Abstract
Background
Since the medullary arteries are of a great neurologic and neurosurgical significance, the aim was to perform a detailed microanatomic study of these vessels, as well as of the medullary infarctions in a group of patients.
Methods
The arteries of 26 halves of the brain stem were injected with India ink and gelatin, microdissected and measured with an ocular micrometer. Neurologic and magnetic resonance imaging (MRI) examinations were performed in 11 patients.
Results
The perforating medullary arteries, averaging 6.7 in number and 0.26 mm in diameter, most often originated from the anterior spinal artery (ASA), and rarely from the vertebral (VA) (38.5%) and the basilar artery (BA) (11.6%). They supplied the medial medullary region. The anterolateral arteries, 4.8 in number and 0.2 mm in size, most often arose from the ASA and PerfAs, and nourished the anterolateral region. The lateral arteries, 2.2 in number and 0.31 mm in diameter, usually originated from the VA and the posterior inferior cerebellar artery (PICA). They supplied the lateral medullary region. The dorsal arteries, which mainly arose from the PICA and the posterior spinal artery (PSA), nourished the dorsal region, including the roof of the 4th ventricle. The anastomotic channels, averaging 0.3 mm in size, were noted in 42.3%. Among the medullary infarctions, the lateral ones were most frequently present (72.8%).
Conclusion
The obtained anatomic data, which can explain the medullary infarctions symptomatology, are also important in order to avoid damage to the medullary arteries during neurosurgical and neuroradiologic interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many authors examined the vertebral artery (VA) [3, 5, 6, 9, 26. 31], the anterior (ASA) and posterior spinal arteries (PSA) [3, 5, 18, 21], and the posterior inferior cerebellar artery (PICA) [5, 6, 18, 22, 28]. However, only a few authors reported anatomic aspects of the medullary branches of the mentioned parent arteries [2, 6, 19, 26, 28], and therefore detailed morphometric data are still lacking.
Certain investigators have examined morphology and the supplying region of the medullary branches [6, 26]. Some others measured their diameter and length, but without dividing them into the corresponding types [2]. Still others measured only the perforating medullary vessels [19]. Finally, a comparison of the vascular anatomy, the location and extent of certain medullary infarctions and their symptomatology were infrequently reported [13, 29].
Accordingly, the aim of our investigation was first to examine the microanatomic characteristics of the medullary arteries and their region of supply. Second, to compare the obtained anatomic data with the medullary infarctions in our patients and in some reported groups [11, 13,14,15]. Third, to analyze the potential clinical significance of the obtained data.
Methods
Anatomic examination
Thirteen brain stems (26 halves) of 7 males and 6 females aged 42–67 years were provided during the autopsy, after the removal of the brain from the skull. This procedure during a routine autopsy caused damage to the initial part of the PICA in several cases, so that this vessel could be completely examined in 8 halves of the specimens. In spite of this, the remaining specimens still provided useful information. Twigs of the other arteries were examined in all the 26 halves of the specimens.
The main inclusion factor was the patients’ relatively younger age (42–66 years) in order to avoid, as much as possible, the eventuality of cerebrovascular disease of the medullary vasculature. The important exclusion criterion was any brain disease or disorder which could damage the brain stem. Nevertheless, demographic data, including the pre-autopsy diagnosis, were shown in a table, along with certain data regarding the clinical patients, which is presented in the result section.
Microcatheters were inserted into the right and left VA of each specimen, and 10 ml of a 10% mixture of India ink and gelatin was injected. The brain stems were fixed in a 10% formaldehyde solution for 3 weeks, and then carefully dissected under the stereomicroscope (Leica MZ6). The arteries were measured by means of an ocular micrometer. We also tried to microdissect some larger intramedullary arteries, in order to examine their ramification and region of supply. They were compared to serial transverse histologic sections of a non-injected brain stem stained after the Klüver-Barrera method, which were examined under a light microscope (Leica DMLS). We also analyzed two representative templates regarding the vascular territories [6, 26]. Photographs of the dissected arteries and histologic slices were used for further analysis.
Patient examination
During ten months in 2022, we enrolled 12 patients with the medullary infarctions in our Stroke Hospital, that is, 8 males and 4 females aged between 56 and 81 years (65.8 on average). In addition to the general, neurologic and laboratory examination, magnetic resonance imaging (MRI) was performed in all patients but one using General Electric, Signa HdX 1.5 T apparatus. The following sequences were applied: T1- and T2-weighted, diffusion-weighted (DWI), fluid-attenuated inversion recovery (FLAIR), and MR angiography (MRA).
Six of our patients had arterial hypertension, 2 diabetes mellitus, 3 hyperlipidemia, and 1 ischemic heart disease, whilst 3 were cigarette smokers. Only 1 patient reported a previous stroke. Demographic data and risk factors were presented in Table 1.
There were two inclusion criteria: a recent medullary infarction, and an MRI examination. Since one of the 12 patients had only computerized tomographic (CT) scans, he was excluded from our study. Finally, a written consent was provided from all clinical patients, which was approved by the authorities of the Neurosurgical Clinic, the Stroke Hospital Sveti Sava, and the Ethics Committee of the Clinical Center.
Statistical analysis
Statistical analyses were performed using statistical software SPSS (version 22.0). Statistical tests are 2-sided, and p values < 0.05 were considered to be statistically significant. Continuous variables are shown using the mean (SD), median (interquartile range [IQR]) and 95% Confidence Interval (95%CI), and compared applying the nonparametric Mann–Whitney U test for all non-normally distributed data.
Results
Autopsy individuals and clinical patients
We have created a summarizing table with the most important data regarding both groups of patients (Table 1). The age of the autopsy individuals was lower (61.9 ± 1.8) compared to that of our patients. There was a similar number of males and females. A pre-autopsy diagnosis was mostly related to ischemic heart disease and tumors, but with no statistical significance (p = 0.442).
Clinical patients were older on average (66.7 ± 8.13) (Table 1), and males predominated. Some of them had two risk factors. Hypertension was the most frequent event, but also with no statistical significance (p = 0.261).
Anatomic, radiologic and neurologic examination
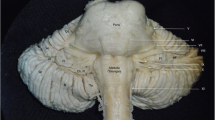
Four groups of the medullary arteries were distinguished, mainly arising from the VA, the anterior spinal artery (ASA) and its roots, the PICA, and the PSA (Fig. 1). These are the perforating arteries (PerfAs), short anterolateral (ALAs), long lateral (LAs), and the dorsal medullary arteries (DAs) (Fig. 1).
A drawing of the basilar (1), vertebral (2), and anterior spinal artery (3) with their medullary branches in the anterior view. Note the perforating (black), anterolateral (white) and lateral twigs (bright grey), and some common stems (stripped). Also note the abducent nerve (4), the foramen caecum (5), the right pyramid (6), the left olive (7), the hypoglossal nerve (8), and the lateral surface (9) of the medulla. Certain perforators (a) enter the foramen caecum or the anterior median sulcus (b and c), whilst some others originate by a common trunk (d), or give rise to the anterolateral branches (e), or originate in common with them (f). There is also a common stem of the anterolateral twigs (g). Note the lateral medullary arteries (h), and a perforator (i) of the basilar artery
As regards their parent vessels, the VA most often was normal in size (Fig. 1), with a mean diameter of 2.64 ± 0.38 mm (range: 2.1–3.2 mm; median: 2.7; IQR: 0.7; 95%CI: 2.42–2.87). A VA hypoplasia, i.e. less than 2.2 mm in size, was rarely observed (7.8%). As for the ASA, most often a typical vessel was seen (Fig. 2A), and less frequently a hypoplastic right (Fig. 2B) or left ASA root, without (Fig. 2C) or with a common stem (Fig. 2D) of certain medullary branches (ASA, LA and sometimes PerfA). A duplication of the ASA (Fig. 2E), its unilateral origin with (Fig. 2F) or without the opposite common stem (Fig. 2G), or a plexiform origin (Fig. 2H) was rarely observed. The ASA averaged 0.62 mm in diameter, and its roots 0.51 mm and 0.41 mm, respectively (Table 2). They arose from the VA 1.6–7.6 mm (mean, 5.1 mm) caudal to the vertebrobasilar junction. They joined together to form the ASA between 4.7 mm and 14.8 mm (mean, 9.1 mm) caudal to the latter junction. The ASA and its roots can give off singular medullary branches or certain common stems (Figs. 1, and 2D and F).
A drawing in the anterior view presenting the basilar (1) and vertebral arteries (2 and 3), the roots (4) of the ASA (5) and their variations in our specimens. Note a typical ASA (A), a hypoplastic right (B) or left root (C) (arrow), which can give rise to a common stem of the anterolateral or lateral arteries (D) (arrow), a double ASA (arrows) interconnected by an anastomotic channel (E) (arrowhead), and a singular ASA origin without the anastomosis but with the mentioned opposite stem (F) (arrow) or without the latter stem (G). Also note a plexiform ASA origin (H)
Perforating arteries and medial medullary infarctions
The PerfAs (Fig. 1), which averaged 6.7 in number and 0.26 mm in diameter, most often originated from the ASA (100%), and less frequently from other vessels (Table 2), including the BA (11.6%) (“i” in Figs. 1, and 3B). The most rostral PerfA originated 3.4 mm on average caudal to the vertebrobasilar junction.
Anterior view of the vertebral and basilar arteries (A), with a magnified detail (B) following the displacement of the large arteries by a pin. Note a basilar origin of the right PICA, the corresponding branches of the vertebral and basilar arteries, including AICA, and an anastomotic channel (arrows in B) between a basilar branch (supplying the medulla) and the twigs within the pontomedullary sulcus
The PerfAs were mainly singular, but in 11.6% they formed common stems (“d” in Fig. 1) (Table 2). Some PerfAs gave rise to the ALAs (“e” in Fig. 1) in all the specimens, or they both originated by a common stem (7.8%) (“f” in Fig. 1). Their common origin with the LAs was noted in 15.4% (Table 2). The PerfAs penetrated the foramen caecum (“a” in Figs. 1 and 3B) and the anterior median sulcus (“b” and “c” in Fig. 1). They nourished the pyramids and deeper structures, following a division into short anteromedial and 2–4 long paramedian branches.
The anteromedial twigs mainly supply the medial part of the pyramidal tract, some corticobulbar fascicles, and parts of the arcuate nucleus (Fig. 4). The paramedian branches nourish certain structures along the raphe, i.e. the medial lemniscus, tectospinal tract, medial longitudinal fasciculus (MLF), and the hypoglossal nucleus, as well as the nucleus prepositus rostrally, and certain paramedian and raphe reticular nuclei (Fig. 4).
A drawing of a transverse section of the upper medulla through the inferior olivary complex to show the extramedullary vessels, including the vertebral artery, PICA, and roots of the anterior spinal artery. Also note the intramedullary branches and its region of supply, i.e. the perforating vessels with the paramedian branches and their territory (a pink region) and the anterolateral twigs with a smaller region (an orange area), the lateral branches and their territory (in blue), and the posterior or dorsal twigs and their region (in green). (Modified after ref. 29)
A medial medullary infarction (MMI) was not observed in our patients. However, a combined partial medial and anterolateral ischemic lesion was noted in one patient (Fig. 5). He presented a hemiparesis with leg predominance, a slight dysarthria, and a mild touch and proprioceptive disorder of the opposite hemi body (Fig. 5A–C). The left VA occlusion was observed, including a multicentric stenosis of the right VA (Fig. 5D).
Anterolateral arteries and medullary infarctions
The short ALAs (Figs. 1 and 3), which averaged unilaterally 4.8 in number and 0.2 mm in size, most often originated from the PerfAs and the ASA, and less frequently from other arteries (Table 2). They rarely branched off from a BA perforator (Fig. 3B). They penetrated the pyramid, the preolivary sulcus, or the olive (Figs. 1 and 3). The ALAs successively arose from a long common trunk (”g” in Figs. 1, and 2D and F) in 19.3% specimens, which sometimes can give off a PerfA or a lateral medullary branch. In addition, some ALAs from the BA (Table 2) terminated in the olivary region, as well as certain descending ALA twigs of the AICA (Fig. 3B).
The ALAs mostly supply the lateral part of the pyramidal and corticobulbar tracts, the hypoglossal nerve, and the ventral part of the inferior olivary complex (Fig. 4). An isolated anterolateral infarction (ALMI) was not seen in our patients.
Lateral arteries and medullary infarctions
The LAs, which averaged unilaterally 2.2 in number and 0.31 mm in diameter, most often originated from the VA (“h” in Figs. 1, and 3A) and the PICA (Fig. 6), and rarely from other vessels (Table 2). They infrequently arose by their own common stems (7.8%), or with a PerfA (15.4%) or ALAs (3.9%). The LAs occasionally gave rise to 1–3 PerfAs, the ALAs, or both.
The LAs nourish the lateral medullary territory (Fig. 4), including certain somatosensory and autonomic pathways (the spinothalamic, spinal trigeminal, trigeminothalamic, and solitary tracts), some somatosensory and autonomic nuclei (the spinal trigeminal, solitary, and dorsal vagal nuclei), certain motor elements (the nucleus ambiguus, and the rubrospinal, vestibulospinal, reticulospinal and central tegmental tracts), sympathetic descending fibers, some ascending pathways (the spinocerebellar and olivocerebellar tracts), portions of the reticular formation, and the lower cranial nerves.
A pure lateral medullary infarction (LMI) was the most frequent type in our study (72.8%) (Fig. 7). In such patients, hemihypesthesia or dysesthesia for pain and temperature commonly appeared, sometimes along with facial hemihypesthesia, dysarthria, dysphagia, ataxia, nausea, vertigo, unsteadiness, and Horner’s syndrome. In addition, the presented patient (Fig. 7), who had the right VA occlusion, also manifested hiccups and vomiting.
Dorsal (posterior) arteries and medullary infarctions
The DAs mainly represent branches of the PICA and the posterior spinal artery (Fig. 8).
Posterior (dorsal) view of the medulla oblongata after removal of the cerebellum by cutting off its peduncles. Note the rhomboid fossa, the inferior cerebellar peduncles, and the left PICA with the caudal (a) and cranial loop (b), and the lateral (c) and medial (d) terminal trunks (cut). Also note the tiny dorsal twigs (arrowheads) and a larger common stem of the dorsal medullary, telar, and choroidal branches. The right PICA was removed to expose certain branches of the right vertebral artery (not visible), including the posterior spinal artery, and the tiny laterodorsal branches (between the two larger arrows). Also note a few anastomoses (smaller arrows) among the dorsal branches of the PSA and PICA
The PICA is the largest branch of the VA (Fig. 6), which encircled the lateral medullary surface and entered the tonsillomedullary fissure, and then coursed along the dorsal surface of the medulla, toward the roof of the 4th ventricle (Fig. 8). It usually formed a caudal and a cranial loop, and bifurcated then into two trunks, the medial and lateral. Larger dorsal branches of the PICA coursed along the inferior cerebellar peduncle toward the tela choroidea, the choroid plexus, and the rhomboid fossa (Fig. 8). Smaller twigs terminated close to the PICA (Fig. 8).
The PSA arose from the VA (Fig. 6) or the PICA. It usually divided into a descending and an ascending branch. The latter branch coursed along the inferior cerebellar peduncle, toward the 4th ventricle, and terminated close to the obex and the foramen of Magendie (Fig. 8). When the ascending branch of the PSA was absent or small (in 2 cases), it was replaced by the lateral VA twigs (Fig. 8, right) and the dorsal PICA branches. Some PSA twigs terminated on the opposite side (Fig. 8, left). In all the cases but one there were the right-left anastomoses among the PICA dorsal branches, occasionally including the PSA twigs (Fig. 8).
The DAs supplied the posterior region of the medulla, that is, the gracile and cuneate fasciculi and nuclei, and the inferior cerebellar peduncle, as well as the vestibular nuclei in the upper medulla (Fig. 4). In addition, they also nourished the inferior medullary velum, the lateral part of the rhomboid fossa and the tela choroidea, including the choroid plexus (Fig. 8).
Isolated dorsal medullary infarctions (DMIs) were not observed in our patients. However, a dorsolateral ischemic lesion was noted in two of them (Fig. 9). In the latter patient, who had a right VA occlusion and a left VA stenosis, a bilateral asymmetric cerebellar infarction was also noted in the PICA territory. Both patients displayed a slight hemihypesthesia, and touch and proprioceptive sense disorders, as well as ataxia, vomiting, vertigo and unsteadiness.
Anastomotic channels
One or 2 anastomoses were noted in 42.3% of the anterior and anterolateral surface of the medulla. They most often involved the PerfAs (30.8%) (Fig. 3B), and rarely the ALAs (15.4%) or the LAs (7.8%). They ranged from 0.1 to 0.4 mm (mean, 0.3 mm) in diameter. Such connections were also present among the dorsal twigs, particularly as the right-left anastomoses (Fig. 8).
Discussion
There were certain differences between the autopsy individuals and the clinical patients (Table 1). As for the age of the former (61.9 ± 1.8), we selected relatively younger people in order to secure brain stems with no disease or disorder. An older age of the latter (66.7 ± 8.13) was expected since ischemic lesions are more frequent in the older population [10, 13, 29]. Although the pre-autopsy diagnosis was mostly related to heart ischemic disease and tumors, there was no statistical significance (p = 0.442). It was the same case with hypertension (p = 0.261) which was the most frequent among the risk factors in our patients. Males predominated, which was also shown by other authors [10, 13, 15, 29].
A short anatomy of the parent arteries
The medulla oblongata is mainly supplied by the branches of the VA [2, 5, 19, 26, 31]. It averages 21.4–33.2 mm in length and 2.85–3.30 mm in diameter [2, 3, 18, 23], which is somewhat higher than our results (mean, 2.64 mm). The clinically important VA hypoplasia incidence in our study (7.8%) is within the reported range (6.5–20.2%) [2, 3, 21, 23]. Hypoplasia is present even in 58.3% of stroke patients [9].
The ASA averaged 0.62 mm in caliber in our study (Table 2), which is similar to certain reports, i.e. 0.59 mm [19], but slightly smaller than in some others, i.e. 0.7- 0.8 mm [2, 3]. Among the variations, a double ASA (Fig. 2E) was noted in 3.9% of our specimens, which is less than in some reports (9.2–23.2%) [3, 7, 21, 25]. Occasionally, there is a transverse communicating anterior spinal artery between the right and left VA, which gives rise to one or two ASAs, and which can be an important anastomotic channel in the case of a VA occlusion [3, 23]. The mentioned common stem of the ALAs, LAs and sometimes the PerfAs (Fig. 2D and F), which arose from an ASA vascular root and coursed parallel to the ASA, was named as the accessory ASA [21] and the second ASA [23]. However, since the common stem is short, there is no true ASA duplication.
A sporadic agenesis of the right or left ASA root has also been mentioned by other authors [2, 3, 7, 19, 21, 23, 31]. In these cases, the ASA originated only from one VA (Fig. 2F and G), as noted in 7.8% in our study, which is similar (9.7–11.1%) to some other findings [7, 21, 23]. As for the ASA roots hypoplasia, to our knowledge, it was not described in the literature.
The initial part of the BA, along with the AICA, may contribute to the supply of the medulla oblongata [2, 6, 19]. This was also noted in our specimens (Table 2) (Fig. 3B).
The PICA has a mean diameter of 1.3–2.0 mm [18, 22, 28]. It most often arises from the VA (85%), and rarely from the BA (12.5%) (Fig. 3) [18, 22, 28]. The PICA was divided into several topographic segments [5, 18, 22, 28].
The paired PSA more often originates from the VA than the PICA [2, 18, 22, 28], as also noted in our specimens, but the opposite was mentioned in another report [30]. The PSA usually divides into a descending and ascending twig. The former twig anastomoses caudally with the posterior longitudinal spinal trunk (5, 30). The latter twig courses to the inferior cerebellar peduncle and sometimes to the tela choroidea [26, 30]. Hypoplasia or an absence of the PSA ascending branch, noted in our study, was not mentioned in the literature.
Medullary branches anatomy
Of the mentioned parent vessels, the VA and ASA give rise to an average of 11 medullary branches of 0.3 mm in size and 9.1 mm in length [2]. Important twigs also originate from the PICA and PSA [5, 6, 30]. We measured the size of various types of medullary branches.
Perforating arteries
These vessels can arise from the VA, the ASA and its roots, and rarely from the BA [2, 6, 19] (Table 2). They averaged 6.7 in number in our specimens, which is almost identical (6.5) to some findings [19], and 0.26 mm in diameter, that is similar (0.25–0.3 mm) to the data of others [2, 3]. They can originate by their own common trunks, or as common stems with the ALAs and/or the LAs (Fig. 1). Such common stems, which usually course parallel to the ASA, have been mentioned but not described in the literature [3, 21, 23]. In any case, the PerfAs supply the medial region of the medulla [6, 26].
Anterolateral arteries
The ALAs are short and tortuous twigs of the VA, the ASA and some other vessels [6, 19, 26]. The ALAs of the PICA were also described by some authors [28], and of the AICA by some others [2, 6]. The ALAs nourish the anterolateral medullary territory [6, 26].
Lateral arteries
The LAs may arise from the VA and PICA [2, 6, 31], and less frequently from the ASA and its roots, as noted in our study. They supply the lateral region of the medulla (Fig. 4).
Dorsal arteries
They originate from the PICA and the PSA [6, 28, 30]. The PICA gives off 3.3 dorsal medullary branches on average, and also several branches to the inferior cerebellar peduncle, tela choroidea, choroid plexus, inferior medullary velum, and adjacent structures (Fig. 8).
The PSA sends 5 dorsal branches on average, with a mean diameter of 0.34 mm [5, 30]. The ascending branch was hypoplastic or absent in two of our specimens. Its territory in these cases was taken over by the terminal twigs of the lateral VA branches, and by the PICA dorsal twigs (Fig. 8). A bilateral supply of the dorsal surface by a single PCA (Fig. 8) seems not to be reported.
Anastomoses
Sporadic anastomotic channels, found in our work, were also mentioned by some other authors [2, 6, 19]. In our study, they were mainly noted among the PerfAs, ALAs, and the LAs on the pyramids and the olives. The anastomoses are more frequent among the dorsal twigs of the PICA and PSA, as shown in our study and some reports [6, 30].
In general, the anastomotic channels can be useful in occlusive cerebrovascular disease, as noted for the anterior communicating spinal artery which interconnects the two VAs [3, 21]. The presence of the anterior and posterior anastomoses is probably the reason for the rare occurrence of medial and dorsal infarctions [11, 13, 15, 16].
However, we revealed only 1–2 anastomoses in 42.3% cases on the ventrolateral surface. Although the ASA roots, the anterior communicating spinal artery, and the mentioned ASA connection with the anterior longitudinal spinal trunk, could also take on the role of the anastomotic channels, the medullary anastomoses are probably not always beneficial. The situation is even worse when the anastomoses are absent.
The neurologic significance of the anatomic data
The ASA can occasionally arise from one VA, without a connection with the opposite VA (Fig. 2F and G) [3, 7, 21, 25]. If the ASA is occluded, bilateral pyramidal damage is possible, which may result in quadriplegia or paraplegia [13].
As for the PerfAs that often give off ALAs, their occlusion may result in both a medial and anterolateral ischemic lesion. An occlusion of a PerfA and LA common stem can lead to medial and lateral infarctions. Finally, the caudal BA perforators can supply the rostral medulla [2, 6, 19] (Fig. 3B), so that their occlusion can cause medullary ischemia [29].
As regards the anterolateral arteries, the upper ALAs from the VA, together with similar twigs of the BA, AICA and occasionally PICA, form a vascular network in the olivary and retro-olivary area [2, 6]. This is why the inferior olivary complex is rarely affected by ischemia [2].
As for the lateral medullary arteries, their occlusion can result in lateral infarctions [13, 14]. If an occlusion affects an LA which gives off the PerfAs, as noted in our study, a combined medial and lateral infarction could be possible.
The dorsal medullary twigs of the PICA and the PSA showed right-left anastomotic channels in our and some others’ specimens [6, 30]. This could be the reason of the rare appearance of dorsal medullary infarctions [10, 11, 13, 16]. Possible ischemia could affect the ipsilateral, but also some contralateral medullary structures (Fig. 8).
In most of our patients there was a VA stenosis or occlusion, as also noted in other reports [13, 29]. This causes a hypoperfusion of the medulla and a resulting ischemia. In general, if an infarction corresponds to the supplying region of a medullary artery, the usual cause of such an ischemia is small vessel disease, artery-to-artery embolism, or an atheromatous occlusion of its orifice [10, 13, 14].
Medial medullary infarction
Pure MMIs were not diagnosed in our small group of patients, since they occur very rarely [15, 29]. If an entire perforating artery is occluded (Fig. 4), hemiparesis with arm predominance can appear [29]. Supranuclear palsies (lesion of the corticobulbar fascicles) are also possible, and touch and proprioceptive sense disorders (lesion of the medial lemniscus), lingual paresis (damaged hypoglossal nucleus), and occasionally gaze disturbances (lesion of the MLF, or the nucleus prepositus) [13, 15, 29].
Anterolateral medullary infarction
The ALMI, which is also rare, can be caused by an ALA occlusion [13, 29]. The main symptoms are commonly hemiparesis with leg predominance and some supranuclear palsies, and occasionally a peripheral hypoglossal nerve paresis.
Lateral medullary infarctions
The LMI occurred in 72.8% of our patients, which is within the reported range of 54.9–78.0% [13, 14, 29]. Sensory disorders appeared in over 90%, due to damage of the spinothalamic tract (contralateral hypesthesia of the body), and the spinal trigeminal nucleus and tract or the trigeminothalamic tract (facial hemihypesthesia). Supranuclear palsies can occur, e.g. dysarthria, dysphagia, and facial paresis (lesion of the nucleus ambiguus, or the corticobulbar tract), and ataxia (damage to the spinocerebellar tracts), as well as Horner’s syndrome (a lesion of descending sympathetic fibers) (Fig. 4). Ischemia can also affect the vasomotor and respiratory centers [13, 14, 29]. Most of the mentioned signs are known as Wallenberg’s syndrome [13, 14].
Dorsal medullary infarctions
No pure DMIs appeared in our patients, though they did emerge in combination with LMIs in two patients. A pure infarction is caused by compromised blood flow through the dorsal medullary branches of the PICA and PSA, which are anastomosed in many cases [6, 30]. This is probably why DMIs occur in only 1.6–16.0% [11, 13, 16]. In any case, damage to the gracile and cuneate nuclei results in diminished tactile discrimination, as well as a proprioceptive and vibration sense. Lesion of the vestibular nuclei or the inferior cerebellar peduncle leads to vertigo, nystagmus, ataxia, and unsteadiness [11, 16, 29].
Neurosurgical significance
First of all, a diseased VA and its branches, or the adjacent pathologic processes, must be reached by corresponding surgical approaches. This can be done by certain transclival approaches, e.g. by various modifications of the transoral, transnasal, transsphenoidal, and anterior cervical routes [5, 8].
As for lateral approaches, transmastoid techniques can be applied, but rarely retrolabyrhintine and translabyrinthine, and often the retrosigmoid and the simple, mid-lateral, far-lateral, or extreme lateral approach, including occasionally the retrocondylar, transcondylar, and transtubercular routes [8, 24, 27].
Among the posterior routes, midline and paramedian suboccipital approach are often used [5, 8, 22, 30]. In this approach, neurosurgeons can access the dorsal and lateral medullary surface, PICA, and the 4th ventricle through the cerebellomedullary fissure and the intertonsillar route, without vermian splitting [8, 22, 28]. Finally, endoscopic surgery is also applied in some patients [4].
As regards the vascular pathology, an atherosclerotic stenosis, dissection, occlusion, or artery-to-artery embolism of the VA and some branches can appear [13, 14, 29], as noted in some of our patients. Stenting can be performed in many of these cases, as well as endarterectomy, angioplasty, or various bypass procedures [8, 20].
Aneurysms of the VA, PICA, ASA or PSA can also be present [17, 22, 24, 27, 31], whilst some of the mentioned vessels or their branches are occasionally the feeding arteries of certain arteriovenous malformations (AVM) or fistulas [8, 32]. The AVMs and aneurysms can be treated surgically, by clipping, trapping, and resection [8, 24, 27, 31], or by various methods of endovascular embolization [1, 12, 17, 22, 25].
Care must be taken during surgical and neuroradiologic interventions to preserve the orifices of the important VA medullary branches, and also the parent vessels themselves, in order to avoid medullary infarction occurrence [1, 3, 12, 25]. Neurosurgeons should have in mind the following facts. First, commonly just a few small-sized medullary anastomoses are present on the anterior and lateral surface in some specimens. It means that most of the anastomoses are not a fully reliable source of the collateral circulation in the case of a vascular occlusion. Second, the anastomoses are absent among the intramedullary twigs. Third, if a small anterior or anterolateral superficial medullary artery is damaged, only mild hemiparesis or monoparesis will appear [13]. However, when a common stem of the perforating arteries is damaged, hemiplegia and supranuclear paresis will occur. If a PerfA, ALA and LA common trunk is damaged, the resultant ischemia will also affect a part of the lateral territory, which will add hemihypesthesia to the mentioned symptomatology [10, 13, 29].
Accordingly, we have only one recommendation to neurosurgeons: a careful inspection of the operating field, the identification of such a common stem and other medullary vessels, an estimate of their relationship with the lesion to be operated on, and an attempt to avoid an iatrogenic injury of these vessels.
Conclusions
The medulla oblongata is supplied by the perforating, anterolateral, lateral, and dorsal branches, which are occasionally interconnected by anastomotic channels. Their morphometric characteristics and region of supply can explain the symptomatology of the medial, anterolateral, lateral, and dorsal medullary infarctions. They are also the basis for medullary branches preservation during surgical and endovascular interventions.
References
Aihara M, Naito I, Shimizu T, Matsumoto M, Asakura K, Miyamoto N, Yoshimoto Y (2018) Predictive factors of medullary infarction after endovascular internal trapping using coils for vertebral artery dissecting aneurysms. J Neurosurg 129:107–113
Akar ZC, Dujovny M, Slavin KV, Gomez-Tortosa E, Ausman JI (1994) Microsurgical anatomy of the intracranial part of the vertebral artery. Progr Neurosurg Neurol Neurosci 16(3):171–180
Ballesteros L, Forrero P, Quintero I (2013) Morphological expression of the anterior spinal artery and the intracranial segment of the vertebral artery: a direct anatomic study. Rom J Morphol Embryol 54(3):513–518
Bossi Todeschini A, Montaser AS, Hardesty DA, Carrau RL, Prevedello DM (2018) The limits of the endoscopic endonasal transclival approach for posterior fossa tumors. J Neuosurg Sci 62(3):322–311
de Oliveira E, Rhoton AL, Peace D (1985) Microsurgical anatomy of the region of the foramen magnum. Surg Neurol 24(3):293–352
Duvernoy HM (1978) Human brainstem vessels. Springer-Verlag, Berlin
Er U, Fraser K, Lanzino G (2008) The anterior spinal artery origin: a microanatomical study. Spinal Cord 46:45–49
Fosset DT, Caputy AJ (2002) Operative neurosurgical anatomy. Thieme New York, Stuttgart
Gaigalaite V, Vilimas A, Ozeraitiene V, Dementaviciene J, Janilionis R, Kalibatiene D, Rocka S (2016) Association between vertebral artery hypoplasia and posterior circulation stroke. BMC Neurol 16:118
Gökçal E, Baran G, Niftaliyev E, Guzel V, Asil T (2017) Risk factors, etiological classification, topographical location, and outcome in medullary infarctions. Neurologist 22(4):116–119
Heckmann JG, Lang CJG, Huk W, Neundorfer T (2003) Dorsal medullary infarction. Cerebrovasc Dis 16(2):176–177
Inamasu J, Guiot BH (2005) Iatrogenic vertebral artery injury. Acta Neurol Scand 112(6):349–357
Kim K, Lee HS, Jung YH, Kim YD, Nam HS, Nam CM, Kim SM et al (2012) Mechanism of medullary infarction based on arterial territory involvement. J Clin Neurol 8(2):116–122
Kim JS (2003) Pure lateral medullary infarction: Clinical-radiological correlation of 130 acute, consecutive patients. Brain 126(8):1864–1872
Kumral E, Afsar N, Kırbas D, Balkır K, Özdemirkıran T (2002) Spectrum of medial medullary infarction: clinical and magnetic resonance imaging findings. J Neurol 249:85–93
Lee SU, Park SH, Park JJ, Kim HJ, Jan MK, Bae HJ, Kim JS (2015) Dorsal medullary infarction. Distinct syndrome of isolated central vestibulopathy. Stroke 46(11):3081–3087
Lehto H, Niemelä M, Kivisaari R, Laakso A, Jahromi BR, Ferzat J, Hugo H et al (2015) Intracranial vertebral artery aneurysms: Clinical features and outcome of 190 patients. World Neurosurg 84(2):380–389
Lister JR, Rhoton AL, Matsushima T, Peace DA (1982) Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery 10(2):170–199
Marinković S, Milisavljević M, Gibo H, Maliković A, Djulejić V (2004) Microsurgical anatomy of the perforating branches of the vertebral artery. Surg Neurol 61:190–197
Mohamadiann R, Sharifpour E, Mansourizadeh R, Shrabi B, Nayebi AR, Harrian S (2013) Angioplasty and stenting of symptomatic vertebral artery stenosis clinical and angiographic follow-up of 206 cases from Northwest Iran. Neurol J 26(4):1–12
Santos-Franco J, de Oliveira E, Mercado R, Ortiz-Velazquez RI, Revuelta-Gutierres R, Gomez-Llata S (2006) Microsurgical considerations of the anterior spinal and the anterior-ventral spinal arteries. Acta Neurochir (Wien) 148:329–338
Singh R, Behari S, Kumar V, Jaiswal A, Jain V (2012) Posterior inferior cerebellar artery aneurysms: Anatomical variations and surgical strategies. Asian J Neurosurg 7(1):2–11
Songur A, Gonul Y, Ozen OA, Kucuker H, Uzun B, Bas O, Toktas M (2008) Variations in the intracranial vertebrobasilar system. Surg Radiol Anat 30:257–264
Sugiyama T, Mizutani T, Sumi K, Matsumoto M, Yabusaki H, Kusyamae M, Irie R et al (2016) Trapping of vertebral aneurysms using mid-lateral suboccipital approach, with emphasis on securing the distal end. Surg Cereb Stroke (Jpn) 44:461–468
Tanoue S, Endo H, Hiramatsu M, Matsumaru Y, Matsumoto Y, Sato K, Tsuruta W et al (2021) Delineability and anatomical variations of perforating arteries from normal vertebral artery on 3D DSA: implications for endovascular treatment of dissecting aneurysms. Neuroradiology 63:609–617
Tatu L, Moulin T, Bogousslavsky J, Duvernoy H (1996) Arterial territories of human brain: brainstem and cerebellum. Neurology 47:1125–1135
Tjahjadi M, Jahromi BR, Serrone J, Nurminen V, Choque-Velasquez J, Kivisaari R, Lehto H et al (2017) Simple lateral suboccipital approach and modification for vertebral artery aneurysms: A study of 52 cases over 10 years. World Neurosurg 108:336–346
Ucerler H, Saylam C, Cagli S, Orhan M (2008) The posterior inferior cerebellar artery and its branches in relation to the cerebellomedullary fissure. Clin Anat 21(2):119–126
Vlašković T, GeorgievskiBrkić B, Stević Z, Kostić D, Stanisavljević N, Marinković I, Vojvodić A et al (2022) Anatomic and MRI bases for medullary infarctions with patients’ presentation. J Stroke Cerebrovasc Dis 31(10):106730
Wang C, Cironi K, Mathkour M, Lockwood J, Aysenne A, Iwanaga J, Loukas M (2021) Anatomical study of the posterior spinal artery branches to the medulla oblongata. World Neurosurg 149:e1098–e1104
Yasargil MG (1984) Microneurosurgery, vol I. Georg Thieme Verlag Stuttgart, New York
Yasargil MG (1987) Microneurosurgery, vol IIIA. Georg Thieme Verlag Stuttgart, New York
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Research involving human participants
All procedures performed in this study involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Grants
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Comments
This is a very interesting study that showed clinical and imagenological correlation of anatomical findings in the compromise of the medulla oblongata arterial irrigation. The authors used specimen injection to show the different and more frequent patterns of the irrigation of this portion of the brainstem. Moreover, they correlate the MRI findings in a small clinical series of patients with medullary infarctions with the anatomical data obtained in the study. This data and the very clear figures of the paper are very useful in order to understand the more frequent ischemic findings in the medulla oblongata imaging in the specific occlusion of the vessels in this vital region.
Jorge Mura
Providencia RM
Chile
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Djukić, B., Djukić-Macut, N., Djulejić, V. et al. Medullary branches of the vertebral artery: microsurgical anatomy and clinical significance. Acta Neurochir 165, 1807–1819 (2023). https://doi.org/10.1007/s00701-023-05613-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05613-7