Abstract
Background
The sphenoid wing dural arteriovenous fistula (AVF) is rare, and can manifest with severe symptoms, particularly in cases classified as greater sphenoid wing type. Endovascular therapy is generally employed, however, open surgical intervention could be warranted in cases with complex fistula.
Method
We present a case with ruptured greater sphenoid wing dural AVF (Cognard type IV), in which endovascular embolization using liquid material was performed, followed by open surgery to concurrently disconnect the fistula and evacuate the hematoma.
Conclusion
The sphenoid wing dural AVFs may be effectively cured by open surgery for fistula disconnection in conjunction with endovascular embolization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Relevant surgical anatomy
Sphenoid wing dural arteriovenous fistulas (AVFs) are extremely uncommon, representing approximately 1% of all intracranial dural AVFs [7]. They can be classified into two types: lesser wing and greater sphenoid wing region. The greater wing dural AVF exhibits more cortical venous reflux, which received venous drainage from the laterocavernous sinus (sphenobasal and sphenopetrosal sinus) to the superficial middle cerebral vein (SMCV), as well as harboring the venous varice which manifests more aggressively [4]. In contrast to the lesser wing dural AVF, which often collects the venous drainage from the sinus of the lesser sphenoid wing and empties into the cavernous sinus or SMCV, this type is less likely to drain directly to the cortical veins [1, 4, 7]. As a result, the greater sphenoid wing dural AVFs are considered as the non-sinus type AVF, where the shunting has the potential to drain directly to the cortical vein without entering the cavernous sinus.
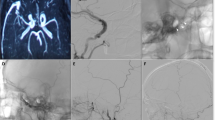
In this case, a 50-year-old female patient presented with a sudden severe headache. The brain computed tomography (CT) showed a diffuse subarachnoid hemorrhage (SAH) with temporal lobe hematoma (Fig. 1a). The CT angiography showed an abnormal dilatation of sphenobasal vein and sphenoid emissary veins at the greater sphenoid wing region with the cortical venous reflux to the SMCV (Fig. 1b, c). The digital subtraction angiography (DSA) showed the greater sphenoid wing dural AVF which was supplied by ECA branches (middle meningeal artery and accessory meningeal artery) with cortical venous reflux. There was no arterial supply from the right ICA, left carotid system, and vertebrobasilar arteries (Fig. 1d-h). The diagnosis was ruptured greater sphenoid wing dural AVF (Borden III, Cognard IV) due to having venous ectasia. A schematic illustration of this case is shown in Fig. 2. Following the cerebral angiography, the patient had mental status changed to drowsy with left hemiparesis. The follow-up CT revealed re-ruptured of the dural AVF despite the strict blood pressure control (Fig. 3a).
a Axial view of the non-contrast CT shows a diffuse subarachnoid hemorrhage and temporal lobe hematoma. b, c Axial and sagittal view of CT angiography reveal abnormal dilatation of the sphenobasal vein (red arrow) and cortical venous reflux to the Sylvian vein (superficial middle cerebral vein, SMCV) with venous ectasia (white arrowheads). d, e Digital subtraction angiography (DSA) demonstrated the greater sphenoid wing dural AVF supply from the middle meningeal artery (black arrowhead), accessory meningeal artery (white arrow) with reflux to the Sylvian vein (white arrowheads) harboring the venous ectasia (white asterisk), which drain to Labbe vein and transverse-sigmoid sinus (black arrow). f–h DSA of the right ICA, left common carotid artery, and the vertebrobasilar system shows no arterial supply to the fistula, respectively
Schematic illustration. SMCV, superficial middle cerebral vein; IMA, internal maxillary artery; MMA, middle meningeal artery; AMA, accessory meningeal artery; SOV, superior ophthalmic vein; SLSW, sinus of lateral sphenoid wing; CS, cavernous sinus; SpBV, sphenobasal vein; SpPV, sphenopetrosal vein; FO, foramen ovale; IPS, inferior petrosal sinus; SPS, superior petrosal sinus; SS, sigmoid sinus; TS, transverse sinus
a Axial view of the non-contrast CT shows a large temporal lobe hematoma with subfalcine herniation. b Three-dimensional digital subtraction angiography (DSA) shows arterial feeders from ECA (white arrowheads) and venous ectasia (white arrow). c Post-embolization ECA run shows gradual filling of the contrast into the Sylvian vein (superficial middle cerebral vein, SMCV) with remaining arterial supply from the deep temporal artery (white arrow). Intraoperative findings after frontotemporal craniotomy. d The sphenoid emissary veins (black asterisk) were identified at the temporal base following the subtemporal approach with evidence of the previously injected Onyx. e The sphenobasal vein (black asterisk) was identified at the proximal segment before emptying into the SMCV
Description of the technique
We opted for a combined approach combining endovascular treatment (EVT) and open surgery to concurrently evacuate the hematoma. The open surgery alone might be used to obliterate the fistula along with clot evacuation. However, there was a re-rupture risk when manipulating the venous varix during hematoma removal since the shunt was in a high-flow state. Therefore, EVT was performed to reduce the flow and also facilitate the surgical disconnection. The change in the flow condition was not a concern since we can check the obliteration status by an intraoperative DSA.
The transvenous approach was deemed risky in light of the SMCV venous ectasia and a ruptured presentation. The transarterial venous approach was considered unfeasible due to the small and tortuous of the sphenoid emissary veins. The procedures were performed by the first author (G.D.).
Endovascular procedure
The procedure was conducted in the hybrid operating suite. The patient was set in a supine position under the general anesthesia. The cranium was sterile and draped to prepare for the frontotemporal craniotomy The transfemoral approach was used to access the right external carotid artery (ECA) using a 6 Fr Envoy guiding catheter (Cerenovus, Johnson & Johnson, USA). The intraoperative DSA run in three dimensions revealed arterial feeders originating from the middle meningeal artery, and accessory meningeal artery (Fig. 3b). The 1.5 Fr microcatheter (Marathon, Medtronic, USA) and 0.008″ hydrophilic guidewire (Mirage, Medtronic, USA) were navigated to the middle meningeal artery in a wedge configuration. Subsequently, the Onyx 18™ (Medtronic, USA) was introduced under the roadmap to obliterate the fistula (Operative Video). The injection of liquid material managed to enter the fistula pocket by partially penetrating the sphenoid emissary veins (Fig. 3c). The post-embolization DSA revealed a gradual flow of contrast material into the remaining fistula (Fig. 3d). The decision was made to disconnect the fistula by transcranial approach since it was determined that it would not be possible to completely embolize the fistula via the transarterial route.
Craniotomy with AVF disconnection
The curvilinear incision was made then the frontotemporal craniotomy was performed. After appropriate hemostasis, the dural incision was made. A partial evacuation of the hematoma was performed to decrease brain edema and aid in subsequent dissection. The subtemporal approach was utilized to expose the vascular malformation and the primary venous drainage with evidence of the liquid material in the fistula channels (Fig. 3f, g). The sphenobasal vein as the primary venous drainage was cauterized and disconnected for complete fistula obliteration (Fig. 3h). The ECA run demonstrated complete fistula obliteration (Fig. 4a, b). Although the fistula had disappeared, there was still bleeding originating from the venous varice, which was believed to be due to the retrograde flow of normal venous drainage. The venous varice was cauterized and safely disconnected since it is not utilized for normal brain venous drainage.
Intraoperative digital subtraction angiography. a, b Anteroposterior (AP) view of the right common carotid artery contrast injection demonstrated complete fistula obliteration. c An intraoperative CT shows significant resolution of brain edema. Follow-up digital subtraction angiography at 12 months postoperation. d Lateral view of right ECA injection shows no residual or recurrent fistula. e, f AP view of right ICA and left common carotid artery injection shows no arteriovenous shunting
The intraoperative brain CT showed appropriate clot evacuation and resolution of the brain edema (Fig. 4c). The postoperative course was uneventful, and the modified Rankin scale was evaluated at 1 upon discharge. At 12 months follow-up, the modified Rankin scale was evaluated at 0. Additionally, the DSA revealed no remaining or recurrent fistula (Fig. 4d-f).
Indications
Utilizing a combination of open surgery and EVT can be advantageous for treating complex dural AVFs. The transcranial approach can facilitate access to the fistula [2] and allows for simultaneous eradication of the fistula along with hematoma evacuation as demonstrated in this particular case. EVT is the primary treatment for sphenoid dural AVFs. However, in cases when the liquid substance cannot reach the fistula site through the sphenoid emissary vein, open surgery is necessary.
Limitations
The dually trained hybrid neurovascular surgeon is necessitated in performing combined open and endovascular surgery. In addition, there is a need for a hybrid operating room to facilitate complex operations.
How to avoid complications and specific perioperative considerations
There are various techniques to eliminate the fistula, including transarterial, transvenous, and transarterial venous embolization, or surgical disconnection [3, 5, 6]. Considering the presence of a large amount of subarachnoid hemorrhage caused by venous ectasia in the SMCV, we believe that transvenous embolization was deemed high-risk in this particular case. The objective of transarterial embolization is to mitigate the excessive blood flow in the fistula, which is then followed by surgical disconnection. It is important to carefully assess the dangerous anastomoses when embolizing the ECA branches to prevent cranial nerve palsy, particularly concerning the middle meningeal artery.
Specific information to give to the patient about surgery and potential risks
The greater sphenoid wing dural AVF is associated with a significant incidence of cortical venous reflux, which is indicative of a more aggressive presentation and a greater likelihood of intracerebral hemorrhage when compared to the lesser sphenoid wing dural AVF. The probability of experiencing cortical venous reflux and venous ectasia can reach up to 65% and 100%, respectively [1, 7].
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- AVF:
-
Arteriovenous fistula
- CT:
-
Computed tomography
- DSA:
-
Digital subtraction angiography
- ECA:
-
External carotid artery
- EVT:
-
Endovascular treatment
- ICA:
-
Internal carotid artery
- SAH:
-
Subarachnoid hemorrhage
- SMCV:
-
Superficial middle cerebral vein
References
Akioka N, Kuwayama N, Kuroda S (2023) Sphenoid Wing Dural Arteriovenous Fistulas. Journal of Neuroendovascular Therapy advpub. https://doi.org/10.5797/jnet.ra.2023-0034
Duangprasert G, Tantongtip D (2024) Direct superior sagittal sinus puncture via a surgical burr hole for curative embolization of the complex transverse-sigmoid sinus dural arteriovenous fistula: How I do it. Acta Neurochir (Wien) 166:131. https://doi.org/10.1007/s00701-024-06020-2
Fukuda H, Miyake K, Kunieda T, Murao K (2014) Endovascular treatment of sphenoid wing dural arteriovenous fistula with pure cortical venous drainage. J Stroke Cerebrovasc Dis 23:1730–1735. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.12.037
Ghali MGZ (2021) Sphenoid dural arteriovenous fistulas. Neurosurg Rev 44:77–96. https://doi.org/10.1007/s10143-019-01209-x
Inoue S, Fujita A, Shinoda K, Yamashita S, Lee TJ, Kuroda R, Urui S, Kurihara E, Sasayama T (2022) Non-Sinus-Type Laterocavernous Sinus Dural Arteriovenous Fistula Treated by Transarterial Venous Coil Embolization: A Case Report. J Neuroendovasc Ther 16:225–231. https://doi.org/10.5797/jnet.cr.2021-0021
Nomura S, Anegawa S, Nakagawa S, Tomokiyo M, Koga H, Hayashi T (2002) Subarachnoid hemorrhage caused by dural arteriovenous fistula of the sphenobasal sinus–case report. Neurol Med Chir (Tokyo) 42:255–258. https://doi.org/10.2176/nmc.42.255
Shi ZS, Ziegler J, Feng L, Gonzalez NR, Tateshima S, Jahan R, Martin NA, Viñuela F, Duckwiler GR (2013) Middle cranial fossa sphenoidal region dural arteriovenous fistulas: anatomic and treatment considerations. AJNR Am J Neuroradiol 34:373–380. https://doi.org/10.3174/ajnr.A3193
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Gahn Duangprasert – Operating physician, Conceptualization, Writing an original draft, Revise the manuscript, Investigation, Data curation, Visualization, Project administration, Supervision. Phichayaphong Durongkaweroj – Investigation, Data curation. Pasinee Chotsakulthong – Visualization. Dilok Tantongtip – Investigation. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The research project was conducted following the principles outlined in the Declaration of Helsinki. The ethics approval was waived by the local university hospital review board according to an anonymized presentation of a single case.
Consent to participate
Informed consent was obtained.
Consent statement
The patient has consented to the submission of this How I Do It to the Acta Neurochirurgica.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
1. Differentiating between the lesser and greater wing dural AVF is crucial because of their differences in natural history, morphology, and prognosis.
2. Dural AVF affecting the greater sphenoid wing is associated with a considerably higher likelihood of intracerebral hemorrhage and a high propensity for cortical venous reflux.
3. Endovascular treatment comprised of transarterial, transvenous, and transarterial venous approaches have been utilized for the treatment of sphenoid wing dural AVF.
4. In cases where endovascular therapy is insufficient to cure the fistula or when there is a surgical hematoma, open surgery might be necessary.
5. It is crucial to carefully assess the dangerous anastomoses between the external carotid artery (ECA) and internal carotid artery (ICA) while using liquid material embolization.
6. A hybrid operating room is advantageous for complex neurovascular conditions, particularly when employing the combined approach.
7. Open surgery can be employed in conjunction with endovascular therapy to facilitate access and provide concurrent treatment.
8. Intraoperative digital subtraction angiography is beneficial for diagnosis and confirms the completeness of fistula obliteration.
9. Embolization via the transarterial route can encounter challenges when attempting a complete cure due to the small size and tortuous nature of the sphenoid emissary veins. However, caution must be taken when performing transvenous access in cases presenting with ruptured AVF and the presence of venous ectasia.
10. A dually trained neurovascular surgeon has an advantage in treating complex diseases by being fully equipped with both open surgery and endovascular skills.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 430313 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duangprasert, G., Durongkaweroj, P., Chotsakulthong, P. et al. Combined open surgery and endovascular embolization for a ruptured sphenoid wing dural arteriovenous fistula. Acta Neurochir 166, 333 (2024). https://doi.org/10.1007/s00701-024-06226-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00701-024-06226-4