Abstract
Background
Aneurysmal rebleed is the most dreaded complication following subarachnoid hemorrhage. Being a cause of devastating outcome, the stratification of risk factors can be used to prioritize patients, especially at high volume centers.
Method
A total of 99 patients with aneurysmal rebleed were analyzed in this study both prospectively and retrospectively from August 2010 to July 2014. In the control group, 100 patients were selected randomly from the patient registry. A total of 25 variables from the demographic, historical, clinical and radiological data were compared and analyzed by univariate and multivariate logistic regression analysis.
Results
Significant independent predictors of aneurysm rebleed were the presence of known hypertension (p = 0.023), diastolic blood pressure of >90 mmHg on admission (p = 0.008); presence of loss of consciousness (p = 0.013) or seizures (p = 0.002) at first ictus; history of warning headaches (p = 0.005); higher Fisher grade (p < 0.001); presence of multiple aneurysms (p = 0.021); irregular aneurysm surface (0.002).
Conclusions
Identification of high risk factors can help in stratifying patients in the high risk group. The risk stratification strategy with early intervention can prevent rebleeds. This in turn may translate into better outcomes of patients with intracranial aneurysms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysm is a common intracranial pathology, and the incidence reported in autopsy studies varies from 0.2 % to 7.9 % (with the mean frequency being 5 %) [1]. General population-based studies have shown the incidence of aneurysms from 6 to 21.6 cases per 1,00,000 persons per year [2–4]. Intracranial aneurysms are the most common cause of spontaneous SAH accounting for 75 % to 80 % of such cases. Although the most common clinical aneurysm presentation is subarachnoid hemorrhage (SAH), intraparenchymal hematoma, intraventricular hemorrhage and, less commonly, acute subdural hematoma are also other radiological features of an intracranial aneurysm rupture. Clinical deficits due to mass effect, although less frequent, may also be a presenting feature of an aneurysm irrespective of rupture.
The aneurysmal subarachnoid rebleed is the most common complication of aneurysmal SAH. It is a major cause of morbidity and mortality with devastating outcome. The reported mortality is very high, ranging from 50 %–80 % [5]. A third of survivors have moderate to severe disabilities.
The aim of all the interventions meant for treatment of ruptured aneurysms is to prevent rebleeding. The incidence of rebleed is highest in the first 2 weeks with the maximum being within the first 24 h following the first bleed [6–9]. Therefore, early intervention is the only means to prevent rebleeding. It is very important to know the risk factors, the presence of which can predict the likelihood of rebleeding. These predictors may be useful to stratify patients as high risk and subject them to the earliest possible intervention.
Since the era of Sir Walter Dandy, who was the first person to clip an intracranial aneurysm in 1937, there has been significant improvement in the knowledge about intracranial aneurysms, their diagnosis and management strategies. Early diagnosis, better critical care treatment and emergent management with modern surgical as well as endovascular modalities have definitely improved the outcome. Still, factors such as vasospasm and rebleeding are significant causes of morbidity and mortality. The understanding of these factors is still unclear, which needs to be addressed. If high risk factors for aneurysmal subarachnoid rebleeding are known, stratification of patients to high and low risk may be possible, and thus the prioritized approach can be translated into better overall patient outcome.
Materials and methods
Patients with aneurysmal subarachnoid hemorrhage were selected retrospectively from August 2010 as well as prospectively up to July 2014. A picture archival communication system (PACS) was used to review the computerized tomography (CT) scans, magnetic resonance (MR) images and digital subtraction angiograms (DSAs). All patients with rebleeding were selected as cases who fulfilled all inclusion criteria.
Inclusion criteria
The inclusion criteria were:
-
(1)
The first SAH as well as rebleeding was confirmed by CT scan, MR imaging or lumbar puncture (LP).
-
(2)
The rebleed should only be attributable to a ruptured aneurysm.
-
(3)
Patients should not have undergone any surgical or endovascular intervention before the rebleed.
Exclusion criteria
-
(1)
Patients who experienced rebleeding following or during surgery or endovascular intervention for a diagnosed aneurysm were excluded from the study.
-
(2)
Patients who did not undergo definitive aneurysm treatment were not included in the study.
The inclusion and exclusion criteria of the randomly selected control group of 100 patients from a total of 790 patients were as follows:
Inclusion criteria
-
(1)
The SAH was confirmed by imaging (i.e., CT scan or MRI) or diagnostic lumbar puncture (LP).
-
(2)
The intracranial bleed should only be due to an aneurysm.
-
(3)
There should be only one episode of objectively proven aneurysmal rupture.
Exclusion criteria
-
(1)
Patients who were on antiplatelet drugs or anticoagulants for any reason were excluded from the study.
-
(2)
Patients who did not undergo definitive aneurysm treatment were not included in the study.
Statistical analysis
A total of 25 variables were studied, which included demographic, historical, clinical and radiological data. Each variable was analyzed by univariate analysis using Student’s t-test and the chi-square test. Also all the risk factors were analyzed by multivariate analysis using logistic regression to derive significant risk factors. A double-tailed P value of <0.05 was considered as a threshold for statistical significance.
The following data were collected: demographic profile (age, sex, presence of hypertension and/or diabetes, smoking, alcohol intake, tobacco chewing, family history); clinical profile [systolic and diastolic blood pressure on presentation, history of loss of consciousness, seizure or warning headaches, World Federation of Neurological Surgeons (WFNS) grade on presentation]; radiological profile (Fisher grade of SAH on presentation and the aneurysm number, location in the anterior or posterior circulation, height, size of dome and neck, aspect ratio, bottleneck factor and height:width ratio, and wall surface irregularity) and external ventricular drainage placement.
Results
In our institute, a total of 943 patients with aneurysmal subarachnoid hemorrhage were managed during the study period. Of these, 889 (94.27 %) patients were managed by aneurysm clipping or coiling. The rest of the 54 patients were not managed surgically or by endovascular coiling because of either the very poor grade or denial of the patient or relatives. These patients were not included in the study. Of 889 patients, a total of 99 (11.13 %) had aneurysmal rebleeds. Of these, 36 (4.04 % of total SAHs and 36.37 % of all patients with rebleeding) had in-hospital bleeds while awaiting surgery, and the remaining 63 (7.08 % of the total SAHs and 63.63 % of all patients with rebleeding) patients had re-bled before admission either during the stay at another referral center or during transportation.
In the rebleed group, 86 patients had undergone CT angiography or digital subtraction angiography (DSA) to determine the number of aneurysms. The rest of the 13 patients had clear CT evidence of aneurysmal rebleeding but, due to the unavailability of CT angiography or DSA, the presence and number of aneurysms could not be noted. In the rebleed group, 19 (22.1 %) out of 86 had multiple aneurysms as compared to 8 (8 %) patients in the control group.
An external ventricular drain (EVD) was placed in patients with symptomatic hydrocephalus or intraventricular hemorrhage with a depressed level of consciousness before aneurysm clipping or endovascular coiling.
Presentation to the hospital after the first bleed and time to treat
Because ours is a tertiary referral center, many patients presented after 3 days of initial bleeding. Thirty (30.3 %) patients out of 99 presented to our hospital within 72 h of the first bleed. Of those, 17 (17.17 %) patients were admitted within 24 h of ictus, while 26 (26.26 %) were admitted within 48 h of the first bleed. Of all the patients, 36 (36.37 %) had in-hospital rebleeding, while the rest had rebleeding before presentation to the hospital. Of 99 patients, 35 (35.36 %) underwent definitive intervention within 24 h, while 58 (58.59 %) were managed within 48 h, and 6 died before any intervention. The average time to treat patients in the rebleed group was 44.23 h after admission. In the control group, 41 (41 %) patients were treated within 24 h and 47 (47 %) in 24 to 48 h, while 12 (12 %) were treated in 48 to 72 h. The average time to treat in this group was 51.18 h. Therefore, the difference in time to treat in both groups was not statistically significant (p = 0.0831).
Interval between the first bleed and rebleed
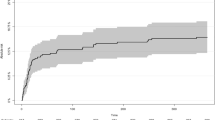
It was found that 61 (61.62 %) patients re-bled within the first 2 weeks of the initial bleed, and of those, nearly half, i.e., 32 (32.32 %) patients, re-bled within the first 72 h. The histogram of the rebleed interval shows the declining trend with the passage of time (Fig. 1).
These factors were evaluated individually in the early rebleed (rebleed <72 h) and late rebleed (rebleed after 72 h) groups. Four factors, i.e., diastolic blood pressure of >90 mmHg (p = 0.0471), presence of a history of LOC (p = 0.049), higher Fisher grade (p = 0.0289) and aneurysmal surface irregularity (p = 0.035), were statistically significant. Therefore, these factors, apart from predicting a high risk of rebleeding, also portend the possibility of early rebleeding.
Univariate and multivariate analysis
On univariate analysis (Table 1), alcohol intake, tobacco chewing, smoking, systolic BP >160 mmHg, diastolic BP >90 mmHg, presence of LOC, warning headache, higher Fisher grade, multiple aneurysms, height and dome of aneurysm >8.5 mm, bottleneck factor >2, presence of surface irregularity and EVD placement were significant factors associated with aneurysmal rebleeding. All these factors were subjected to multivariate linear logistic regression analysis.
The multivariate logistic regression analysis (Table 2) indicated that presence of a history of hypertension, diastolic blood pressure >90 mmHg, presence of loss of consciousness and/or seizures at first bleed, history of warning headaches, higher Fisher’s grade, presence of multiple aneurysms and aneurysm wall surface irregularity are independent risk factors for rebleeding (p < 0.05).
Discussion
Several authors have attempted to identify the risk factors for aneurysmal rebleeding, which may help to prioritize these patients for early interventions, especially at high volume centers. Because our hospital is a tertiary referrel center, many patients are referred from other centers after diagnosis and initial management. We treated 889 patients during the study period. The incidence of rebleeding at our institute was 11.13 % (99 out of 889). Previous studies have reported the incidence of rebleeding from 17.3 % to 19.9 % [10–12], but recent studies show lower incidences ranging from 4 % to 10 % due to improvement in treatment strategies and early intervention protocols [13–15]. A meta-analysis of seven studies showed an average incidence of 11.3 % [16]. The incidence of rebleeding in our study is similar to these results. The actual “in-hospital” rebleeding incidence was 4.04 % in our study. This calls for emphasis on the need for referring the patients early and managing them at a tertiary center after the first ictus.
The maximum incidence of rebleeding was within first 2 weeks in our study. Of 99 patients, 62 (62.63 %) had bled within the 2 weeks following the first bleed, of which 31 (31.31 %) patients bled in the first 72 h. Although there is no clear consensus regarding the period of highest risk for rebleeding, it occurs more frequently in the earlier period after the initial SAH. Several reports have shown the peak incidence of rebleeding on the same day within hours of the first bleed [17–20], while on the contrary some have shown that the peak of rebleeding is not on the day of the original hemorrhage but at the end of 1st week and the beginning of the 2nd week [21–24]. It can be inferred from our study that about a third of rebleeds occur within 72 h of the first bleed. The maximum risk of rebleeding is in the initial 14 days after the ictus with almost 2/3 (61.62 %) of the rebleeds occurring within this time frame.
We analyzed various risk factors that may predispose patients to a higher risk of rebleeding. We found the systolic blood pressure of >160 mmHg and diastolic blood pressure of >90 mmHg on admission as independent risk factors on univariate analysis. But on multivariate analysis only diastolic blood pressure was found to be significant. We propose that the diastolic blood pressure is an indirect measure of mean arterial pressure (MAP), which is the continuous minimum stress present on the aneurysm. Higher and persistent stress on the aneurysm weakens the wall, which predisposes to a higher risk of rupture. A number of studies have shown that the presence of systolic and diastolic hypertension indicates a higher risk of hemorrhagic and ischemic stroke. They have shown that lowering the diastolic blood pressure reduces the risk of stroke by 29 % to 60 % [25–30]. Therefore, equal emphasis should be given to treating high diastolic blood pressure.
Loss of consciousness (LOC), seizures and a warning headache at the first bleed were found to be risk factors in our study. Of these, LOC and seizures have not been studied adequately so far. In our study, loss of consciousness was defined as loss of awareness of the surroundings or an altered state of consciousness that lasted for a few minutes to hours, following which the patient again regained consciousness as reported by the patient or patient’s relatives at the time of ictus. The average time of LOC in our study was 28.62 min. We found 7.27-fold odds of rebleeding in the presence of a warning headache, 3.71-fold odds for LOC and 10-fold odds for seizures. It can be presumed that these symptoms may be associated with higher grades of SAH. On analyzing the scan findings, which were done at the time of first bleed, we found that all 25 patients with seizures and 51 of 52 with LOC had a higher Fisher SAH grade of III or IV. Several studies have found an increased risk of rebleeding by 2.3 to 10 fold in patients with a history of warning headaches [4, 10, 14, 31]. A higher Fisher grade has been uniformly found to be a significant risk factor for aneurysmal rebleeding in most of the studies, which we also found in our study [32–34].
Irregularity of the wall or surface of the aneurysm was a significant predictor of rebleeding in our study with a 4.5-times increased risk of rebleeding. No studies have considered this particular factor in relation to the risk of rebleeding. The presence of blebs has been frequently found in cases of ruptured aneurysms, which itself predicts a high risk of rebleeding. Apart from this, multilobed aneurysms or the presence of multiple surface irregularities is an indicator of a high risk of re-rupture. The irregular surface may indicate the weakest part of the aneurysm wall that is about to rupture. In bi- or multilobed aneurysms, the increased turbulence of flowing blood and altered hemodynamics may also contribute to the rupture.
There has been a difference of opinion regarding the location of aneurysms and risk of rebleeding. Some studies have reported the location of the aneurysm in the anterior circulation, especially the anterior and posterior communicating arteries, as a risk factor [13, 15, 35]. On the contrary, Cong et al. showed in their study that patients with a posterior circulation aneurysm are at higher risk of rebleeding [32]. We did not find the location of the aneurysm in the anterior or posterior circulation as a risk factor for rebleeding.
Although in our study we found the presence of multiple aneurysms as a risk factor, interestingly, of 19 patients with multiple aneurysms, all had rebleeding from the same aneurysm except one, who had bleeding from a different one. Therefore, we believe that the presence of multiple aneurysms only suggests the inherent weakness of cerebral blood vessels, which predisposes to them to rebleed from the same aneurysm rather than from another one. Beck et al. [36] showed the presence of multiple aneurysms as a risk factor for rebleeding, while Cong et al.[32] did not find any correlation. In our study, in multivariate analysis we did not find any correlation of various parameters of aneurysm size with rebleeding. The association of aneurysm size with rebleeding is not a consistent finding in all studies. Some have found aneurysm size >10 mm or increasing size of the aneurysm on imaging as a risk factor for rebleeding [14, 31, 37]. Guo et al. showed that the risk of rebleeding in patients with aneurysms >10 mm was 1.624-fold greater than those with aneurysms of 10 mm or less [37]. However, some of the studies have not found such a correlation [10, 38]. Most of the studies have taken the maximum aneurysm diameter into account, but this measurement can be arbitrary. Maximum diameter can be of the height, dome or sometimes neck. Therefore, more studies are required to consider the specific size parameters such as the height, dome or neck.
The placement of external ventricular drainage was not found to be a risk factor in our study. Several studies have reported a higher risk of rebleeding following placement of EVD [14, 39, 40]. Pare et al. suggested that CSF drainage in patients with unsecured, recently ruptured aneurysms may increase the transmural pressure across the aneurysm wall, and this may lead to increased likelihood of re-bleeding [41]. Some of the studies have not found such an association [36]. The presence of intraventricular bleeding (i.e., a higher Fisher grade), hydrocephalus and poor WFNS grade usually leads to EVD placement in emergency situations. In the presence of these risk factors, the contribution of EVD to aneurysmal rebleeding per se should be evaluated carefully.
In our study, we did not find patient age of patient to be a significant determining factor for rebleeding. Several studies have reported older age, especially age >70 years, to be a risk factor with almost four times increased risk [9, 42], while others have not [14, 43]. Similarly, sex also did not have any impact on rebleeding in our study. The documentation of a predisposition of females to rebleeding was found in several studies [6, 20, 21], which can be attributed to higher overall incidence of aneurysms in females.
Conclusion
Aneurysmal subarachnoid rebleeding is a devastating complication of a first aneurysmal bleed with significant imminent morbidity and mortality. According to the present study, the presence of a history of hypertension, diastolic blood pressure >90 mmHg on admission the presence of loss of consciousness or seizures at first ictus, history of warning headaches, higher Fisher grade, presence of multiple aneurysms and irregular aneurysm surfaces are significant independent predictors of aneurysmal rebleeding. Of these, high diastolic blood pressure, history of LOC, higher Fisher’s grade and aneurysm surface irregularity also portend early (within 72 h) rebleeding. Identification of these high risk factors can help in stratifying the patients in the high risk group. The risk stratification strategy with early intervention can prevent rebleeding. This in turn may translate into better outcomes for aneurysm patients.
References
McCormick WF, Nofzinger JD (1965) Saccular intracranial aneurysms: an autopsy study. J Neurosurg 22:155–159
Broderick JP, Brott T, Tomsick T, Miller R, Huster G (1993) Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg 78:188–191
Ingall T, Asplund K, Mahonen M, Bonita R (2000) A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke; J Cereb Circ 31:1054–1061
Linn FH, Rinkel GJ, Algra A, van Gijn J (1996) Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta-analysis. Stroke; J Cereb Circ 27:625–629
Greenberg M (2006) Handbook of neurosurgery. Thieme, New York
Graf CJ (1971) Prognosis for patients with nonsurgically-treated aneurysms. Analysis of the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. J Neurosurg 35:438–443
Hijdra A, Vermeulen M, van Gijn J, van Crevel H (1987) Rerupture of intracranial aneurysms: a clinicoanatomic study. J Neurosurg 67:29–33
Locksley HB, Sahs AL, Sandler R (1966) Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. 3. Subarachnoid hemorrhage unrelated to intracranial aneurysm and A-V malformation. A study of associated diseases and prognosis. J Neurosurg 24:1034–1056
Steiger HJ, Fritschi J, Seiler RW (1994) Current pattern of in-hospital aneurysmal rebleeds. Analysis of a series treated with individually timed surgery and intravenous nimodipine. Acta Neurochir 127:21–26
Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Koike T, Tanaka R (1996) Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. J Neurosurg 84:35–42
Herrick IA, Gelb AW (1992) Anesthesia for intracranial aneurysm surgery. J Clin Anesth 4:73–85
Sakaki T, Morimoto T, Hoshida T, Kawaguchi S, Nakase H, Fukuzumi A (1999) Rebleeding during transport of patients with a ruptured intracranial aneurysm. J Stroke Cerebrovasc Dis: Off J Nat Stroke Assoc 8:38–41
Beck J, Raabe A, Szelenyi A, Berkefeld J, Gerlach R, Setzer M, Seifert V (2006) Sentinel headache and the risk of rebleeding after aneurysmal subarachnoid hemorrhage. Stroke; J Cereb Circ 37:2733–2737
Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, Commichau C, Connolly ES, Mayer SA (2005) Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol 62:410–416
Tanno Y, Homma M, Oinuma M, Kodama N, Ymamoto T (2007) Rebleeding from ruptured intracranial aneurysms in North Eastern Province of Japan. A cooperative study. J Neurolog Sci 258:11–16
Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA (2014) Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg
Biller J, Godersky JC, Adams HP Jr (1988) Management of aneurysmal subarachnoid hemorrhage. Stroke; J Cereb Circ 19:1300–1305
Jane JA, Kassell NF, Torner JC, Winn HR (1985) The natural history of aneurysms and arteriovenous malformations. J Neurosurg 62:321–323
Juvela S (1989) Rebleeding from ruptured intracranial aneurysms. Surg Neurol 32:323–326
Kassell NF, Torner JC (1983) Aneurysmal rebleeding: a preliminary report from the Cooperative Aneurysm Study. Neurosurgery 13:479–481
Locksley HB (1966) Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. J Neurosurg 25:321–368
Pakarinen S (1967) Incidence, aetiology, and prognosis of primary subarachnoid haemorrhage. A study based on 589 cases diagnosed in a defined urban population during a defined period. Acta Neurol Scand 43(29):21–28
Rosenorn J, Eskesen V, Schmidt K, Ronde F (1987) The risk of rebleeding from ruptured intracranial aneurysms. J Neurosurg 67:329–332
Vermeulen M, van Gijn J, Hijdra A, van Crevel H (1984) Causes of acute deterioration in patients with a ruptured intracranial aneurysm. A prospective study with serial CT scanning. J Neurosurg 60:935–939
Arima H, Anderson C, Omae T, Woodward M, Hata J, Murakami Y, Macmahon S, Neal B, Chalmers J, Group PC (2011) Effects of blood pressure lowering on major vascular events among patients with isolated diastolic hypertension: the perindopril protection against recurrent stroke study (PROGRESS) trial. Stroke; J Cereb Circ 42:2339–2341
Arima H, Chalmers J (2011) PROGRESS: prevention of recurrent stroke. J Clin Hypertens 13:693–702
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ, Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C (2003) Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 42:1206–1252
Fang XH, Zhang XH, Yang QD, Dai XY, Su FZ, Rao ML, Wu SP, Du XL, Wang WZ, Li SC (2006) Subtype hypertension and risk of stroke in middle-aged and older Chinese: a 10-year follow-up study. Stroke; J Cereb Circ 37:38–43
Kelly TN, Gu D, Chen J, Huang JF, Chen JC, Duan X, Wu X, Yau CL, Whelton PK, He J (2008) Hypertension subtype and risk of cardiovascular disease in Chinese adults. Circulation 118:1558–1566
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B, Management of Arterial Hypertension of the European Society of H, European Society of C (2007) 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of hypertension (ESH) and of the european society of cardiology (ESC). J Hypertens 25:1105–1187
Starke RM, Connolly ES Jr, Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid H (2011) Rebleeding after aneurysmal subarachnoid hemorrhage. Neurocritical Care 15:241–246
Cong W, Zhongxin Z, Tiangui L, Zhang Y, Min H, Chao Y (2012) Risk factors for rebleeding of aneurysmal subarachnoid hemorrhage based on the analysis of on-admission information. Turkish Neurosurg 22:675–681
Ohkuma H, Tsurutani H, Suzuki S (2001) Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke; J Cereb Circ 32:1176–1180
Reynolds ASC (1980) Bleeding Patterns from ruptured intracranial aneurysms: an autopsy series of 205 patients. Surg Neurol 15:4
Nibbelink DW, Torner JC, Henderson WG (1975) Intracranial aneurysms and subarachnoid hemorrhage. A cooperative study Antifibrinolytic therapy in recent onset subarachnoid hemorrhage. Stroke; J Cereb Circ 6:622–629
Beck J, Rohde S, Berkefeld J, Seifert V, Raabe A (2006) Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg Neurol 65:18–25, discussion 25-17
Guo LM, Zhou HY, Xu JW, Wang Y, Qiu YM, Jiang JY (2011) Risk factors related to aneurysmal rebleeding. World Neurosurg 76:292–298, discussion 253-294
Cha KC, Kim JH, Kang HI, Moon BG, Lee SJ, Kim JS (2010) Aneurysmal rebleeding : factors associated with clinical outcome in the rebleeding patients. J Korean Neurosurg Soc 47:119–123
Mehta V, Holness RO, Connolly K, Walling S, Hall R (1996) Acute hydrocephalus following aneurysmal subarachnoid hemorrhage. The Canadian journal of neurological sciences. J Can Sci Neurol 23:40–45
Rajshekhar V, Harbaugh RE (1992) Results of routine ventriculostomy with external ventricular drainage for acute hydrocephalus following subarachnoid haemorrhage. Acta Neurochir 115:8–14
Pare L, Delfino R, Leblanc R (1992) The relationship of ventricular drainage to aneurysmal rebleeding. J Neurosurg 76:422–427
Locksley HB (1966) Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg 25:219–239
Lanzino G, Kassell NF, Germanson TP, Kongable GL, Truskowski LL, Torner JC, Jane JA (1996) Age and outcome after aneurysmal subarachnoid hemorrhage: why do older patients fare worse? J Neurosurg 85:410–418
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patient-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of NIMHANS (National Institute of Mental health and Neuro Sciences, Bengaluru, Karnataka, India) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Additional information
Comment
Spontaneous subarachnoid hemorrhage (SAH), which is most frequently due to aneurysmal rupture, is a significant source of morbidity and mortality. However, the continued evolution of both open and endovascular treatment modalities are actively improving the expected outcome of this disease process. As the authors point out, though, aneurysmal rebleed prior to treatment is both largely unpredictable and clinically devastating to the patient. On both retrospective and prospective review of their own patient series, the authors attempt to identify the risk factors that portend a higher likelihood of rebleed before a patient can receive treatment. By examining a variety of demographic, comorbid and radiographic characteristics, the authors are able to identify clinical variables that reliably identify those patients at a higher risk of early rebleed. They further posit that following these clinical variables in practice may allow for more urgent treatment of high-risk cases and therefore further improve patient outcomes.
The authors make a valiant attempt to more thoroughly delineate the risk factors that predispose patients to this devastating complication of aneurysmal SAH. While the authors attempt to clarify which patients should be targeted to early therapy, the interval at which treatment should be instituted remains ambiguous. It is well documented in the literature that the highest risk of aneurysmal rebleed is in the first 24 h after initial hemorrhage (1–3). The patients in this series vary widely in their times from initial bleeding event to presentation, transfer and eventual treatment (24 to 72 h). Therefore, it would be of more clinical relevance to explore these intervals scrupulously in the context of the risk factors discussed. If patients with risk factors that predispose them to rebleed are also at risk of earlier rebleed, this assists in designing treatment algorithms. Without this analysis, the presence of risk factors is identified and patients are placed in a ‘high risk’ class, but not treated differently from any other aneurysmal rupture. This timing of therapy is the crucial variable that has the most potential impact on patient outcome.
Previous works have attempted to delineate the risk factors for both aneurysmal rupture and rebleed (2, 4, 5). The authors admit as much and compare their sometimes contradictory results with previous work. While this is meaningful to some extent and helps to better define the ‘high-risk’ group, it takes the focus off of the proposed novel factors the authors suggest. The authors do their best, in the discussion, to spend less time focusing on previously documented risks and instead examining if their factors of interest (diastolic hypertension, seizure, LOC, warning headache, etc.) have an additive effect with known risks or impart a higher clinical risk.
The data presented highlight several interesting points regarding the discord between the existing data and the management of SAH. The authors and others cited focus on the highest risk of rebleed being in the first 24 h. The authors then point out that a minority of patients even arrive at their tertiary care center within 24 h of hemorrhage (<20 %), and few of these patients are treated in this time period (35 %). The data presented herein and the current treatment data call for change in the way SAH patients are prioritized, transferred to properly equipped facilities and treated promptly.
The authors are to be commended for their careful, diligent examination of the risk factors that increase the likelihood of aneurysmal rebleed prior to treatment. As we acquire insight into aneurysmal rebleed, its risk factors and the appropriate timing of definitive treatment, this paper holds the potential to serve as a framework to utilize for patient prioritization.
References
1. Pare L, Delfino R, Leblanc R. The relationship of ventricular drainage to aneurysmal rebleeding. Journal of Neurosurgery. 1992;76(3):422–7.
2. Kaptain GJ, Lanzino G, Kassell NF. Subarachnoid haemorrhage: epidemiology, risk factors, and treatment options. Drugs Aging. 2000;17(3):183–99.
3. Robbert M, Hoogmoed J, van Straaten HA, Coert BA, Peter Vandertop W, Verbaan D. Time intervals from aneurysmal subarachnoid hemorrhage to treatment and factors contributing to delay. J Neurol. 2014;261(3):473–9.
4. Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. Journal of Neurosurgery. 2002;97(4):771–8.
5. Juvela S. Rebleeding from ruptured intracranial aneurysms. Surg Neurol. 1989;32(5):323–6.
Drew Spencer, Christopher M. Loftus
Illinois, USA
Rights and permissions
About this article
Cite this article
Solanki, C., Pandey, P. & Rao, K.V.L.N. Predictors of aneurysmal rebleed before definitive surgical or endovascular management. Acta Neurochir 158, 1037–1044 (2016). https://doi.org/10.1007/s00701-016-2784-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2784-6