Abstract
Background
Functional magnetic resonance imaging (fMRI) is a widely used method for research and visualization of the brain function. However, its clinical use is still limited. Our objective was to study fMRI reliability in localizing the primary hand motor cortex (M1) under pathological conditions caused by the proximity of a brain tumour. The results were then compared with standard technique of cortical function mapping—electric cortical stimulation (ECS).
Method
We compared M1 areas localized with the fMRI and ECS in 18 patients with brain tumours in fronto-parietal regions. The 1.5 T blood oxygenation-level dependent (BOLD) fMRI was performed preoperatively using a motor task involving rhythmic touching of the thumb consecutively with other fingers on the same hand contralateral to the affected hemisphere. Each individual fMRI result was displayed at the P < 0.05 significance level corrected for family wise error (more conservative approach) or at the P < 0.001 level uncorrected (less conservative approach) and projected on the T1-weighted image used for neuronavigation.
Findings
In 12 patients (66.6%) we found full agreement between the fMRI and ECS. In 3 patients (16.6%) the overlap was only partial, with one ECS testing position on motor response found outside the BOLD signal cluster. In another 3 cases (16.6%) there was a discrepancy between the two methods. The fMRI sensitivity for localizing the ECS reactive M1 cortex was 71%. The fMRI/ECS consistency was within a 5-mm range in 77% of the testing positions used for ECS which complies with the inherent accuracy of the navigation system.
Conclusions
Because the overlap between the two methods never exceeded 10-mm, we found that the fMRI method correctly guided the ECS to the M1 cortex in 83% of patients. Infiltrative growth of the tumour and collateral oedema were the reasons for the BOLD signal suppression in three patients. Our results support using ECS as a more reliable tool for M1 cortical mapping than fMRI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Functional magnetic resonance imaging (fMRI) is a non-invasive method used to map various cortical functions. A number of techniques are available, the most widely used one utilizes the BOLD (Blood Oxygenation-Level Dependent) effect, which is based on the detection of subtle local changes in the levels of oxy-and deoxy-hemoglobin caused by raised regional neuronal activity [4]. Although the exact mechanism of BOLD effect is not yet fully understood [27] and technical limitations still cause some artifacts [3], fMRI has become a valuable research tool for functional brain imaging of perception, motion control and cognitive processing.
The clinical uses of fMRI, however, are still rather limited. Planning and surgical navigation with fMRI are not widely accepted. The first authors, mostly neuroradiologists, reported the accuracy of fMRI imaging as being excellent when it was used as a landmark for intraoperative identification of the sensorimotor cortex [21, 28, 30] later more discriminating works came from the neurosurgical community using intraoperative navigation methods to validate fMRI [1, 6, 20, 23, 24].
Our objective was to study the accuracy of fMRI in the localization of the primary hand motor cortex under pathological conditions caused by proximity to a brain tumour. The fMRI results were then compared with the standard technique of cortical function mapping—electric cortical stimulation (ECS). The primary goal of our study was to compare the spatial resolution of both methods in locating the same function, and to learn more about fMRI limitations in surgical navigation. In addition, we were interested in clinical consequences of fMRI—navigated surgery manifested by neurological changes in our patients.
Materials and methods
During 2004–2007, we compared the results of fMRI and ECS of the primary hand motor area in 18 patients (12 males, 6 females, age 13–67 years) treated surgically at our department. All patients were enrolled prospectively after collective assessment of conventional T1-and T2-weighted magnetic resonance imaging (MRI) by a neuroanatomist, a neuroradiologist and a neurosurgeon. Each patient had a brain tumour localized within or in close proximity to the precentral gyrus. An informed consent concerning preoperative fMRI and intraoperative use of ECS was obtained from all patients, and the design of the study was approved by the local ethics committee.
Four patients had an isolated palsy of the hand, another four a hemiparesis and in three patients the weakness was accompanied by focal epileptic seizures. Six patients suffered solely from focal epileptic seizures and one from periodic headaches (Table 1). Using MRI volumetric analysis, three patients’ tumours were considered relatively small (not exceeding 10 cm3), twelve patients had medium-sized tumour (10–30 cm3), and in three patients the tumour volume exceeded 30 cm3. On T2-weighted images, the so called Ω-sign, corresponding to pli de passage moyen (PPM) as part of the middle bend of the precentral gyrus, and bulging into the central sulcus, was discernible in 6 patients while in the others it was blurred by oedema and/or infiltrative growth of the tumour.
fMRI was performed on a 1.5T Siemens Symphony scanner (Erlangen, Germany). The blood oxygenation-level dependent (BOLD) signal was detected using a gradient echo–planar T2*—weighted sequence with the following parameters: TR = 6000 ms; TA = 3244 TE = 54 ms; FA = 90°. During each fMRI session, lasting 6 min and 42 s, 64 dynamic scans were obtained consisting of 28 axial slices 4-mm thick. For morphological imaging, T1-weighted (TR = 2140 ms; TE = 3.93 ms; FA = 15°; TI = 1100 ms, 160 axial 1.6-mm thick slices) and T2-weighted (TR = 4930 ms; TE = 86 ms; FA = 150°; 28 axial 4-mm thick slices) sequences were added.
fMRI data preprocessing and statistical analysis were completed with SPM99 software (Wellcome Department of Cognitive Neurology, London, UK). The preprocessing involved realignment of images distorted by motion artifacts, and isotropic smoothing with a 10-mm Gaussian FWHM (full-width at half-maximum) filter. The primary sensorimotor cortex (SM1) was activated by voluntary rhythmic movements consisting of alternating touching between the tip of the thumb consecutively with the other fingers performed during the active phase of the task. Seven patients who were not able to execute this task due to a too severe weakness performed only repetitive opening and clenching of the hand. The data were analyzed using the general linear model in a block design with 4 alternating periods of active and resting phases, each covered by 8 dynamic scans. The results of individual analyses were thresholded at the P < 0.05 level of significance corrected for family wise error (FWE). For less conservative assessment, the uncorrected threshold of P < 0.001 was used. Clusters with a significant BOLD signal increase were subsequently merged to each patient’s T1-weighted image, which was then re-exported back to the DICOM format. This enabled registration of the fMRI results in the frameless stereonavigation system Treon (Medtronic, Minneapolis, MN) allowing visualization of the brain together with activated clusters in three orthogonal planes.
For electric cortical stimulation (ECS), an Ojemann bipolar stimulator (Radionics, Burlington, USA) with an electrode distance of 5-mm was used. The frequency of the stimulation current was 60 Hz with 0.2 ms pulse duration. The current was gradually elevated from 2 mA until a motor response was elicited or the maximum current of 8 mA was reached. This motor response to ECS was evaluated visually when involuntary contractions of the hand muscles occurred. The areas with positive motor response to ECS were never resected. Five patients had cortical stimulation performed during the awake phase of surgery, while the other patients had it performed under general anaesthesia after withdrawal of muscle relaxants. Total intravenous anaesthesia using propofol with ramifentanil was used in all patients.
After the images were co-registered in the neuronagivation system using ‘point merge’ and ‘surface merge’, the results of fMRI and ECS were compared for each patient separately. Sites with ECS-related motor responses were highlighted with sterile markers and registered by the neuronavigation system just before starting the tumour resection (Fig. 1). The distance between the marker representing the hand M1 cortex and the nearest border of the BOLD signal cluster as part of the SM1 cortex was measured using a sterile surgical ruler. The volumes of the tumour and BOLD signal clusters were then calculated using the Treon device software after manual delineation of the borders in each axial plane.
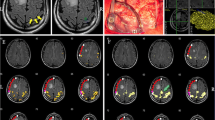
Excellent concordance between fMRI and ECS results in localizing primary hand motor cortex in patient (No.14) with oligodendroglioma (grade II). A, B, C—coronal, sagital, axial projection of the primary hand motor cortex according to ECS covered by BOLD activation as displayed during intraoperative neuronavigation D—intraoperative photograph; asterix = expected primary hand motor cortex according to fMRI; label Δ1 = area of motor response to ECS; TU and blue line = border between tumour and primary motor cortex I—BOLD activated areas (P < 0.05 FWE corrected) elicited by left hand tapping task II—postoperative T2-weighted MRI
MRI was repeated postoperatively in each patient. For metastases or high grade gliomas, the radicality of surgery was assessed using native and post-contrast T1-weighted MRI performed within 72 h after the surgery. For low grade gliomas grade II, the T2-weighted MRI was obtained one month after the surgery. The extent of tumour removal was classified as gross-total resection, near-total resection (≥90%), or subtotal (<90%) resection according to MRI volumetric analysis. Preoperative and postoperative neurological states were compared by the neurosurgeon before and three months after the surgery, using the British Medical Research Council (BMRC) grading scale, modified Rankin scale and Engel grading system.
Results
Clinical results
Only one patient had neurological morbidity in the patient’s group (Table 1). This was due to hemorrhage into the tumour-infiltrated precentral gyrus after partial resection of an anaplastic astrocytoma resulting in severe upper extremity paresis. After two months, however, this patient significantly improved from level 4 to 2 on the modified Rankin scale.
When present, palsies due to metastases (N = 6) improved in all cases, while palsies caused by high-grade gliomas (N = 5) showed temporary improvement only in one case. Low-grade gliomas constituted a specific subgroup (N = 6), in which temporary worsening occurred more frequently after gross-total resection. In this subgroup we observed hand weakness lasting one to three weeks in 3 patients, probably due to a lesion in the supplementary motor area (SMA) or parietal association areas. In the end, muscle strength became normal in all these patients. Moreover, in four low-grade glioma patients, the frequency of epileptic seizures decreased.
fMRI results
A significant BOLD signal increase in the primary motor cortex of the hemisphere ipsilateral to the tumour was observed in 17 patients. In one patient (No.1) it appeared on the contralateral side only, i.e. in the unaffected hemisphere. In 3 patients the fMRI results were adversely affected by motion artifacts.
In 8 patients we exported the fMRI results into the neuronavigation system at the P < 0.05 significance level corrected for FWE. The average volume of BOLD activation representing the SM1 cortex was 9 cm3 (variance: 6–11 cm3) in these patients.
In 10 patients there was no or minimal BOLD signal increase using the corrected threshold. Consequently, the threshold was changed to P < 0.001 level of significance uncorrected. Using this less conservative approach, the average SM1 cluster volume increased to 10 cm3 (2–22 cm3) in these patients (Table 2).
As preoperatively measured using the neuronavigation system, the distance between the BOLD signal cluster of the ipsilateral SM1 cortex and the tumour ranged from 0 to 25 mm.
ECS results
The hand M1 cortex was localized in all of the patients using ECS. As an adverse event, focal epileptic seizures were provoked in six (33%) patients; however, this did not result in any long-term complications, as all seizures rapidly resolved after local administration of ice-cold Ringer’s solution.
Motor response of the contralateral hand was elicited in 10 patients using the ECS in at least one of the two cortical areas (minimum 5 mm apart) within the M1 cortex. Eight patients showed a motor response during the stimulation in only one location.
The surgical approach to the tumour or its resection was less than 5 mm to the eloquent area in 15 patients; in three patients the distance of the tumour resection border from the motor cortex was about 10 mm (No. 1, 8, 18). In 6 patients (No. 2, 11, 14, 15, 16, 17) the border of the resection cavity was defined by a positive motor response to subcortical electric stimulation.
Comparison of the fMRI and ECS results
In twelve patients the results of both methods were in a full agreement, i.e., the motor response was successfully elicited from areas with significantly increased BOLD signal in the M1 cortical area. This was observed in seven patients evaluated conservatively at the P < 0.05 level with FWE correction (Fig. 1). In three patients (two of them at P < 0.05 corrected and in one patient at P < 0.001 uncorrected level) the agreement between the two methods was only partial with one positive motor response elicited from the cortex out of the BOLD signal cluster, albeit within the same gyrus.
The fMRI sensitivity (calculated as: sensitivity = fMRI / ECS * 100; fMRI = 20: number of cortical areas with positive motor response to stimulation elicited from the M1 BOLD cluster; ECS = 28: total number of cortical areas with positive motor response to stimulation regardless of the M1 BOLD cluster) for predicting the motor responses detectable with ECS was 71%. The distance between the positions of M1 cortices determined from both methods was less than 10 mm in 15 of 18 patients; i.e., fMRI provided correct guidance for ECS in 15 of the 18 patients (Table 2). Nonetheless, in the remaining 3 patients (P < 0.001 uncorrected), the results of the two methods were inconsistent. Due to the fact that falsely negative fMRI results might prevent correct M1 cortex localization, these will be discussed in more detail.
The first was a 36-year-old patient (No. 1) with an anaplastic astrocytoma in the right hemisphere resulting from a secondary malignant shift of an originally low-grade glioma infiltrating the central area. In this case BOLD activation was completely missing in the affected hemisphere when corrected thresholding (P < 0.05) was applied. With the significance level of P < 0.001 uncorrected, the SM1 ipsilateral to the affected hand was activated, suggesting a paradoxical compensatory ‘pseudodominance’ of the contralateral SM1 cortex. However, we identified clearly two functionally intact areas of the M1 near the tumour. According to post-resection MRI, these areas were affected by infiltrative growth of the tumour probably suppressing the BOLD signal.
In a 67-year-old patient (No. 6) with a metastasis in the parietal lobe surrounded by oedema, the M1 cortex was expected at the distance of one gyrus (18 mm) posteriorly to the tumour as shown by fMRI results with a threshold of P < 0.05 corrected for FWE. Using ECS, the functionally preserved M1 cortex was identified immediately behind the tumour. The BOLD signal was suppressed in this area even when thresholded at the P < 0.001 without correction.
BOLD signal suppression by collateral oedema also applied to a 50-year-old patient (No. 11) with a glioblastoma. According to fMRI (P < 0.001 uncorrected), the M1 was expected 25 mm in front of the tumour (Fig. 2) when it was actually located only 5 mm anteriorly as documented by ECS.
An example of insufficient concordance between fMRI nad ECS in patient (No.11) with glioblastoma multiforme. A, B—coronal and sagital projection of primary hand motor cortex according to fMRI C—intraoperative photograph demonstrating lack of fMRI / ECS concordance (the same labelling as in Fig. 1) I—BOLD activated areas (P < 0.001 uncorrected) elicited by repetitive left hand grasping II—sagital postoperative contrast T1-weighted MRI III—axial postoperative T2-weighted MRI
Discussion
The validity of fMRI has been evaluated in a number of studies; however, some of these reported only a limited number of patients [17, 30], or failed to compare the fMRI results with neuronavigation [17, 21, 28–30].
One of the largest studies compared the results of fMRI motor areas co-registered into neuronavigation with ECS in 32 patients who underwent surgery for a brain tumour or for chronic pain. There was a good correlation between the two methods in 87% of the patients [23]. In our series of 18 patients, the results were very similar. fMRI correctly guided ECS in 15 of our cases. A recent study [20] compared both methods in 21 patients with neuropathic pain. Concordance between contours of the fMRI activation cluster and ECS combined with the phase reversal of the somatosensory evoked potential (SEP) was found in 20 of the patients. When more restrictive analysis threshold values were used, the optimal concordance decreased to 65%. In our study, the distances between the fMRI activation cluster and ECS targets were within the range of 5 mm in 20/26 of our measurements. This corresponded with the results of a study [1] reporting a mean distance of 4.4–4.7 mm which is within the inherent error for target-localizing accuracy of neuronavigation system [8, 16].
The borders between activated and non-activated areas are not as distinct as the resulting fMRI colour maps might suggest. The outline of the cluster with an enhanced BOLD signal represents merely the statistical level of the map determining the likelihood of each voxel membership of either an active or an inactive area. Lowering the threshold will would cause the clusters to expand with an increased risk of encountering falsely positive areas, while raising the threshold is likely to produce the opposite effect.
The problem of choosing the optimal thresholding of fMRI results occurred in our study as well. While in 8 patients we used a more restrictive method based on the threshold of P < 0.05 with a conservative correction for multiple comparisons (FWE), in 10 patients it was necessary to employ a less conservative technique without FEW correction because otherwise there would have been no activation in the motor areas. However, using uncorrected P < 0.05 threshold usually causes an exaggerated false increase in the number and size of clusters. Consequently, a higher threshold of P < 0.001 was used instead. In our study, the best agreement between fMRI and ECS was obtained with the conservative thresholding (P < 0.05 corrected) which produced excellent or partial fMRI/ECS concordance in all 8 patients. With the BOLD signal suppressed or insufficiently enhanced, we had to use the less conservative approach (P < 0.001 uncorrected), with which were observed concordance in only 7 out of 10 patients. There were various reasons for the lower BOLD signal/noise ratio which will be discussed further.
One of them lies in the limited reproducibility of fMRI results. This applies to findings even in healthy individuals as Havel et al. [10] described during the course of three sessions in four anatomical regions involved in sensorimotor control. They suggested caution when using single session data for neuronavigation purposes. In our single session study, we detected lower interindividual dispersion of the BOLD clusters in cases of more conservative thresholding.
Variable fMRI results may depend on the choice of the motor task or the way it is performed. Finger movements in a patient with distal paresis can only elicit subliminal BOLD signal changes in the SM1 cortex whereas movements in a proximal unaffected part of the limb will produce good-quality fMRI results. When only the finger movement task is carried out, the SM1 cortex for the proximal part of the extremity cannot be visualized. The motor task can also be performed in imagery of motion. It is the premotor and the supplementary motor areas that are activated rather than SM1 in such a case. However, these areas play role in the planning and not in the actual control of movement [14, 22].
In agreement with other studies [28], we considered the persistence of motion artifacts to be another problem of falsely negative or positive fMRI results, despite using additional mathematical procedures to remove them. Moreover, the patient’s motivation and training the paradigm before the fMRI collection are of key importance. Roux et al. [23] recommend excluding from clinical use those evaluations in which the head movements extended beyond 5 mm, rather than correcting them. Haberg et al. [9] described a high occurrence (53%) of unsuccessful results of BOLD acquisition in patients, in whom craniotomy had been performed. We cannot unambiguously confirm this finding. In patients No. 13 and 18, who undergone craniotomy two and 5 years previously for an open biopsy and primary resection, sufficient BOLD signal was achieved after both levels of thresholding. Similarly, for patient No.15 who had craniotomy performed one week before the fMRI acquisition, good fMRI results were obtained. Another problem may be related to susceptibility artifacts caused by titanium plates, residual metallic particles from the drilling or hemorrhage during surgery for the tumour [13]. Therefore, preoperative T2-weighted MRI is recommended. In our study, the susceptibility artifacts were probably minimized by the non-use of titanium plates; all craniotomy bones were fixed with non-absorbable polyester sutures.
Another limitation of the fMRI in surgical navigation rests in the problem of BOLD signal suppression in the regions surrounding the tumour. Such false negatives can be caused by vascular response reduction in the proximity of the tumour caused by oedema or by direct infiltrative growth into still-functional cortex. Holodny et al. [11] considered this signal reduction to be directly proportional to the degree of neurological deficit. Our observations are in agreement with this hypothesis. In all of our three patients, in whom fMRI provided false negative results, moderate monoparesis was present at the time of fMRI acquisition. More recently, Hou et. al. [12] suggested that the BOLD signal decrease in close proximity to high-grade gliomas resulted from a rise in the regional cerebral blood flow, which is accompanied by failure of vasodilatation in response to increased neuronal activity. In addition, local ischemia might occur in the immediate neighbourhood of the tumour, causing the deoxy-hemoglobin level paradoxically to increase, which is likely to be misinterpreted during conventional statistical analysis of the BOLD signal [18].
Brain plasticity is another phenomenon which may affect the fMRI results in patients with brain tumours [5, 6, 20, 26]. Fandino et al. [6] reported functional reorganization of the M1 cortex even in the unaffected hemisphere. In contrast, Ulmer et al. [26] warned against overestimating this phenomenon in pre-surgical planning. In a subgroup of patients they observed a BOLD signal reduction in close proximity of the tumour accompanied by apparently increased contralateral activation suggesting compensatory ‘pseudo-dominance’ of the homotopic M1 cortex in the healthy hemisphere. However, one third of these cases had the BOLD signal around the lesion falsely negative. We came to the same conclusion in our patient No. 1 with the contralateral cortical ‘pseudo-dominance’ (Fig. 3) in whom the functionally preserved M1 cortex adjoining the tumour was localized only by intraoperative ECS.
There have been several attempts to use the fMRI for estimating the risk of postoperative neurological deterioration. The distance between the activated BOLD cluster and the tumour was found to be a significant predictor of such deficits. Neurological deterioration occurs more likely if this distance is less than 5 mm [15]. A more conservative study [9] considered the distance of 20 mm as entirely safe. In our series, no patient in whom the areas of BOLD activation were surgically respected suffered from a worsening of their neurological state postoperatively. We consider the effort to resect a part of the precentral gyrus infiltrated by the tumour, as very risky, especially in a patient with minimal symptoms. On the other hand, if the pial border of the precentral gyrus adjacent to the tumour is spared or the tumour affecting part of precentral gyrus is well circumscribed, resection can proceed to the immediate neighbourhood of the positive stimulation cortical area (Fig. 4).
A radical resection of oligodendroglioma (grade II) located in precentral gyrus in patient No.2 A—central area demonstrating PPM on an example of cadaver dissection (patient not from this study) B—intraoperative photograph: label □ 1, 3, 4—hand movement, label □ 2—elbow flexion I—BOLD activated area (P < 0.05 FWE corrected) elicited by left hand tapping task II—preoperative T2-weighted MRI of tumour partially involving the PPM III—postoperative T2-weighted MRI
Efforts to achieve maximum safety may involve visualizing the subcortical pathways using diffusion-weighted MRI tractography [19] or performing subcortical stimulation intraoperatively [5]. Methods for localizing the central sulcus by motor evoked potentials (MEP) elicited by transcranial magnetic stimulation or by reversal of the SEP in combination with continuous monitoring of the MEP elicited by high frequency cortical stimulation were found to be helpful in deeply situated lesions [2, 25]. The SEP phase reversal technique was a reliable method especially in very young children, in whom the motor pathways were not yet fully myelinated [7]. In addition, the Ω-sign (PPM)—a simple morphological MRI sign of primary hand motor area—should not be overlooked [1]. We had six patients in whom the PPM was clearly distinguishable on a preoperative conventional MRI, the hand motor response elicited with ECS was within this area. On the other hand, there was a higher probability of mismatch between fMRI and ECS results in cases where it was impossible to distinguish the PPM preoperatively due to oedema and/or tumour infiltration.
Conclusions
In our study the preoperative fMRI correctly localized the primary hand motor cortex in 15 patients who underwent surgery for a tumour in close proximity. Our results confirmed that the fMRI accuracy in surgical planning was mainly related to contamination of the BOLD signal by various artifacts. With high quality of input data, which were obtained in 8 patients, we could use a conservative approach for thresholding. In such patients the fMRI technique had good reliability and the fMRI / ECS concordance was reached in all these patients . With the BOLD signal suppressed or insufficiently enhanced, we had to use the less conservative approach, with which concordance was observed in only 7 out of 10 patients . On the other hand, in 3 cases we noticed a falsely negative fMRI, probably caused by local BOLD signal suppression due to collateral oedema and/or infiltrative tumour growth. These examples lead us to urge caution when using the results of fMRI in intraoperative navigation. We believe, that fMRI should be combined with ECS, which still remains the most reliable method for cortical mapping.
References
Boling W, Parsons M, Kraszpulski M, Cantrell C, Puce A (2008) Whole-hand sensorimotor area: cortical stimulation localization and correlation with functional magnetic resonance imaging. J Neurosurg 108:491–500. doi:10.3171/JNS/2008/108/3/0491
Cedzich C, Taniguchi M, Schafer S, Schramm J (1996) Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery 38:962–970. doi:10.1097/00006123-199605000-00023
Cohen MS (2000) Echo-Planar Imaging and Functional MRI. In: Moonen CTW, Bandettini PA (eds) Functional MRI. Springer-Verlag, Berlin Heidelberg, pp 137–148
DeYoe EA, Bandettini P, Neitz J, Miller D, Winans P (1994) Functional magnetic resonance imaging (FMRI) of the human brain. J Neurosci Methods 54:171–187. doi:10.1016/0165-0270(94)90191-0
Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Taillandier L, Lopes M, Mitchell MC, Roche S, Muller JC, Bitar A, Sichez JP, van Effenterre R (2003) Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg 98:764–778
Fandino J, Kollias SS, Wieser HG, Valavanis A, Yonekawa Y (1999) Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J Neurosurg 91:238–250
Goldring S (1978) A method for surgical management of focal epilepsy, especially as it relates to children. J Neurosurg 49:344–356
Gumprecht HK, Widenka DC, Lumenta CB et al (1999) BrainLab VectorVision Neuronavigation System: technology and clinical experiences in 131 cases. Neurosurgery 44:97–104. doi:10.1097/00006123-199901000-00056 discussion 104-105
Haberg A, Kvistad KA, Unsgard G, Haraldseth O (2004) Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery 54:902–914. doi:10.1227/01.NEU.0000114510.05922.F8 discussion 914-905
Havel P, Braun B, Rau S, Tonn JC, Fesl G, Bruckmann H, Ilmberger J (2006) Reproducibility of activation in four motor paradigms. An fMRI study. J Neurol 253:471–476. doi:10.1007/s00415-005-0028-4
Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA, Kalnin AJ (2000) The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 21:1415–1422
Hou BL, Bradbury M, Peck KK, Petrovich NM, Gutin PH, Holodny AI (2006) Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage 32:489–497. doi:10.1016/j.neuroimage.2006.04.188
Kim MJ, Holodny AI, Hou BL, Peck KK, Moskowitz CS, Bogomolny DL, Gutin PH (2005) The effect of prior surgery on blood oxygen level-dependent functional MR imaging in the preoperative assessment of brain tumors. AJNR Am J Neuroradiol 26:1980–1985
Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP (1993) Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science 261:615–617. doi:10.1126/science.8342027
Krishnan R, Raabe A, Hattingen E, Szelenyi A, Yahya H, Hermann E, Zimmermann M, Seifert V (2004) Functional magnetic resonance imaging-integrated neuronavigation: correlation between lesion-to-motor cortex distance and outcome. Neurosurgery 55:904–914 discusssion 914-905
Mascott CR, Sol JC, Bousquet P, Lagarrigue J, Lazorthes Y, Lauwers-Cances V (2006) Quantification of true in vivo (application) accuracy in cranial image-guided surgery: influence of mode of patient registration. Neurosurgery 59:ONS146–ONS156. doi:10.1227/01.NEU.0000220089.39533.4E discussion ONS146-156
Mueller WM, Yetkin FZ, Hammeke TA, Morris GL 3rd, Swanson SJ, Reichert K, Cox R, Haughton VM (1996) Functional magnetic resonance imaging mapping of the motor cortex in patients with cerebral tumors. Neurosurgery 39:515–520. doi:10.1097/00006123-199609000-00015 discussion 520-511
Murata Y, Sakatani K, Katayama Y, Fujiwara N, Hoshino T, Fukaya C, Yamamoto T (2004) Decreases of blood oxygenation level–dependent signal in the activated motor cortex during functional recovery after resection of a glioma. AJNR Am J Neuroradiol 25:1242–1246
Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, Fahlbusch R (2005) Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery 56:130–137 discussion 138
Pirotte B, Neugroschl C, Metens T, Wikler D, Denolin V, Voordecker P, Joffroy A, Massager N, Brotchi J, Levivier M, Baleriaux D (2005) Comparison of functional MR imaging guidance to electrical cortical mapping for targeting selective motor cortex areas in neuropathic pain: a study based on intraoperative stereotactic navigation. AJNR Am J Neuroradiol 26:2256–2266
Pujol J, Conesa G, Deus J, Vendrell P, Isamat F, Zannoli G, Marti-Vilalta JL, Capdevila A (1996) Presurgical identification of the primary sensorimotor cortex by functional magnetic resonance imaging. J Neurosurg 84:7–13
Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD et al (1993) Functional magnetic resonance imaging of complex human movements. Neurology 43:2311–2318
Roux FE, Ibarrola D, Tremoulet M, Lazorthes Y, Henry P, Sol JC, Berry I (2001) Methodological and technical issues for integrating functional magnetic resonance imaging data in a neuronavigational system. Neurosurgery 49:1145–1156. doi:10.1097/00006123-200111000-00025 discussion 1156-1147
Schulder M, Maldjian JA, Liu WC, Holodny AI, Kalnin AT, Mun IK, Carmel PW (1998) Functional image-guided surgery of intracranial tumors located in or near the sensorimotor cortex. J Neurosurg 89:412–418
Taniguchi M, Cedzich C, Schramm J (1993) Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery 32:219–226
Ulmer JL, Hacein-Bey L, Mathews VP, Mueller WM, DeYoe EA, Prost RW, Meyer GA, Krouwer HG, Schmainda KM (2004) Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery 55:569–579. doi:10.1227/01.NEU.0000134384.94749.B2 discussion 580-561
Villringer A (2000) Physiological changes during brain activation. In: Moonen CTW, Bandettini PA (eds) Functional MRI. Springer-Verlag, Berlin Heidelberg, pp 3–13
Yetkin FZ, Mueller WM, Morris GL, McAuliffe TL, Ulmer JL, Cox RW, Daniels DL, Haughton VM (1997) Functional MR activation correlated with intraoperative cortical mapping. AJNR Am J Neuroradiol 18:1311–1315
Yetkin FZ, Papke RA, Mark LP, Daniels DL, Mueller WM, Haughton VM (1995) Location of the sensorimotor cortex: functional and conventional MR compared. AJNR Am J Neuroradiol 16:2109–2113
Yousry TA, Schmid UD, Jassoy AG, Schmidt D, Eisner WE, Reulen HJ, Reiser MF, Lissner J (1995) Topography of the cortical motor hand area: prospective study with functional MR imaging and direct motor mapping at surgery. Radiology 195:23–29
Acknowledgment
This study was supported by the Czech Ministry of Education (research program MŠM 0021620849) and by the Grant Agency of Czech Republic 309/09/1145.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The authors report a series of 18 consecutive patients who underwent surgery for a central tumor. Using a neuronavigation system, they compared the results provided by preoperative motor functional MRI (fMRI) and intraoperative electrical stimulation. They showed that the fMRI sensitivity for localizing the primary motor cortex detected by electrostimulation was 71%.
Although such comparison has been made in many previous studies, the present results provide additional arguments supporting the fact that fMRI is not so reliable. The mechanisms of such low reliability have been well discussed by the authors. Therefore, on the basis of these data, it is important to keep in mind that intraoperative electrical mapping remains the gold standard, and that neurosurgeons have to be cautious when using solely fMRI for brain surgery within eloquent areas, even in motor region—indeed, it has already been demonstrated that the reliability of fMRI was poor for language and cognitive functions. As a consequence, combination of “classical” intrasurgical electrophysiology with new neuroimaging methods (fMRI and diffusion tensor imaging) is presently still mandatory.
Hugues Duffau,
Montpellier, France
Rights and permissions
About this article
Cite this article
Bartoš, R., Jech, R., Vymazal, J. et al. Validity of primary motor area localization with fMRI versus electric cortical stimulation: A comparative study. Acta Neurochir 151, 1071–1080 (2009). https://doi.org/10.1007/s00701-009-0368-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-009-0368-4