Abstract
Objective
The aim of this study was to evaluate the anatomy of the central myelin portion and the central myelin-peripheral myelin transitional zone of the trigeminal, facial, glossopharyngeal and vagus nerves from fresh cadavers. The aim was also to investigate the relationship between the length and volume of the central myelin portion of these nerves with the incidences of the corresponding cranial dysfunctional syndromes caused by their compression to provide some more insights for a better understanding of mechanisms.
Methods
The trigeminal, facial, glossopharyngeal and vagus nerves from six fresh cadavers were examined. The length of these nerves from the brainstem to the foramen that they exit were measured. Longitudinal sections were stained and photographed to make measurements. The diameters of the nerves where they exit/enter from/to brainstem, the diameters where the transitional zone begins, the distances to the most distal part of transitional zone from brainstem and depths of the transitional zones were measured. Most importantly, the volume of the central myelin portion of the nerves was calculated. Correlation between length and volume of the central myelin portion of these nerves and the incidences of the corresponding hyperactive dysfunctional syndromes as reported in the literature were studied.
Results
The distance of the most distal part of the transitional zone from the brainstem was 4.19 ± 0.81 mm for the trigeminal nerve, 2.86 ± 1.19 mm for the facial nerve, 1.51 ± 0.39 mm for the glossopharyngeal nerve, and 1.63 ± 1.15 mm for the vagus nerve. The volume of central myelin portion was 24.54 ± 9.82 mm3 in trigeminal nerve; 4.43 ± 2.55 mm3 in facial nerve; 1.55 ± 1.08 mm3 in glossopharyngeal nerve; 2.56 ± 1.32 mm3 in vagus nerve.
Correlations (p < 0.001) have been found between the length or volume of central myelin portions of the trigeminal, facial, glossopharyngeal and vagus nerves and incidences of the corresponding diseases.
Conclusion
At present it is rather well-established that primary trigeminal neuralgia, hemifacial spasm and vago-glossopharyngeal neuralgia have as one of the main causes a vascular compression. The strong correlations found between the lengths and volumes of the central myelin portions of the nerves and the incidences of the corresponding diseases is a plea for the role played by this anatomical region in the mechanism of these diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperactive dysfunctional syndromes affecting cranial nerves were described centuries ago, and their pathophysiology has been subject to much debate ever since. Causes and mechanisms are likely to be various, probably multiple and intricate in a majority of cases. However, the most popular theory is that these diseases are likely to be caused—at least as the main factor—by vascular compression at the root level [4, 6, 7, 10]. Indeed, over the past decades a number of publications on imaging data, as well as on intra-operative anatomical observations, have brought evidence that a majority of so-called “primary” trigeminal neuralgias, hemifacial spasms and vago-glossopharyngeal neuralgias are related to a vascular compression of the corresponding root. An even larger number of reports on the valuable long-term results of the microvascular decompression (MVD) procedure on the trigeminal nerves, facial nerves and glossopharyngeal nerves and vagus nerves confirmed the validity of this hypothesis for the correspondig diseases. On the other hand, for vertigo and tinnitus, definitively convincing studies are lacking, with still a need for defining clear-cut indications. As regard to dysfunctional syndromes of the other cranial nerves, they are not very common and literature data remain rare. For these reasons, the present study was limited to consider only trigeminal, facial and vago-glossopharyngeal nerves.
Following Jannetta’s observations [10, 12], most authors dealing with MVD stressed on the fact that in a majority of cases the vascular compression was found located where the authors named the root entry/exit zone (REZ). Although frequently used, first for spinal roots [21, 28, 41, 42], then for cranial nerves [5, 12, 21, 30, 31], to our knowledge the REZ has not received any official taxonomic definition, at least for cranial nerves. Nevertheless, when dealing with this term, it is generally concerned that the term includes the transitional zone itself, the central portion of the root, together with its subpial adjacent part at the brainstem. The hypothesis is that this region as a whole harbours greater anatomical-physiological “sensitiveness” [2, 6, 7, 10, 12, 18].
The purpose of the present work was first to provide additional knowledge on structural anatomy (shapes, dimensions and, above all, volumes) of the central myelin portion and central myelin-peripheral myelin transitional zone, of the trigeminal, facial, glossopharyngeal and vagus nerves. To our knowledge, this is the first reported anatomical study measuring and comparing not only the lengths (which had been done before) but also the volumes of the central myelin portion of these cranial nerves, and to relate these lengths and volumes with the incidences (as reported in the literature) of the corresponding dysfunctional syndromes caused by vascular compression.
Materials and methods
Anatomical study
All procedures were conducted at the Anatomy and Pathology Departments of the University of Lyon I. Six brains were obtained from three male and three female fresh cadavers, whose ages were between 81 and 91 at the time of death. All the cadavers were handled in less than 24 h after death and until that time they were put in the refrigerator. None of the deaths were caused by neurological diseases. Our findings were based on the examination of ten trigeminal nerves, eight facial nerves, and seven glossopharyngeal, eight vagus nerve rootlets. Also, ten vestibulo-cochlear nerves were studied, but they were not included in this study, as explained in the “Introduction”.
The calvaria was opened; the dura and brain were removed down to the level of cerebral peduncles and oculomotor nerves. The skull with attached brainstem and cranial nerves was put into 4% formol for 21 days. After fixation, all nerves were severed at their foramen and the spinal cord was cut at the level of the foramen magnum. Then brainstem with attached nerves was removed from the calvaria and arteries; veins and arachnoid membrane were removed from the brainstem without harming the nerves. The lengths of the nerves from the brainstem to the exiting foramina were measured. A tissue block consisting of the nerve and adjoining brainstem was removed for each nerve. Each tissue block was embedded in paraffin and serially and horizontally sectioned. The thickness of sections was 6 µm. The number of sections was five for the trigeminal and vagus nerves, and three for the facial and glossopharyngeal nerves. The distance between sections was 50 µm for the trigeminal nerve, and 10 µm for the facial, glossopharyngeal and vagus nerves. The reason for taking different sections was that we wanted the maximum measurements of these nerves. If we had taken only one section of each nerve, we would have missed the maximum measurements of that nerve. Also, different sections were used to calculate the volume of the central myelin portion of the nerves. Each section was stained with hematoxylin phloxine saffron and luxol fast blue. After staining, a photomicrograph of each section was obtained with Leica DMB 108.
For each gross specimen, we measured the length of the nerve from the brainstem (pons or medulla) to the foramen that the nerve exits, designated as L. In each photomicrograph, we measured four distances, designated A, B, F and f (Fig. 1). On each photomicrograph, (1) the point where the nerve met the brainstem on the medial edge of the nerve was located, and a line was drawn from that point to the corresponding point on the lateral edge making segment A, (2) the point where the central myelin met the peripheral myelin on the medial edge of the nerve was located, and a line was drawn from that point to the corresponding point on the lateral edge making segment B, (3) from the middle of the A line, a line was drawn to the tip of the arc-shaped transitional zone, making segment F, (4) a perpendicular line was drawn from the B line to the tip of the arch-shaped transitional zone, making segment f. Also, the volume of the central myelin portion of each nerve was calculated by using A, B, F and f of that nerve for volume determination. For each specimen, the length of central myelin from brainstem to the tip of transitional zone was calculated relative to the length of the nerve from brainstem to foramen that the nerve exits (F/L) and the depth of transitional zone was calculated relatively to the maximum length of central myelin (f/F).
Drawing demonstrating A, B, F and f. The point where the nerve met the brainstem on the medial edge of the nerve was located, and a line was drawn from that point to the corresponding point on the lateral edge, making segment A. The point where the central myelin met the peripheral myelin on the medial edge of the nerve was located, and a line was drawn from that point to the corresponding point on the lateral edge, making segment B. From the middle of the A line, a line was drawn to the tip of arc shaped transitional zone making segment F. A perpendicular line was drawn from the B line to the the tip arch-shaped transitional zone, making segment f
The trigeminal and facial nerves exit from the brainstem as single entities. The glossopharyngeal and vagus nerves, on the other hand, exit fom the brainstem as rootlets (three to five for the glossopharyngeal nerve, seven to ten for the vagus nerve); these rootlets later unite to form the glossopharyngeal and vagus nerves, which receive the common perineurium derived from the arachnoid. We chose well-preserved and stained rootlets to make measurements for the glossopharyngeal and vagus nerves. The volumes of the central myelin portion of glossopharyngeal and vagus nerves were found by multiplying the volume of central myelin portion of one rootlet with the number of rootlets.
In this study, distances and volumes were expressed as the mean values ± standard deviations. To correct for shrinkage of nerve tissue during fixation and embedding, we utilised oculomotor nerves as control nerves. We measured each oculomotor nerve before fixation and after fixation, and calculated the percentage of shrinkage of this nerve. We then substituted the percentage of shrinkage of the oculomotor nerve for all other nerves.
Comparison between anatomical features of cranial nerves V, VII and IX-X
Comparisons between the L, A, B, F, f and V values of cranial nerves V, VII and IX-X were made. Also, comparisons between the F/L and f/F ratios for these cranial nerves were made. They are presented in Table 1 (Fig. 2).
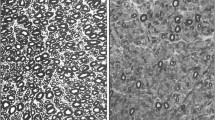
Photomicrographs of longitudinal sections of trigeminal and facial nerve roots, and glossopharyngeal and vagus nerve rootlets. To establish comparison, all the nerve roots and rootlets were magnified at the same scale. All the nerve roots and rootlets were stained with luxol fast blue staining. The length of the central myelin portion (L) and a line drawn from the point where the nerve met the brainstem on the medial edge of the nerve to a corresponding point on the lateral edge (A) were marked to easily understand the anatomy
Correlations of anatomical features of cranial nerves V, VII and IX-X with incidences of the corresponding vascular compression syndromes
To give insights to the pathophysiological debate on mechanisms of the cranial nerve hyperactive dysfunctional syndromes, we correlated length and volume of the central myelin portion of the trigeminal, facial, glossopharyngeal and vagus nerves with the respective incidences of primary trigeminal neuralgia, hemifacial spasm and vago-glossopharyngeal neuralgia. For the source of incidences of these syndromes, we chose what we thought the most classical—namely, the most frequently quoted incidences in the literature. Incidence for trigeminal neuralgia was 4.3 new cases per 100,000 persons per year [14]. Incidence of hemifacial spasm was 0.74 and 0.81 for 100,000 persons per year for men and women, respectively, 0.77 on average [1]. The incidence of vago-glossopharyngeal neuralgia was one case for every 70 cases of trigeminal neuralgia [40], 0.062 per 100,000 persons per year. For a statistical correlation, Pearson product-moment correlation coefficient (typically denoted by r) was used as a measure of the strength of the linear dependence between length/volume of the central myelin portion of cranial nerves and diseases caused by their compression.
Results
Measurements
Trigeminal nerve
The lengths of ten trigeminal nerves from the pons to Meckel’s cave were between 11.9 and 15.2 mm (L: 13.11 ± 1.12 mm). A for the trigeminal nerve was between 2.02 and 4.43 mm (A: 3.11 ± 0.83 mm), B was between 2.26 and 3.67 mm (B: 3.08 ± 0.39 mm), F was between 3.24 and 5.65 mm (F: 4.19 ± 0.81 mm) and f was between 0.89 and 2.38 mm (f: 1.53 ± 0.45 mm). F calculated relative to L was between 22.2% and 51.8% (F/L: 36.61 ± 10.38%) and f calculated relative to F was between 24.6% and 39.7% (f/F: 31.42 ± 4.86%).
The volume of the central myelin portion for trigeminal nerve was between 10.11 and 38.82 mm3 (24.54 ± 9.82 mm3).
Facial nerve
The lengths of eight facial nerves from the pontomedullary junction to the internal auditory meatus were between 14.8 and 20.9 mm (L: 17.93 ± 2.29 mm). A for the facial nerve was between 0.87 and 3.11 mm (A: 1.60 ± 0.66 mm), B was between 1.01 and 2.34 mm (B: 1.42 ± 0,41 mm), F was between 1.18 and 5.93 mm (F: 2.86 ± 1.19 mm) and f was between 0.46 and 1.82 mm (f: 1.03 ± 0.55 mm). F calculated relative to L was between 8.1% and 30.6% (F/L: 15.33 ± 8.21%) and f calculated relative to F was between 20.4% and 57.8% (f/F: 38.79 ± 10.14%).
The volume of central myelin portion of the facial nerve was between 1.49 and 8.85 mm3 (4.43 ± 2.55 mm3).
Glossopharyngeal nerve
The lengths of seven glossopharyngeal nerve rootlets from the medulla oblongata to the jugular foramen were between 14.2 and 19.9 mm (L: 16.36 ± 2.53 mm). A for the glossopharyngeal nerve was between 0.62 and 1.11 mm (A: 0.93 ± 0.17 mm), B was between 0.39 and 1.10 mm (B: 0.78 ± 0.26 mm), F was between 0.97 and 2.09 mm (F: 1.51 ± 0.39 mm) and f was between 0.25 and 0.44 mm (f: 0.36 ± 0.08 mm) (Fig. 1). F calculated relative to L was between 4.8% and 13.3% (F/L: 9.38 ± 3.27%) and f calculated relative to F was between 13.7% and 35.4% (f/F: 25.34 ± 7.45%).
The volume of central myelin portion for glossopharyngeal nerve formed by four rootlets on average was between 0.6 and 3.26 mm3 (1.55 ± 1.08 mm3).
Vagus nerve
The lengths of eight vagus nerve rootlets from the medulla oblongata to the jugular foramen were between 13.9 and 20.6 mm (L: 13.42 ± 2.42 mm). A for the vagus nerve was between 0.62 and 1.16 mm (A: 0.90 ± 0.19 mm), B was between 0.50 and 0.86 mm (B: 0.73 ± 0.15 mm), F was between 0.45 and 4.22 mm (F: 1.63 ± 1.15 mm) and f was between 0.22 and 0.82 mm (f: 0.42 ± 0.22 mm). F calculated relative to L was between 3.9% and 27.7% (F/L: 11.86 ± 8.65%), and f calculated relative to F was between 17.8% and 49.6% (f/F: 30.48 ± 12.36%).
The volume of the central myelin portion for the vagus nerve formed by eight rootlets on average was between 0.56 and 4.89 mm3 (2.56 ± 1.32 mm3).
Comparisons of anatomical features of cranial nerves V, VII and IX-X
The facial nerve in the cerebellopontine angle cistern was the longest, L: 17.93 ± 2.29 mm; the trigeminal nerve was the shortest, L: 13.11 ± 1.12 mm.
The distance of the most distal part of the transitional zone from brainstem was longest in the trigeminal nerve, F: 4.19 ± 0.81 mm, shortest in the glossopharyngeal nerve, F: 1.51 ± 0.39 mm (Fig. 3).
The depth of the transitional zone was greatest in the trigeminal nerve, f: 1.53 ± 0.45 mm, and least in the glossopharyngeal nerve, f: 0.36 ± 0.08 mm.
The proportion of the central myelin to length of the nerve was highest in the trigeminal nerve, F/L: 36.61 ± 10.38%, lowest in the glossopharyngeal nerve, F/L: 9.38 ± 3.27%. The proportion of depth of transitional zone to length of the central myelin was largest in the facial nerve, f/F: 38.79 ± 10.14%, smallest in the vagus nerve, 30.48 ± 12.36%.
The trigeminal nerve was the widest of these nerves at exit/entry from/to brainstem, A: 3.11 ± 0.83 mm; the vagus nerve was the narrowest, A: 0.90 ± 0.19 mm. Where the transitional zone began, the trigeminal nerve was also the widest, B: 3.08 ± 0.39 mm; the glossopharygeal nerve was also the narrowest, B: 0.68 ± 0.26 mm (Fig. 4).
The volume of the central myelin portion was biggest in the trigeminal nerve with a volume of 24.54 ± 9.82 mm3; and smallest in the glossopharyngeal nerve with a volume of 1.55 ± 1.08 mm3) .
Correlations of anatomical features with incidences of corresponding vascular compression syndromes
A very strong correlation was found between the lengths of the central myelin portion of these nerves and the incidences of “primary” dysfunctional syndromes (r +0.796, p < 0.001). Also, a very strong correlation was found between volumes of the central myelin portion of these nerves and the incidences of “primary” dysfunctional syndromes (r +0.894, p < 0.001) (Fig. 5).
a The mean values of the lengths of the central myelin portions of the trigeminal (TN), facial (FN), glossopharyngeal (GPN) and vagus (VN) nerves. b The mean values of volumes of central myelin portions of trigeminal (TN), facial (FN), glossopharyngeal (GPN) and vagus (VN) nerves. c The incidences of hyperactive cranial dysfunctional syndromes
Discussion
The present report was limited to consider the trigeminal, facial and vago-glossopharyngeal nerves only, and the correlations between their anatomical features and respective incidences of their corresponding hyperactive dysfunctional syndromes, for the following reasons. Primary trigeminal neuralgia, hemifacial spasm, vago-glossopharyngeal neuralgia are relatively well-defined syndromes from a clinical standpoint [3, 4, 7, 15]. A large number of literature reports on the long-term curative effect of MVD (reviewed in [33–37]) have brought strong evidence that in a majority of cases, vascular compression is the main factor of the disease. This does not hold true for tinnitus and vertigo; there is no such a clear-cut evidence that vascular compression of the vestibulocochlear nerve is the most frequent responsible factor [9, 19, 20, 44]. Ocular motor synkinesis, third nerve palsy with cyclic spasms, superior oblique myokymia and ocular neuromyotonia are very rarely observed diseases [16, 17, 26, 27].
Publications evaluating the anatomy of the central myelin portion and central myelin-peripheral myelin transitional zone of cranial nerves, date back around century ago. Obersteiner and Redlich [21] stated that there was a transitional zone between the peripheral nervous system and the central nervous system in the brain and the spinal cord. In the peripheral portion , myelin sheaths are produced by Schwann cells and the supporting tissues include fibroblasts, collagen fibrils and root sheath cells. In the central portion, myelin sheaths are produced by oligodendrocytes and nerve fibres are partly separated from one another by astrocytes [21, 41]. Skinner [38] measured the length of the central myelin portion of all cranial nerves and observed a dome shape of the central myelin-peripheral myelin transitional zone; he stated that the central myelin portion was longer in sensory nerves than in motor nerves. Tarlov [41, 42] stated that the central glial segment of a nerve presented essentially the structure of a fibre tract of the brain, its peripheral segment corresponding to a peripheral nerve. In a “stimulating” paper on the epidemiological plane, De Ridder et al. [5] considered positively the correlation between the length of central nervous system segment, which differed among cranial nerves, and the incidence of the corresponding vascular compression syndromes. Tomii et al. [43] defined the exact borders of the root exit zone of the facial nerve and measured the distribution of myelin histologically. Peker et al. [23] showed that central myelin occupied only the initial one-fourth of the trigeminal nerve length. More recently we carried out anatomical (structural) studies of cranial nerves (V, VII, VIII, IX, X) in cadavers [8], for enlarging our surgical anatomical background and better understanding cranial nerve vascular compression syndromes that we have to deal with frequently [36].
To our knowledge, REZ region did not receive formal definition for cranial nerves. However, for most surgeons dealing with MVD surgery, including us, the term involves the transitional zone, the central myelin portion of the root and the adjacent surface of brainstem where root fibres have subpial paths on their way from or to their respective nuclei (Fig. 6). Whatever the definition might be, it is classically agreed that cranial nerve vascular compression syndromes have their conflicting vessel(s) situated at that REZ region considered—on the whole—as harbouring anatomical-physiological sensitiveness and excitability [4, 6, 7, 10, 12, 18].
As an illustration from personal series, of the 117 patients who underwent MVD for hemifacial spasm, and that we assessed in 2005 for evaluating outcome, 96% had the vascular compression at the REZ [32, 37]. Of the 23 patients with vago-glossopharyngeal neuralgia, and that we assessed in 2008, 95% had their compression at the REZ [35]. Regarding the patients with trigeminal neuralgia, the percentage of neurovascular conflict at the REZ itself was not as important. In a consecutive series of 579 patients in whom surgery was perfomed for MVD, no obvious vascular conflict was found in 19 cases (3.3%). In the remaining 560 cases (96.7%), one or several vessel(s) could be identified. Location of the vascular compressions were in the trigeminal REZ in 52.3% of the patients, in the cisternal mid third of the root in 54.3%, and at exit of the root from Meckel’s cave in 9.8% [30, 31].
A puzzling problem is why some hyperactive dysfunctional syndromes are more frequent than others. This question has previously been adressed by de Ridder et al. [5], who—on literature data—suggested that incidences were in accordance with lengths of the central myelin portion of the respective cranial nerves. The study included also the vestibulo-cochlear nerve. The authors concluded that a positive correlation existed between the high incidence of Meniere disease (15.3 per 100,000 persons) and the length of the central myelin portion of the vestibulo-cochlear nerve, considering that approximately one-third to one-half of the patients with Meniere disease could be of vascular compression origin [24]. From a personal anatomical study on the vestibulo-coclear nerves [8], we found a similar proportionality on ten vestibulo-cochlear nerves, the mean length of central myelin portion was 10.21 ± 2.52 mm, and the mean volume of central myelin portion was 31.38 ± 15.93 mm3. For the present report, we excluded the vestibulo-cochlear nerve from the correlation calculation; if some syndromes can be related to a vascular compression [9, 11, 13, 44], it is widely estimated that a large number of tinnitus and vertigo cases are actually secondary to a variety of pathologies, the majority of them not being vascular compression of the root. Contrarily, there is a solid consensus to consider that most of the so-called “primary” trigeminal neuralgias, vago-glossopharyngeal neuralgias, and hemifacial spasms are linked to a vascular compression.
Therefore, for the present report, we retained only trigeminal, facial, glossopharyngeal and vagus nerves. Trigeminal neuralgia is the commonest of the neurovascular compression syndromes; its incidence can be estimated at 4.3 per 100,000 persons per year [14]. Skinner [38] stated that the trigeminal nerve had a considerably long central myelin portion. According to our measurements, the length of central myelin of the trigeminal nerve is 4.19 ± 0.81 mm. It has the largest diameter (A: 3.11 ± 0.83 mm, B: 3.08 ± 0.39 mm) and the biggest volume of central myelin portion (volume: 24.54 ± 9.82 mm3). These relatively large dimensions may give a large surface and a big volume for vessels to exert compression; this might be a reason for the high incidence of trigeminal neuralgia, compared with other cranial dysfunctional syndromes. Compressing vessels are mostly found at the REZ, but they can also be seen anywhere along the root [30, 31]. In the cases with the neurovascular conflict at the REZ, the compression was generally exerted directly by a loop of a neigbouring elongated superior cerebellar artery coming from above or by the anterior inferior cerebellar artery coming from below. For neurovascular conflicts in the mid portion of the root, generally a superior cerebellar artery pushing down the root, the mechanism could be stretching exerted on the REZ [30, 31]. In the cases with the neurovascular conflict at the porus of Meckel’s cave, the compressive vessel was most often the inferior transverse pontine vein making a pronounced groove on the inferior surface of the root after its exit from Meckel’s cave. In addition to the vascular compression, other anatomical factors can be observed; sometimes the nerve is squeezed between the pons and the petrous bone, due to the small size of the posterior fossa, with cross-compressing veins engrooving the surface of the nerve. Additionally, other abnormalities may play a role, especially global atrophy of the root, also focal arachnoid thickening, and also a ribbon-shaped and stretched, angulated, root on crossing over the petrous ridge. In most of these cases, one can observe a segmental grayish demyelination of the root under the compressive vessel [30, 31].
The incidence of hemifacial spasm can be estimated at 0.74 per 100,000 men and 0.81 per 100,000 women [1]. Skinner [38] stated that the facial nerve had the longest central myelin portion compared with the other cranial motor nerves. Our data (F: 2.86 ± 1.19 mm) are similar to his findings (F: 2.5 mm). We found the volume of the central myelin portion to be 4.43 ± 2.55 mm3. In more than 96% of hemifacial spasm cases, vascular structures compress the REZ; in the remaining few cases, the distal part of the facial nerve is compressed. For hemifacial spasm also, anatomical factors other than neurovascular conflict may play roles as causative factors, especially thickening of the arachnoid membranes or hypertrophic choroid plexus or voluminous flocculonodular lobe. Nervus intermedius is a tiny part of the facial nerve; vascular compression of this nerve may cause geniculate neuralgia; but this is a very infrequent disease [25].
Vago-glossopharyngeal neuralgia is rare with one case for every 70 cases of trigeminal neuralgia [40]. We would like to stress that glossopharyngeal neuralgia is mostly in fact vago-glossopharyngeal neuralgia, as the sensory rootlets of the vagus nerve are frequently implicated at the same time as the glossopharyngeal nerve [3, 22, 28, 29, 35, 39]. Central myelin portions of glossopharyngeal and vagus nerves are very short: glossopharyngeal nerve: F: 1.51 ± 0.39 mm, vagus nerve: F: 1.63 ± 1.15 mm. The volumes of the central myelin portions of the glossopharyngeal and vagus nerves are 1.55 ± 1.08 mm3 and 2.56 ± 1.32 mm3, respectively.
The central myelin portions of these nerves are shorter than the central myelin portion of the trigeminal and facial nerves, and also the volumes of the central myelin portion are smaller. De Ridder et al. [5] also stated that the low incidence of glossopharyngeal neuralgia was likely related to the short length of central myelin portion of these nerves.
Conclusion
The trigeminal nerve is the biggest nerve with regards to the length and volume of the central myelin portion; its dysfunction, which causes trigeminal neuralgia, is the most frequently recognized hyperactive dysfunctional syndrome, with an incidence of 4.3 per 100,000 persons per year. The large volume of the central myelin portion and comparably long central myelin portion of the trigeminal nerve may offer a bigger area for vascular compression. The facial nerve is the sole motor cranial nerve frequently causing a dysfunctional syndrome with an incidence of 0.77 per 100,000 persons per year; its central myelin portion is the longer and the volume of the central myelin portion is the bigger of the motor nerves. Short length and small volume of central myelin portion of glossopharyngeal and vagus nerves might explain the low incidence of vago-glossopharyngeal neuralgia with an incidence of 0.062 per 100,000 persons per year. Although not probably the sole implicated group of factors, our study shows a positive correlation (p < 0.001) between the length and volume of the central myelin portion of the nerves and the incidences of their corresponding hyperactive dysfunctional syndromes. Much further studies are needed to understand better the complex mechanisms and the high differences in incidences of these syndromes.
References
Auger RG (1990) Whisnant JP: hemifacial spasm in Rochester and Olmsted County, Minnesota, 1960 to 1984. Arch Neurol 47(11):1233–1234
Burchiel KJ (1980) Abnormal impulse generation in focally demyelinated trigeminal roots. J Neurosurg 53(5):674–683
Chawla JC, Falconer MA (1967) Glossopharyngeal and vagal neuralgia. Br Med J 3(5564):529–531
Dandy WE (1934) Concerning the cause of trigeminal. Am J Surg 24:447–495
De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder L (2002) Is the root entry/exit zone important in microvascular compression syndromes? Neurosurgery 51(2):427–434
Gardner WJ, Miklos MV (1959) Response of trigeminal neuralgia to decompression of sensory root; discussion of cause of trigeminal neuralgia. J Am Med Assoc 170(15):1773–1776
Gardner WJ (1962) Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg 19:947–958
Guclu B, Meyronet D, Simon E, Streichenberger N, Sindou M, Mertens P (2009) Structural anatomy of cranial nerves (V, VII, VIII, IX, X). Neurochirurgie 55(2):92–98
Guevara N, Deveze A, Buza V, Laffont B, Magnan J (2008) Microvascular decompression of cochlear nerve for tinnitus incapacity: pre-surgical data, surgical analyses and long-term follow-up of 15 patients. Eur Arch Otorhinolaryngol 265(4):397–401
Jannetta PJ (1967) Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg 26(1):Suppl:159–162
Jannetta PJ (1975) Neurovascular cross-compression in patients with hyperactive dysfunction symptoms of the eighth cranial nerve. Surg Forum 26:467–469
Jannetta PJ (1979) Microsurgery of cranial nerve cross-compression. Clin Neurosurg 26:607–615
Jannetta PJ, Møller MB, Møller AR (1984) Disabling positional vertigo. N Engl J Med 310(26):1700–1705
Katusic S, Beard CM, Bergstralh E, Kurland LT (1990) Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol 27(1):89–95
Laha RK, Jannetta PJ (1977) Glossopharyngeal neuralgia. J Neurosurg 47(3):316–320
Kommerell G, Mehdorn E, Ketelsen UP (1985) Oculomotor paralysis with cyclic spasms; electromyographic and electron microscopic indications of chronic peripheral nerve irritation. Fortschr Ophthalmol 82(2):203–204
Mikami T, Minamida Y, Ohtsuka K, Houkin K (2005) Resolution of superior oblique myokymia following microvascular decompression of trochlear nerve. Acta Neurochir (Wien) 147(9):1005–1006
Møller AR (1999) Vascular compression of cranial nerves: II: pathophysiology. Neurol Res 21(5):439–443
Møller MB, Møller AR, Jannetta PJ, Jho HD (1993) Vascular decompression surgery for severe tinnitus: selection criteria and results. Laryngoscope 103(4 Pt 1):421–417
Møller MB, Møller AR, Jannetta PJ, Jho HD, Sekhar LN (1993) Microvascular decompression of the eighth nerve in patients with disabling positional vertigo: selection criteria and operative results in 207 patients. Acta Neurochir (Wien) 125(1–4):75–82
Obersteiner H, Redlich E (1984) Uber Wesen und Pathogenese der Tabischen Hinterstrangsdegeneration. Arb Neurol Inst Wien Univ 1–3:158–172
Patel A, Kassam A, Horowitz M, Chang YF (2002) Microvascular decompression in the management of glossopharyngeal neuralgia:analysis of 217 cases. Neurosurgery 50(4):705–711
Peker S, Kurtkaya O, Uzün I, Pamir MN (2006) Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery 59(2):354–359
Ryu H, Yamamoto S, Sugiyama K, Nozue M (1998) Neurovascular compression syndrome of the eighth cranial nerve. What are the most reliable diagnostic signs? Acta Neurochir (Wien) 140(12):1279–1286
Sakas DE, Panourias IG, Stranjalis G, Stefanatou MP, Maratheftis N, Bontozoglou N (2007) Paroxysmal otalgia due to compression of the intermediate nerve: a distinct syndrome of neurovascular conflict confirmed by neuroimaging. Case report. J Neurosurg 107(6):1228–1230
Samii M, Rosahl SK, Carvalho GA, Krzizok T (1998) Microvascular decompression for superior oblique myokymia: first experience. Case report. J Neurosurg 89(6):1020–1024
Scharwey K, Krzizok T, Samii M, Rosahl SK, Kaufmann H (2000) Remission of superior oblique myokymia after microvascular decompression. Ophthalmologica 214(6):426–428
Sindou M, Quoex C, Baleydier C (1974) Fiber organization at the posterior spinal cord-rootlet junction in man. J Comp Neurol 153(1):15–26
Sindou M, Henry JF, Blanchard P (1991) Idiopathic neuralgia of the glossopharyngeal nerve. Study of a series of 14 cases and review of the literature. Neurochirurgie 37(1):18–25
Sindou M, Chiha M, Mertens P (1995) Anatomical findings in microsurgical vascular decompression for trigeminal neuralgia. Correlations between topography of pain and site of the neuro-vascular conflict. Acta Neurochir Suppl 64:125–127
Sindou M, Howeidy T, Acevedo G (2002) Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir (Wien) 144(1):1–13
Sindou MP (2005) Microvascular decompression for primary hemifacial spasm. Importance of intraoperative neurophysiological monitoring. Acta Neurochir (Wien) 147(10):1019–1026
Sindou M, Leston J, Decullier E, Chapuis F (2007) Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg 107(6):1144–1153
Sindou M, Leston JM, Decullier E, Chapuis F (2008) Microvascular decompression for trigeminal neuralgia: the importance of a noncompressive technique—Kaplan-Meier analysis in a consecutive series of 330 patients. Neurosurgery 63(4 Suppl 2):341–351
Sindou M, Keravel Y (2009) Neurosurgical treatment of vago-glossopharyngeal neuralgia. Neurochirurgie 55(2):231–235
Sindou M, Keravel Y (2009) Functional neurosurgery in the cranial nerve hyperactivity syndromes. Neurochirurgie 55(2):75–291
Sindou M, Keravel Y (2009) Neurosurgical treatment of primary hemifacial spasm with microvascular decompression. Neurochirurgie 55(2):236–247
Skinner HA (1931) Some histological features of the cranial nerves. Arch Neurol Psychiatry 25:356–372
Słoniewski P, Korejwo G, Zieliński P, Moryś J, Krzyzanowski M (1999) Measurements of the Obersteiner-Redlich zone of the vagus nerve and their possible clinical applications. Folia Morphol (Warsz) 58(1):37–41
Spurling RG, Grantham EG (1942) Glossopharyngeal neuralgia. South Med J 35:509–512
Tarlov IM (1937) Structure of the nerve root. I. Nature of the junction between the central and the peripheral nervous system. Archs Neurol Psychiat 37:555–583
Tarlov IM (1937) Structure of the nerve root. II. Differentiation of sensory from motor roots; observations on identification of function in roots of mixed cranial nerves. Arch Neurol Psychiatry 37:1338–1355
Tomii M, Onoue H, Yasue M, Tokudome S, Abe T (2003) Microscopic measurement of the facial nerve root exit zone from central glial myelin to peripheral Schwann cell myelin. J Neurosurg 99(1):121–124
Vasama JP, Moller MB, Moller AR (1998) Microvascular decompression of the cochlear nerve in patients with severe tinnitus. Preoperative findings and operativeoutcome in 22 patients. Neurol Res 20(3):242–248
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guclu, B., Sindou, M., Meyronet, D. et al. Cranial nerve vascular compression syndromes of the trigeminal, facial and vago-glossopharyngeal nerves: comparative anatomical study of the central myelin portion and transitional zone; correlations with incidences of corresponding hyperactive dysfunctional syndromes. Acta Neurochir 153, 2365–2375 (2011). https://doi.org/10.1007/s00701-011-1168-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1168-1