Abstract
Introduction
Dehydroepiandrosterone sulfate (DHEA(S)) is a multi-functional steroid implicated in a broad range of biological effects, including obesity, diabetes, bone metabolism, neuroprotection, and anti-tumorigenesis. It has not yet undergone wider research in the context of Cushing’s disease. The objective of this study was to determine if perioperative blood levels of DHEA(S) correlate with levels of ACTH and cortisol, and therefore may be useful as a new, additional marker for the early definition of cure in patients suffering from Cushing’s disease.
Methods
Forty-two consecutive patients undergoing transsphenoidal surgery for Cushing’s disease from September 2009 to September 2010 were perioperatively monitored for ACTH, cortisol, and DHEA(S).
Results
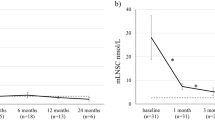
Pre-operative blood samples revealed ACTH levels of median 65 ng/l (range 11–1,183 ng/l, standard deviation 183.76), cortisol of median 257 μg/l (range 93–803 μg/l, standard deviation 140.88), and DHEA(S) of median 2.22 mg/l (range 0.44–7.79 mg/l, standard deviation 1.82) according to the pathology of Cushing’s disease. Postoperative blood samples drawn over a 7-day time span showed a drop in median ACTH to just 14.5 % (median: 9 ng/l, range 2–44, standard deviation 12.75) of its median preoperative figure. Median cortisol levels were reduced to 6.9 % (median: 18 μg/l, range 10–190 μg/l, standard deviation 38.04) of their preoperative values and DHEA(S) levels decreased to 17 % (median: 0.38 mg/l, range 0.05–2.29, standard deviation 0.51). In persistent disease, no patient showed a drop in DHEA(S) below 38 % of its preoperative figures.
Conclusions
DHEA(S) shows the potential to become an additional marker in the diagnosis and follow-up of patients. However, it needs to be examined further, including whether DHEA(S) may also be a useful predictor of recovery of the HPA-axis after successful surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the development of high-resolution MRI, intraoperative microscopy/endoscopy, and intraoperative imaging techniques like ultrasound and MRI, the treatment of minute microadenomas or invasive adenomas in Cushing’s disease remains challenging regarding cure. Transsphenoidal surgery for Cushing’s disease is the therapy of choice [6], with healing rates in microadenomas of up to 95 %. Early determination of cure after surgical treatment is of great importance, but may be challenging to prove. Different postoperative metrics have been published to define cure, e.g., the measurement of early postoperative cortisol [2] and ACTH [14, 18], as well as a low-dose dexamethasone test. In most patients, a period of secondary hypocortisolism follows successful surgical treatment [3, 19].
It has been the author’s policy for decades to establish biochemical cure by early postoperative measurement of ACTH and cortisol prior to hydrocortisone substitution [13, 14]. Remission is assumed if morning cortisol levels reach subnormal levels; however, in a minority of patients, normocortisolism is reached without the need for hydrocortisone substitution. In these patients, a differentiation from persistent disease may be difficult and therefore close follow-up is needed. Recurrent disease is common, with rates from 5 to 19 % being published [3, 17]. Esposito and coworkers published that ACTH is a somewhat unreliable predictor of remission, as only up to 30 % of patients who go into sustained remission show subnormal levels of ACTH [2]. Contrary to these results, we reported that different early postoperative ACTH levels correlate with different cure and recurrence rates [3]. Similar results have been published subsequently. In cases of unsuccessful initial surgery, one may consider early re-operation with remission rates of up to 67 % [10, 12, 15].
DHEA, the most common adrenal steroid in the human body, is ACTH-dependent and therefore its serum levels follow a circadian rhythm. DHEA has a half-life of approximately 25 min and similar to cortisol peaks in the morning following a diurnal rhythm while DHEA(S) has a half-life of 10 h and therefore its levels are mostly stable throughout the day. DHEA(S) levels reach their maximum at the age of 25 years and decline to 15–20 % of their maximum level by the age of 70 years [1, 8]. Kleiber et al. [9] reported that after transsphenoidal surgery for Cushing’s disease, DHEA levels dropped and remained low during the follow-up period. In this study, we investigated a possible correlation of peri-operative ACTH, cortisol, and DHEA(S) levels, and the feasibility of DHEA(S) as an additional marker for successful surgery for Cushing’s disease.
Patients and methods
Levels of ACTH, cortisol, and DHEA(S) in 42 consecutive patients suffering from Cushing’s disease were perioperatively monitored and prospectively analyzed. Blood samples were analyzed via chemiluminescence. All patients were admitted for transsphenoidal surgery after pituitary-dependent hypercortisolism was proven. The average age at surgery was 42 years, with a range from 11 to 73 years, and a sex ratio of ten males to 32 females. MRI findings were classified according to radiological standards as macroadenoma (n = 4), MRI-positive microadenoma (n = 27), and uncertain or negative MRI (n = 11). Nine tumors showed radiographic signs of invasive growth into the surrounding structures of the cavernous sinus.
Eight of 42 (19 %) patients suffered from recurrent disease and had been surgically treated at least once before; seven of these patients were treated via transsphenoidal surgery in the author’s department, one patient had undergone transcranial surgery in another department 7 years prior to treatment at the author’s facility.
All 42 patients underwent transsphenoidal microscopic surgery and were pre- and postoperatively monitored for levels of ACTH, cortisol, and DHEA(S). Preoperative samples were drawn the day before the surgery, and postoperative blood checks were done on the first postoperative day and between days 4 and 6 postoperatively (prior to discharge).
Statistical analysis
Descriptive data are presented as median and quartiles. Wilcoxon signed-rank tests were performed to evaluate changes between pre- and post-operative figures. Spearman’s rank correlations were computed on the relative changes to relate the responses of the three markers. A value of p < 0.05 was considered significant. R 2.14 [16] was used for calculations.
Results
Overall
Pre-operative blood samples (n = 42) were taken the day before surgery and revealed ACTH levels of median 65 ng/l (range 11–1,182 ng/l), cortisol of median 258 μg/l (range 93–803 μg/l), and DHEA(S) of median 2.23 mg/l (range 0.44–7.79 mg/l) according to the pathology of Cushing’s disease. Postoperative blood checks showed decreased median levels of ACTH down to 9 ng/l (range 2–44 ng/l). Cortisol levels were reduced to a median of 18 μg/l (range 10–190 μg/l) and DHEA(S) levels decreased down to a median of 0.38 mg/l (range 0.1–2.29 mg/l) (Fig. 1 and Table 2).
The degree of resection was intraoperatively classified as complete in 35 cases, as uncertain in six cases, and as partial resection in one case. Histology confirmed the clinical diagnosis in all 42 cases.
Tumor size
Four tumors were classified as macroadenomas; median preoperative levels of ACTH were 50.5 ng/l, median cortisol levels were 172.5 μg/l, and DHEA(S) reached a median of 1.21 mg/l. Median postoperative levels were 8 ng/l for ACTH, 43.5 μg/l for cortisol, and 0.48 mg/l for DHEA(S). All four patients showed postoperative early morning cortisol levels of less than 100 μg/l, thus fulfilling the criteria for early remission and the need for hydrocortisone substitution.
Twenty-seven lesions were classified as MRI-positive microadenoma not exceeding 9 mm in any diameter. Preoperative blood tests revealed median ACTH levels of 75.5 ng/l, cortisol levels of 275 μg/l, and DHEA(S) reached a median of 1.85 mg/l. Median postoperative levels were 12 ng/l ACTH, 18 μg/l cortisol, and 0.355 mg/l DHEA(S). One of these patients did not go into early remission, as early morning cortisol levels did not decline to 100 μg/l or less (early morning cortisol 139 μg/l, ACTH 20 ng/l and DHEA(S) of 0.51 mg/l). Further evaluation confirmed persistent disease. This patient is currently undergoing radiotherapy of the left cavernous sinus, as pre-operative and follow-up MRI revealed invasive growth of the tumor into this region.
Eleven patients had to be classified as MRI-negative. Preoperative blood tests revealed elevated median levels of ACTH of 48 ng/l, cortisol 241 μg/l, and DHEA(S) 3.04 mg/l. Postoperatively, these levels declined to 6 ng/l ACTH, 18 μg/l cortisol, and 0.43 mg/l DHEA(S).
Of these 11 patients, one did not fulfill early remission criteria, with cortisol levels of 190 μg/l. Without further therapy, this patient went into delayed remission later on, as salivary cortisol tests revealed a subnormal circadian rhythm of low cortisol levels, which was confirmed by blood sampling (ACTH 2 ng/l and early morning cortisol under 50 μg/l). Low-dose dexamethasone-suppression test (1 mg) showed complete suppression of cortisol <10 μg/l.
Early remission vs. no early remission/persistent disease
In cases of early remission (postoperative early morning cortisol levels under 100 μg/l), (n = 40) cortisol levels reached a median of 18 μg/l; DHEA(S) levels in these patients declined to a median of 0.38 mg/l.
Patients who did not reach the cut-off in terms of early remission (n = 2) showed postoperative levels of cortisol decrease to median 164.5 μg/l and a decrease in DHEA(S) down to median 0.4 mg/l. In both cases, persistent disease was confirmed by further investigation and these patients were treated by radiation therapy.
Statistical analysis revealed a strong correlation between percent changes in DHEA(S) and cortisol (Spearman’s correlation coefficient rho = 0.482, p < 0.001, Chart 2). An even stronger correlation was present between changes in DHEA(S) and ACTH (rho = 0.590, p < 0.0001, Fig. 2 and Tables 1 and 2).
Discussion
Early determination of surgical cure or failure in Cushing’s disease is very important. It has been shown that early repeat surgery for Cushing’s disease has a high success rate in cases of failure of a previous attempt. Due to the near absence of scarring, the pituitary gland can be rapidly accessed, and this is combined with a low surgical morbidity. In most patients, a successful surgery can be demonstrated the day after surgery by biochemical means. Different methods have been published, including early morning cortisol measurement and ACTH determination, as well as an early postoperative low-dose dexamethasone suppression test. There are a small number of patients where early postoperative results may not show clear-cut cure or failure. For these patients, additional biochemical markers like DHEA(S) may be helpful in the assessment. Our statistical analysis revealed a highly significant correlation between perioperative levels of DHEA(S) and levels of cortisol and ACTH, thus making DHEA(S) a possible new marker for diagnosis, course, and possibly long-term evaluation in Cushing’s disease. In cases of persistent disease and only partial removal of the tumor, the drop in DHEA(S) did not fall below 38 % of its preoperative levels, compared to an average drop to 17 % after successful surgeries. Whether this is statistically significant cannot be determined due to the small sample number of just two patients with persistent disease in this series.
Another aspect is the role of DHEA(S) in the postoperative hypocortisolemic phase of these patients. Kleiber et al. [9] have shown in a small number of patients that after surgery for Cushing’s disease, DHEA(S) levels were low in the follow-up period of 15 months and remained suppressed for a longer period of time than cortisol. Hunt and coworkers [7] concluded that DHEA(S) replacement in patients suffering from Addison’s disease may not only raise DHEA(S) levels back to normal age-adjusted values but have a positive clinical impact on such patients in terms of psychological well-being, mood, and fatigue. Low levels of DHEA(S) supposedly have a strong correlation with increased cardiovascular risk [11], malignancy [4, 21], and risk of osteoporosis. Low levels of DHEA(S) may also result in a relative glucocorticoid excess [20], especially in patients who need cortisol replacement or patients who have been successfully treated for Cushing’s and whose cortisol levels return to normal, whereas DHEA(S) does not. This may explain at least in part the long-term consequences such as psychopathology and maladaptive personality [5] characteristic of Cushing’s disease, even if treated successfully. This of course raises the question of an additional need for DHEA(S) substitution after successful treatment of Cushing’s disease. So far, no study has been performed with regard to this question.
Conclusions
From the author’s standpoint, DHEA(S) is a useful additional marker in the perioperative phase of patients suffering from Cushing’s disease. It needs to be further examined whether DHEA(S) substitution may play a role in the well-being of patients after successful treatment during the phase of secondary adrenal insufficiency, and whether DHEA(S) levels may also be a potential predictor of recovery of the HPA-axis after successful surgery.
References
Barrett-Connor E, Edelstein SL (1994) A prospective study of dehydroepiandrosterone sulfate and cognitive function in an older population: The Rancho Bernardo study. J Am Geriatr Soc 42:420–423
Esposito F, Dusick JR, Cohan P (2006) Clinical review: Early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 91:7–13
Flitsch J, Knappe UJ, Lüdecke DK (2003) The use of postoperative ACTH levels as a marker for successful transsphenoidal microsurgery in Cushing’s disease. Zentralbl Neurochir 64:6–11
Gordon GB, Bush TL, Helzlsouer KJ, Miller SR, Comstock GW (1990) Relationship of serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing postmenopausal breast cancer. Cancer Res 50:3859–3862
Heald AH, Ghosh S, Bray S, Gibson C, Anderson SG, Buckler H, Fowler HL (2004) Long-term negative impact on quality of life in patients with successfully treated Cushing’s disease. Clin Endocrinol 61:458–465
Hofmann BM, Hlavac M, Martinez R, Buchfelder M, Müller OA, Fahlbusch R (2008) Long-term results after microsurgery for Cushing disease: experience with 426 primary operations over 35 years. J Neurosurg 108(1):9–18
Hunt PJ, Gurnell EM, Huppert FA, Richards C, Prevost AT, Wass JA, Herbert J, Chatterje VK (2000) Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double-blind trial. J Clin Endocrinol Metab 85:4650–4656
Khorram O (1996) DHEA: A hormone with multiple effects. Curr Opin Obstet Gynecol 8:351–354
Kleiber H, Rey F, Temler E, Gomez F (1991) Dissociated recovery of cortisol and dehydroepiandrosterone sulphate after treatment for Cushing’s syndrome. J Endocrinol Invest 14:489–492
Knappe UJ, Lüdecke DK (1996) Persistent and recurrent hypercortisolism after transsphenoidal surgery for Cushing’s disease. Acta Neurochir Suppl 65:31–34
Lacroix AZ, Yano K, Reed DM (1992) Dehydroepiandrosterone sulfate, incidence of myocardial infarction, and extent of atherosclerosis in men. Circulation 86:1529–1535
Locatelli M, Vance ML, Laws ER (2001) Clinical review: the strategy of immediate reoperation for transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 90:5478–5482
Lüdecke DK, Flitsch J, Knappe UJ, Saeger W (2001) Cushing’s disease: a surgical view. J Neurooncol 54(2):151–166
Lüdecke DK, Niedworok G (1985) Results of microneurosurgery in Cushing’s disease and effect on hypertension. Cardiology 72(suppl 1):91–94
Ram Z, Nieman LK, Cutler GB Jr (1994) Early repeat surgery for persistent Cushing’s disease. J Neurosurg 80:37–45
R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/
Sonino N, Zielezny M, Fava GA (1996) Risk factors and long-term outcome in pituitary-dependent Cushing’s disease. J Clin Endocrinol Metab 81:2647–2652
Srinivasan L, Laws ER, Dodd RL, Monita MM, Tannenbaum CE, Kirkeby KM, Chu OS, Harsh GR 4th, Katznelson L (2011) The dynamics of post-operative plasma ACTH values following transsphenoidal surgery for Cushing’s disease. Pituitary 14(4):312–317
Valassi E, Biller BM, Swearingen B, Pecori Giraldi F, Losa M, Mortini P, Hayden D, Cavagnini F, Klibanski A (2010) Delayed remission after transsphenoidal surgery in patients with Cushing’s disease. J Clin Endocrinol Metab 95(2):601–610
Valenti G (2002) Adrenopause: an imbalance between dehydroepiandrosterone (DHEA) and cortisol secretion. J Endocrinol Invest 25(10 Suppl):29–35
Zumoff B, Levin J, Rosenfeld RS, Markham M, Strain GW, Fukushima DK (1981) Abnormal 24-hr mean plasma concentrations of dehydroisoandrosterone and dehydroisoandrosterone sulfate in women with primary operable breast cancer. Cancer Res 41:3360–3363
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
This is a thought-provoking and interesting article that any surgeon in the Cushing’s field will find of great interest. Where DHEA will take us may prove of importance. It is also worth noting how a center that can find 42 Cushing’s cases in a year and cure the vast majority, can really focus on ways to improve outcome in this dangerous and difficult disease, underlining the often-ignored fact that experience in a rare disease usually improves outcome. Specialist centers really make a difference.
Michael Powell,
London, UK
Rights and permissions
About this article
Cite this article
Burkhardt, T., Schmidt, N.O., Vettorazzi, E. et al. DHEA(S)—a novel marker in Cushing’s disease. Acta Neurochir 155, 479–484 (2013). https://doi.org/10.1007/s00701-012-1596-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1596-6