI) of 3 or mock infected with three major clonal Toxoplasma strains: RH-2F (type I), PRU (type II), CTG (type III). After 20-h incubation, RNA transcript levels were quantified by microarray analyses using Affymetrix GeneChip human Exon 1.0 ST arrays (GEO accession number GSE22986). A two way analysis of variance (ANOVA) was used to detect genes with statistically significant expression levels between each Toxoplasma strain and their corresponding set of mock-infected cells. Due to the exploratory nature of the study and the fact that the brain normally shows small changes in gene expression (Marvanová et al. 2004; Guiry et al. 2010), genes were judged to be differentially expressed if their transcripts differed from that of controls with an ANOVA P < 0.01 and a fold change >1.2-fold in either direction. Based on the differentially expressed gene list, a further group of genes coding for NNS was selected according to the KEGG PATHWAY database (hsa04080, Neuroactive ligand–receptor interaction-Homo sapiens: http://www.genome.jp/kegg-bin/show_pathway?hsa04080). This database consists of genes coding for (a) ligand-gated ion channels, (b) G protein-coupled receptors for classical neurotransmitters, (c) G protein-coupled receptors for neuropeptides, lipids, and nucleotides, (d) classical neurotransmitter synthases and (e) neuropeptides. Moreover, genes involved in regulation of synthesis, packaging, release, and degradation (or removal) of neurotransmitter and neuroactive molecules were also screened.
Real-time PCR
Validation by qPCR and western blot was performed in a separate cohort of control and infected cells. The type of cells and the infection conditions were the same as described above except the cells were infected at MOI = 5. All differentially expressed genes coding for NNS indicated by microarray analysis have been measured. Quantitative PCR analysis was following previously published procedures (Xiao et al. 2011). In brief, the RNA was first treated with TURBO DNA-free DNase (Applied Biosystem, Foster City, CA, USA) to remove trace amounts of genomic DNA. Reverse transcription was performed using Multiscribe reverse transcriptase and random primers as recommended by the manufacturer (Applied Biosystem, Foster City, CA, USA). The fold changes between groups were evaluated using relative quantization (delta Ct method) with β-actin endogenous controls. All the qPCR analyses were repeated at least three times for three biological replicates to confirm differences in the expression levels and only results obtained in all three analyses were considered valid. The amount of Toxoplasma DNA in each group of infected cells was measured to check the replication difference among the infected cells (Xiao et al. 2011).
Western blot
Cells were lysed in RIPA buffer containing protease inhibitor followed by sonication at 4 °C for 5 min (15 s “on” and 45 s “off”) at higher power (Bioruptor UCD-300). Cellular debris was removed by centrifugation (10,000g, 4 °C, 5 min), and then the supernatant was boiled in LDS sample buffer for 10 min. Proteins were first separated on a precast 4–12 % Bis–Tris polyacrylamide Ready gel (Invitrogen, Carlsbad, CA, USA) and subsequently electrotransferred onto nitrocellulose membranes. Membranes were blocked with PBS Blocking Buffer (Thermo) for 60 min at room temperature (RT) followed by incubation with primary antibodies overnight at 4 °C (Table 1). After washing blots in PBS-Tween (PBST), antibody binding was detected with an HRP-conjugated secondary antibody (Table 1) for 1 h at RT. After washing blots in PBST, bands were visualized using an enhanced chemiluminescence (West Femto Chemiluminescent, Substrate Thermo scientific).
Immunoblots were probed with successive antibodies. To remove prior antibody reactions the transfer nitrocellulose membrane were stripped using Restore Plus Western Blot Stripping Buffer (Thermo Scientific, Waltham, MA, USA). Following stripping, membranes were probed with antibodies as described in Table 1. Anti-β-actin was used to confirm equal loading. Western blot analyses were repeated at least three times to confirm differences in the expression levels and only results obtained in all three analyses were considered valid.
The intensity of immunoreactive bands obtained in ECL-exposed film (GE Healthcare) was quantitated by imaging analysis using a scanner and Scanalytics image analysis software (Bio-Rad, Hercule, CA, USA)
Statistics
The statistical significance of differences between Toxoplasma infection and control was analyzed using Student’s t test. A mixed regression model was used to analyze repeated-measures of qPCR and western blot. A P value of <0.05 was considered to represent a significant difference among groups.
Results
Modulation of receptors for neurotransmitters
After 20 h, no significant differences were found in terms of the amount of Toxoplasma DNA in each group of infected SK-N-MC cells (data not shown). However, the three Toxoplasma strains caused different patterns of gene expression and protein generation related to neurotransmitter receptors (Table 2; Fig. 1). Microarray analysis suggested that cells infected with type I strain have altered transcriptional expression for dopamine receptor 1 (DRD1), glutamate receptor, ionotropic, N-methyl D-aspartate 2A (GRIN2A) and 5-hydroxytryptamine receptors 1 (HTR1D) and 3 (HTR3E) when compared with mock infected cells. Infection with type III exhibited altered expression for GRIN2A, adrenergic receptor, beta-3-(ADRB3), and alpha-1A-(ADRA1A). Type II infection did not yield any significant change of neurotransmitter receptors.
Validation was performed in a separate cohort of control and infected cells by real-time PCR (each strain has three biological replicates). The mRNA expression of the four neurotransmitter receptor genes (DRD1, GRIN2A, HTR3E, and HTR1D) apparently altered by type I infection was determined to be significantly downregulated. However, the alterations of mRNA transcription in the context of type III infection, as suggested by microarray data, did not reach statistical significance (Table 2).
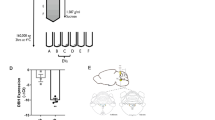
In order to evaluate how these changes of mRNA expression correlated with changes in the levels of protein expression, we performed western blot analysis on proteins whose gene expression change has been confirmed by qPCR. The protein measurement was thus conducted on type I strain-infected samples, focusing on DRD1, GRIN2A, HTR3E, and HTR1D. We found protein expression levels in GRIN2A was 48 % lower, in HTR3E was 37 % lower, and in DRD1 was 64 % lower in cells infected by type I compared to mock infected controls (Fig. 1a–c), which correspond to the decrease shown in mRNA expression level. We were not able to identify changes of HTR1D at protein level in cells infected by type I strain.
Western blot analysis of the expression of neuroactive proteins in human neuroepithelial cells 20 h following infection with Toxoplasma (+) or mock infection (–). a–g Toxoplasma type I elicits strain-dependent changes on proteins of GRIN2A (150–250 kDa), HTR3E (~50 kDa), DRD1 (60–80 kDa), PROK2 (20–25 kDa), TAC1 (20–25 kDa), SLC1A3 (50–75 kDa), and MAOA (~60 kDa); h type III elicits a strain-dependent change on TDO2 (~50 kDa); β-actin immunoreactivity (42 kDa) is displayed as loading controls. i Levels of proteins were calculated by densitometric analysis of the immunoreactive band. Each protein was compared to β-actin and normalized to untreated. Data, expressed as percent of control, are the mean ± SD of three separate preparations of infected cells. *P < 0.05; **P < 0.01 versus control
Modulation of ligand-receptor system for neuropeptides, lipids, and nucleotides
Microarray analysis indicated a differing pattern of expression on ligand-receptor system for neuropeptides, lipids, and nucleotides in cells infected with the 3 Toxoplasma strains (Table 3). In cells infected by type I, the expression of four receptors containing neuropeptide [vasoactive intestinal peptide receptor 2 (VIPR2), prolactin releasing hormone receptor (PRLHR)], hormone [prostaglandin E receptor 4 (PTGER4)], and nucleotide [adenosine A1 receptor (ADORA1)] was modulated. In cells infected by type II, only the expression of VIPR2 was modulated. In cells infected by type III, 3 receptors including neuropeptide (VIPR2) and nucleotide [purinergic receptor P2Y, 1 (P2RY1) and P2X, 6 (P2RX6)] were affected. The expression profile of modulated neuropeptides also varied according to Toxoplasma strains. Type I infection resulted in the modulation of tachykinin, precursor 1 (TAC1) and prokineticin 2 (PROK2), while type II infection caused a change in the growth hormone releasing hormone (GHRH).
Real-time PCR confirmed that type I infection results in an upregulation of transcripts for PTGER4, TAC1, and PROK2. In cells infected by type III, an altered transcriptional expression of P2RY1 was validated (Table 3).
According to the results obtained by qPCR, we determined protein levels for PTGER4, TAC1, PROK2, and P2RY1 in corresponding samples. We found that the expression of PROK2 has a dramatic increase in cells infected by type I strain compared to mock infected controls (1,200 %, Fig. 1d). In contrast, although the expression of TAC1 was highly increased at mRNA level (2,300 %, Table 3), we only observed a slight increase at protein level (18 % higher, Fig. 1e).
Modulation of genes involved in the pathway of neurotransmitters and neuroactive molecules
Cells infected by the three strains displayed different gene-expression patterns for genes involved in the pathway of neurotransmitters and neuroactive molecules (Table 4). Microarray analysis indicated that type I infection altered the expression of monoamine oxidase A (MAOA), paired mesoderm homeobox protein 2A (PHOX2A) and solute carrier family 1, member 3 (SLC1A3). Type III infection modulated the expression of tryptophan 2,3-dioxygenase (TDO2).
The dysregulation of MAOA and SLC1A3 by type I infection and that of TDO2 by type III infection were validated by qPCR (Table 4).
Protein measurements revealed that the expression of MAOA, SLC1A3, and TDO2 corresponded to the results obtained by qPCR. Cells infected by type I exhibited a higher protein level for SLC1A3 (213 %, Fig. 1f), while a lower protein level for MAOA (51 %, Fig. 1g). Cells infected by type III displayed a higher expression level for TDO2 (53 %, Fig. 1h) compared to controls.
Correlation between gene transcription and protein levels
Using the genes validated by qPCR as a standard reference, we examined the extent to which transcriptional expression was comparable to corresponding protein abundance. The comparison was performed on 11 genes with altered transcription level (Tables 2, 3, 4). Of these, 72.7 % (8 out of 11) agreed in the direction of change, i.e. both gene and protein expression were up-regulated due to the presence of the parasite or both gene and protein expression were down-regulated after infection. We were unable to detect significant differences at protein level for the remaining 27.3 % (3 out of 11) of the modulated genes.
We also performed a more quantitative comparison involving the actual volume differences in gene and protein expression. The matched proteins and genes were compared by volume using the Spearman’s rank correlation coefficient to quantify the association between the two variables. 8 modulated proteins and matched genes were compared (DRD1, GRIN2A, HTR3E, TAC1, PROK2, SLC1A3, MAOA, TDO2). The calculated correlation co-efficient (ρ) value was 0.60, showing a moderate positive correlation between protein and transcriptional expression.
Discussion
Because Toxoplasma can infect the brain and establish persistent infection within the central nervous system of intermediate hosts, the goal of this study was to profile the abnormal NNS in human neuroepithelioma cells infected with 3 Toxoplasma strains. Potential candidates involved in the NNS were selected from previous microarray gene expression data and validated at both mRNA and protein levels in order to obtain a more thorough insight into the changes affected by Toxoplasma. We found that disturbance of NNS, including presence, magnitude, and profile, following Toxoplasma infection, depended upon the specific-type strain used for infection. Toxoplasma type I infection induced the largest changes in neuroinformation processing in which several neurotransmitter systems (dopamine, glutamate and serotonin) and a number of neuropeptides (PROK2 and TAC1) were affected. Type III infection led to the change of a critical enzyme, TDO2, in the kynurenine pathway. No significant effects of type II infection were found in the NNS. These discrepancies are unlikely to be due to the differential growth rates of the three strains, as consistent with our previous reports (Xiao et al. 2011), parasite DNA levels measured 20 h following infection did not significantly differ among strains.
There was a poor correlation between microarray data and qPCR in this particularly small subset of genes. These discrepancies may be related to the different MOI (3 vs 5) used by the two cohort samples. In addition, the variability of qPCR could also contribute to these discrepancies. Most of these neuroactive molecules (e.g. VIPR2, ADORA1, PRLHR, GHRH, P2RX6, PHOX2A) have a very low abundance (Ct around 38–40); this could yield internal PCR variability, resulting in a less sensitive measurement of relative expression (delta delta Ct). Moreover, the degree of concordant regulation on RNA (generated by qPCR) and protein level are moderate. Several reasons involving post-transcriptional mechanisms and the rate of protein turnover might account for the lack of a perfect correlation between mRNA and protein levels (Greenbaum et al. 2003). However, even with these limitations, the data from the present study indicate that Toxoplasma infection could cause impairment in several aspects of the neuronal information processing. To our knowledge, the thorough examination of possible NNS affected by different Toxoplasma strains has not been reported.
Significant NNS changes specific to type I infection
Neurotransmitter systems
Three significant changes of neurotransmitter receptor did occur at both mRNA and protein level in type I infected cells. One of these was a 48 % decrease at the protein level for GRIN2A. GRIN2A encodes a subunit of the NMDA-glutamate-receptor that is well known for regulating excitatory neurotransmission in the brain and for controlling movement and behavior. Previous research has indicated negative feedback in NMDA receptor expression (Gascón et al. 2005). The decreased expression of GRIN2A may interpret as an indicator of increased glutamate levels in infected cells. This interpretation is further supported by a more than 200 % increase in the expression of SLC1A3 in infected cells since glutamate can act as a stimulus for increasing the expression of SLC1A3 (Duan et al. 1999). SLC1A3 plays an important role in terminating the postsynaptic action of glutamate by removing released glutamate from the synaptic cleft. The upregulation of SLC1A3 expression might efficiently remove excessive glutamate in order to maintain normal synaptic functions.
The protein level of DRD1 was 64 % lower in type I infected cells relative to controls. Since DRD1 is involved in the negative feedback regulation of dopamine release in the brain (Saklayen et al. 2004), an increased level of dopamine might be expected. This increased dopamine level may be suggest an increase in the rate of dopamine synthesis. In support of this, a previous study found that Toxoplasma can potentially supply a rate-limiting enzyme, tyrosine hydroxylase, in dopamine synthesis (Gaskell et al. 2009). It is possible that the infected cells synthesize greater amounts of this neurotransmitter. The reason why type I infection might be related to an increase in dopamine levels is not clear, but notably, dopamine was recently shown to stimulate type I strain multiplication in primary neonatal rat astrocyte cells (Strobl et al. 2012). This suggests that dopamine plays a supportive role in type I infection and may explain the downregulation of DRD1.
Another reason for the possible increase of dopamine level could due to decreased turnover rate of dopamine in the homovanillic acid (HVA) pathway. MAOA has a primary role in regulating monoamine turnover. The current study found a decreased expression of MAOA in infected cells, which may result in increased extracellular monoamine levels. Consequently, this would lead to a decrease in monoamine receptors such as DRD1 and HTR3E as suggested by the current study. Taken together, the present study suggested that alterations in dopamine, glutamate, and serotonin transmission might be involved in Toxoplasma type I infection.
Neuropeptide systems
The level of PROK2 and TAC1 changed significantly in cells infected by type I strain. Surprisingly, the protein expression of PROK2 increased dramatically compared to mock-infected controls (1,200 %). The PROK2 receptor has been found to be involved in the regulation of circadian rhythm behavior by the suprachiasmatic nuclei (Prosser et al. 2007). As PROK2 is abundantly produced by infected cells, this could potentially influence the level of its receptor. Consequently, this could lead to circadian abnormalities. In addition, an association between major depressive disorder and PROK2 gene expression was reported before (Spijker et al. 2010). In contrast, TAC1 displayed a dramatic increase at transcript level (2,400 %) but showed only a slight increase at protein level (18 %) compared to controls. The reasons for the final protein concentration being somewhat independent of mRNA levels cannot currently be explained but may be partly due to posttranslational modifications. TAC1 encodes a precursor containing substance P and other neurokinins (Neurokinin A, Neurokinin K, and Neuropeptide γ). Tachykinin system has been known to be involved in the pathophysiology of mood disorder (Kramer et al. 1998). Using mice with a targeted deletion of the gene TAC1, Bilkei-Gorzo et al. (2002) have demonstrated that the tachykinin system plays a major role in depression and anxiety in mice.
Significant NNS changes specific to type III infection
The protein level of TDO2 was 53 % higher in the cells infected by type III compared to controls. TDO2 plays a role in catalyzing the first and rate-limiting step in the kynurenine pathway, the major pathway of tryptophan metabolism (Dantzer et al. 2008). It has been reported that interferon-gamma suppresses the growth of Toxoplasma through acceleration of L-tryptophan–L-kynurenine pathway metabolism (Pfefferkorn et al. 1986; Fujigaki et al. 2002). Interestingly, the enhanced expression of TDO2 would conceivably lead to an acceleration of this pathway. While a previous study showed that the reduction of tryptophan levels is due to an activation of the enzyme indoleamine 2,3-dioxygenase (IDO) in type II (Fukaya strain) infection (Fujigaki et al. 2002), the current study observed an increased expression of TDO2 in type III infected cells. Since both TDO2 and IDO are critical enzymes that catalyze the first step in the conversion of kynurenine to tryptophan, it is possible that the mechanisms used by the immune system to inhibit the proliferation of Toxoplasma are strain-dependent. Notably, levels of mRNA encoding for TDO2 are elevated in the brain of individuals with schizophrenia (Miller et al. 2004).
Apart from TDO2, the absence of other neurochemical changes in cells infected with Toxoplasma type III is consistent with the findings reported by Goodwin et al. (2012), who found no changes in the monoamine systems in mice congenitally infected with a type III strain. However, in the study by Stibbs (1985), several neurochemical changes were indeed observed in the post-natally type III-infected mice. Moreover, we were not able to identify significant effects type II infection had on the NNS of human neural cells, while Gatkowska et al. (2013) found that type II strain-induced alterations in monoamine systems in both acutely and chronically infected mice. These differences may be related to the context of infection models used in the different studies. Further studies with different infection models in concurrent experiments utilizing the three main Toxoplasma genotypes are needed to further define the role of neurochemical effects of Toxoplasma infection.
Conclusions
Although a convincing body of evidence exists to indicate that Toxoplasma can cause specific behavioral changes in its host, the underlying mechanisms still remain poorly studied and explained. It is possible that abnormal NNS observed during Toxoplasma infection may contribute to Toxoplasma-associated behavioral changes. These observations raise important implications for the direction of future research.
References
Barnes EN, Biedler JL, Spengler BA, Lyser KM (1981) The fine structure of continuous human neuroblastoma lines SK-N-SH, SK-N-BE(2), and SK-N-MC. In Vitro 17:619–631
Bilkei-Gorzo A, Racz I, Michel K, Zimmer A (2002) Diminished anxiety- and depression-related behaviors in mice with selective deletion of the Tac1 gene. J Neurosci 22:10046–10052
Carruthers VB, Suzuki Y (2007) Effects of Toxoplasma gondii infection on the brain. Schizophr Bull 33:745–751
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56
Duan S, Anderson CM, Stein BA, Swanson RA (1999) Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci 19:10193–10200
Flegr J, Havlícek J, Kodym P, Malý M, Smahel Z (2002) Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study. BMC Infect Dis 2:11
Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, Wada H, Seishima M (2002) L-tryptophan–L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect Immun 70:779–786
Gascón S, Deogracias R, Sobrado M, Roda JM, Renart J, Rodríguez-Peña A, Díaz-Guerra M (2005) Transcription of the NR1 subunit of the N-methyl-D-aspartate receptor is down-regulated by excitotoxic stimulation and cerebral ischemia. J Biol Chem 280:35018–35027
Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA (2009) A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One 4:e4801
Gatkowska J, Wieczorek M, Dziadek B, Dzitko K, Dlugonska H (2013) Sex-dependent neurotransmitter level changes in brains of Toxoplasma gondii infected mice. Exp Parasitol 133:1–7
Goodwin D, Hrubec TC, Klein BG, Strobl JS, Werre SR, Han Q, Zajac AM, Lindsay DS (2012) Congenital infection of mice with Toxoplasma gondii induces minimal change in behavior and no change in neurotransmitter concentrations. J Parasitol 98:706–712
Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4:117
Guiry A, Flynn D, Hubert S, O’Keeffe AM, LeProvost O, White SL, Forde PF, Davoren P, Houeix B, Smith TJ, Cotter D, Wilkins NP, Cairns MT (2010) Testes and brain gene expression in precocious male and adult maturing Atlantic salmon (Salmo salar). BMC Genomics 11:211
Havlícek J, Gasová ZG, Smith AP, Zvára K, Flegr J (2001) Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology 122:515–520
Howe DK, Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172:1561–1566
Kannan G, Moldovan K, Xiao JC, Yolken RH, Jones-Brando L, Pletnikov MV (2010) Toxoplasma gondii strain-dependent effects on mouse behaviour. Folia Parasitol (Praha) 57:151–155
Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ, Reines SA, Liu G, Snavely D, Wyatt-Knowles E, Hale JJ, Mills SG, MacCoss M, Swain CJ, Harrison T, Hill RG, Hefti F, Scolnick EM, Cascieri MA, Chicchi GG, Sadowski S, Williams AR, Hewson L, Smith D, Carlson EJ, Hargreaves RJ, Rupniak NM (1998) Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 281:1640–1645
Marvanová M, Lakso M, Wong G (2004) Identification of genes regulated by memantine and MK-801 in adult rat brain by cDNA microarray analysis. Neuropsychopharmacology 29:1070–1079
Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S (2004) Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol Dis 15:618–629
Pfefferkorn ER, Eckel M, Rebhun S (1986) Interferon-gamma suppresses the growth of Toxoplasma gondii in human fibroblasts through starvation for tryptophan. Mol Biochem Parasitol 20:215–224
Prosser HM, Bradley A, Chesham JE, Ebling FJ, Hastings MH, Maywood ES (2007) Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc Natl Acad Sci U S A 104:648–653
Saeij JP, Boyle JP, Boothroyd JC (2005) Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol 21:476–481
Saklayen SS, Mabrouk OS, Pehek EA (2004) Negative feedback regulation of nigrostriatal dopamine release: mediation by striatal D1 receptors. J Pharmacol Exp Ther 311:342–348
Spijker S, Van Zanten JS, De Jong S, Penninx BW, van Dyck R, Zitman FG, Smit JH, Ylstra B, Smit AB, Hoogendijk WJ (2010) Stimulated gene expression profiles as a blood marker of major depressive disorder. Biol Psychiatry 68:179–186
Stibbs HH (1985) Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol 79:153–157
Strobl JS, Goodwin DG, Rzigalinski BA, Lindsay DS (2012) Dopamine stimulates propagation of Toxoplasma gondii tachyzoites in human fibroblast and primary neonatal rat astrocyte cell cultures. J Parasitol 98:1296–1299
Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM (2007) Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A 104:6442–6447
Xiao JC, Jones-Brando L, Talbot C Jr, Yolken R (2011) Differential effects of three canonical Toxoplasma strains on gene expression in human neuroepithelial cells. Infect Immun 79:1363–1373
Acknowledgments
This work was supported by Stanley Medical Research Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, J., Li, Y., Jones-Brando, L. et al. Abnormalities of neurotransmitter and neuropeptide systems in human neuroepithelioma cells infected by three Toxoplasma strains. J Neural Transm 120, 1631–1639 (2013). https://doi.org/10.1007/s00702-013-1064-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-1064-3