Abstract
Intracranial endovascular procedures are less invasive and relatively safe; however, these procedures do carry a risk of complications, such as thromboembolization, arterial injury, and vessel occlusion. We present a case of carotid-cavernous fistula development secondary to injury of the cavernous segment of the internal carotid artery (ICA) during stent angioplasty and its treatment by transarterial coil embolization. Probable causes of this complication and its treatment method are discussed. To the best of our knowledge, this is the first report of such a case.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracranial stent angioplasty is relatively easy and safe for well-selected cases and medically unstable or elderly patients can be treated under local anesthesia; however, it harbors many possible complications [5, 7, 13, 19, 20]. In this report, the authors present a case of direct carotid-cavernous fistula (DCCF) by arterial injury during stent angioplasty and its endovascular treatment. Here, the probable causes of DCCF and its treatment methods are discussed.
Case report
An 81-year-old man was admitted complaining of grade IV right hemiparesis with motor dysphasia. These symptoms had fluctuated over the past 2 weeks. In addition, the patient had a past medical history significant for hypertension, hyperlipidemia, diabetes mellitus, and chronic obstructive pulmonary disease. Brain magnetic resonance imaging (MRI) showed acute hemodynamic infarction scattered in the left cerebral hemisphere and perfusion studies showed delayed time to peak and decreased perfusion reservoir (Fig. 1a–c). Standard cerebral digital subtraction angiogram (DSA) revealed complete occlusion of left internal carotid artery (ICA) with collateral circulation from the right side through the anterior communicating (a-com) artery and moderate stenosis in the posterior genu of the right cavernous ICA (Fig. 1d, e). All of these symptoms and results of imaging studies indicated that this patient needed revascularization for the left cerebral hemisphere. As opposed to a bypass surgical procedure under general anesthesia, the authors attempted angioplastic dilatation of the stenotic segment of the right ICA in order to augment left cerebral hemisphere collateral flow through the a-com artery for this medically unstable patient.
a The perfusion computed tomography (CT) shows time-to-peak flow delay on left cerebral hemisphere, which is 0.69:1.46 compared with the right middle cerebral artery (MCA) territory. b Basal brain SPECT images. c Diamox-challenging SPECT images. These pictures indicate a decreased perfusion reservoir in the left cerebral hemisphere. Anteroposterior (d) and lateral (e) projection of right ICA angiography. Blood supply to the left cerebral hemisphere is totally dependent on the right ICA system (arrowheads in d). The cavernous segment of the right ICA is moderately stenotic (arrow in e)
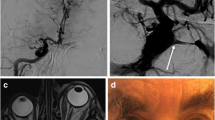
Preoperative routine CT angiography showed multiple calcification of the right cavernous ICA in its two-dimensional multi-sliced images (Fig. 2). The authors obtained a measured diameter and length of the ICA to be covered by stent from CT angiogram and cerebral DSA as 4.5 mm and 13 mm, respectively. The procedure was performed according to the standard technique of stent angioplasty with caution not to injure the ICA of this old-age, tortuous and calcified lesion. Because of the tortuousness of the ICA, it was very difficult to advance the stent delivery catheter. Deployment of a balloon-mounted bare-metal DRIVER stent 4.5-mm wide and 15-mm long (Medtronic, Minn., USA) was followed by balloon dilatation. It was dilated to subnominal pressure as a 7-atm dilating stent to 4.34 mm. It was ballooned in a graded manner lasting about 15 s. During balloon dilation, the patient experienced a generalized tonic clonic seizure that lasted for approximately 10 s. Angiography confirmed proper positioning of the implant and sufficient widening of the stenotic lesion. However, in the early arterial phase of the angiogram, DCCF was found without cortical reflux (Fig. 3a). A follow-up brain perfusion CT the next morning showed aggravated time-to-peak flow delay in the left cerebral hemisphere, indicative of flow-steal by CCF (Fig. 3b). The patient’s neurological status was also deteriorating, and the authors decided to occlude the fistula.
a A DCCF occurred immediately after stent deployment. The fistula drains mainly into the inferior petrosal sinus and pterygoid plexus. There is no reflux to the superior ophthalmic vein or cortical veins. b More aggravated time-to-peak flow delay on left cerebral hemisphere and this indicates steal of flow by CCF
The exact fistulous site could not be located because of its fast shunt. Thus, embolization with Tornado coils (Cook, Bloomington, Ind., USA) via the inferior petrosal sinus for reduction of cavernous sinus reflux was followed by transarterial GDC coil (Boston Scientific Target, Calif., USA) embolization through the fistulous point. The DCCF was completely occluded without stent luminal narrowing or coil migration and clinical status improved with recovery of perfusion delay (Fig. 4). Ten months of clinical follow-up showed a modified Rankin scale of grade 2 without any fluctuation of ischemic symptoms.
a Complete obliteration of DCCF by coil embolization with preservation of in-stent luminal integrity. The stenotic lesion is fully dilated and there is no coil herniation. b Improved perfusion to the left cerebral hemisphere. Time-to-peak ratio compared with the right MCA territory improved from 0.60:1.67 of perfusion CT before coil embolization of DCCF to 0.72:1.38. This is also an improved result compared with initial perfusion CT in Fig. 1
Discussion
There are many possible complications of intracranial stent angioplasties, most commonly thromboembolization; however, arterial injury, hyperperfusion syndrome and stent occlusion by acute or subacute thrombosis could also occur [5, 13, 19, 20]. Arterial wall injury can cause particularly devastating clinical results, such as subarachnoid hemorrhage, thrombosis, and infarction. However, the reports of iatrogenic DCCF during endovascular or other intracranial surgical procedures are a few in number [8, 9, 12, 15, 21], and there are even fewer reports of stent-graft treatment of DCCF which occurred during neurointervention [8, 12]. To our knowledge, the development of DCCF after a stent angioplasty and its treatment with transarterial coil embolization has not been reported.
It is very important to predict the factors that would lead to this complication in order to avoid it. In this case, we could consider factors related to arterial wall injury into two categories. One category is patient factors, such as vascular tortuousness, calcification and intolerance to ischemia. The tortuousness weakens the tracking force of the stent delivery catheter by dispensing it to various vectors. Struggling to advance the catheter accumulates force on the tip of the stent delivery catheter, which could eventually injure the arterial wall [3, 6, 17]. Calcification of the vessel wall is another factor, which was observed in the ICA on the axial raw data of initial CT angiography (Fig. 3). Calcification is an outgrowth of atherosclerosis that reduces vascular elasticity and compliance [17]. During ballooning, the genu portion of the ICA could be stretched to some degree, and the concave portion of the stenotic calcified ICA might have been ruptured. A third factor is intolerance to ischemia because the patient’s brain is totally dependent on a unilateral blood supply [2]. Therefore, when the balloon was dilated, the ischemic time might exceed the threshold of ischemic tolerance, causing seizure. This could cause the operator to hurry through the procedure and eventually over-dilate the balloon. The seizure might occur not by ischemic intolerance but also by sudden occurrence of DCCF. The other category includes technical and stent factors. Thus, the stiffness of the coronary stent could harm the arterial wall. Excessive balloon dilatation by overestimation of vessel diameter could also be a cause [3, 8, 15]. Sufficient preoperative evaluation of the risk factors and indication, and experience of highly trained surgeons is very important for overcoming all of these obstacles. Moreover, even though its long-term follow-up result is not quite satisfactory nor established, future consideration of a newly developed self-expending stent is warranted. Predilatation provides a route of atraumatic passage for the soft-profiled self-expandable stent and reduces the resistance to expansion of the stent [3, 11, 22].
Since Serbinenko [18] reported his experience with a detachable balloon, endovascular treatment has become the first choice for the treatment of DCCF. Fistula occlusion using a detachable balloon is the preferred method for treating DCCF while preserving a patent parent ICA; however, this method may fail if the fistula is too small or large or cannot be reached due to its location [1]. Additionally, the balloon sometimes deflates within a few hours after deployment, which may result in recurrence of the fistula. Several reports about stent-graft for treatment of DCCF with various causes have been published recently [8, 9]. A covered stent graft is an ideal material theoretically because it isolates blood flow from the fistula. However, because of its thick profile, trackability is very low in a tortuous vascular environment. Deployment of the stent graft for this case could cause another arterial wall injury and additional luminal narrowing.
Transarterial coil embolization of the DCCF is feasible for small fistulas, and can be accomplished safely with the aid of a stent to prevent coil migration into the parent artery [1, 4, 10, 14, 16]. In this case, it was performed easily by selecting a fistula site with a microcatheter after reducing the shunt velocity by transvenous partial embolization. A previously placed coronary stent was helpful in detaching the coil safely without coil migration into the ICA.
Vascular tortuousness, calcification, intolerance to ischemia, overestimation of the size of the artery, overdilatation and other specific characters of stents should be considered as predictive factors of procedure-related arterial injury. Here, the authors report a rare case of transarterial coil embolization of DCCF, which was caused by stent angioplasty.
References
Ahn JY, Lee BH, Joo JY (2003) Stent-assisted Guglielmi detachable coils embolisation for the treatment of a traumatic carotid cavernous fistula. J Clin Neurosci 10:96–98
Chaer RA, Trocciola S, DeRubertis B, Lin SC, Kent KC, Faries PL (2006) Cerebral ischemia associated with PercuSurge balloon occlusion balloon during carotid stenting: Incidence and possible mechanisms. J Vasc Surg 43:946–952 discussion 952
Chiam PT, Samuelson RM, Mocco J, Hanel RA, Siddiqui AH, Hopkins LN, Levy EI (2008) Navigability trumps all: stenting of acute middle cerebral artery occlusions with a new self-expandable stent. AJNR Am J Neuroradiol
Eddleman CS, Surdell D, Miller J, Shaibani A, Bendok BR (2007) Endovascular management of a ruptured cavernous carotid artery aneurysm associated with a carotid cavernous fistula with an intracranial self-expanding microstent and hydrogel-coated coil embolization: case report and review of the literature. Surg Neurol 68:562–567
Friedman JA, Kallmes DF, Wijdicks EF (2004) Thalamic hemorrhage following carotid angioplasty and stenting. Neuroradiology 46:399–403
Georganos SA, Guilbert F, Salazkin I, Gevry G, Raymond J (2004) Surgical construction of an in vivo carotid siphon model to test neurovascular devices. Neurosurgery 54:1239–1243 discussion 1243
Kim JK, Ahn JY, Lee BH, Chung YS, Chung SS, Kim OJ, Kim WC, Joo JY (2004) Elective stenting for symptomatic middle cerebral artery stenosis presenting as transient ischaemic deficits or stroke attacks: short term arteriographical and clinical outcome. J Neurol Neurosurg Psychiatry 75:847–851
Kim SH, Qureshi AI, Boulos AS, Bendok BR, Levy EL, Yahia AM, Guterman LR, Hopkins LN (2003) Intracranial stent placement for the treatment of a carotid-cavernous fistula associated with intracranial angioplasty. Case report. J Neurosurg 98:1116–1119
Kocer N, Kizilkilic O, Albayram S, Adaletli I, Kantarci F, Islak C (2002) Treatment of iatrogenic internal carotid artery laceration and carotid cavernous fistula with endovascular stent-graft placement. AJNR Am J Neuroradiol 23:442–446
Koyanagi M, Nishi S, Hattori I, Horikawa F, Iwasaki K (2004) Stent-supported coil embolization for carotid artery pseudoaneurysm as a complication of endovascular surgery—case report. Neurol Med Chir (Tokyo) 44:544–547
Layton KF, Hise JH, Thacker IC (2008) Recurrent intracranial stenosis induced by the Wingspan stent: comparison with balloon angioplasty alone in a single patient. AJNR Am J Neuroradiol 29:1050–1052
Lopez-Quinones M, Bargallo X, Blasco J, Real MI, Gonzalez S, Bunesch L, Gilabert R (2006) Iatrogenic carotid-jugular arteriovenous fistula: color Doppler sonographic findings and treatment with covered stent. J Clin Ultrasound 34:301–305
Macdonald S (2007) Brain injury secondary to carotid intervention. J Endovasc Ther 14:219–231
Men S, Ozturk H, Hekimoglu B, Sekerci Z (2003) Traumatic carotid-cavernous fistula treated by combined transarterial and transvenous coil embolization and associated cavernous internal carotid artery dissection treated with stent placement. Case report. J Neurosurg 99:584–586
Mollers MO, Reith W (2004) Intracranial arteriovenous fistula caused by endovascular stent-grafting and dilatation. Neuroradiology 46:323–325
Moron FE, Klucznik RP, Mawad ME, Strother CM (2005) Endovascular treatment of high-flow carotid cavernous fistulas by stent-assisted coil placement. AJNR Am J Neuroradiol 26:1399–1404
Nicita-Mauro V, Maltese G, Nicita-Mauro C, Basile G (2007) Vascular aging and geriatric patient. Minerva Cardioangiol 55:497–502
Serbinenko FA (1971) Occlusion of the cavernous portion of the carotid artery with a balloon as a method of treating carotid-cavernous anastomosis. Vopr Neirokhir 35:3–9
Terada T, Tsuura M, Matsumoto H, Masuo O, Tsumoto T, Yamaga H, Ohura Y, Itakura T (2006) Hemorrhagic complications after endovascular therapy for atherosclerotic intracranial arterial stenoses. Neurosurgery 59:310–318 discussion 310–318
Theron J, Guimaraens L, Coskun O, Sola T, Martin JB, Rufenacht DA (1998) Complications of carotid angioplasty and stenting. Neurosurg Focus 5:e4
Vanninen RL, Manninen HI, Rinne J (2003) Intrasellar latrogenic carotid pseudoaneurysm: endovascular treatment with a polytetrafluoroethylene-covered stent. Cardiovasc Intervent Radiol 26:298–301
Zaidat OO, Wolfe T, Hussain SI, Lynch JR, Gupta R, Delap J, Torbey MT, Fitzsimmons BF (2008) Interventional acute ischemic stroke therapy with intracranial self-expanding stent. Stroke 39:2392–2395
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
This paper reports an imaginative and technically challenging case describing an endovascular approach in which a high-flow CCF was closed by using detachable coils that were introduced into the cavernous sinus from a venous and an arterial approach, while preserving the ICA and restoring the normal hemispheric ICA flow. Most interestingly, none of this was originally planned, but rather executed “à la Carte” to sequentially solve ensuing problems. However, contrary to the authors claim, there have been several case reports of patients with direct CCFs as a result of iatrogenic internal carotid artery laceration associated with intracranial angioplasty [1–3] or after transsphenoidal surgery for pituitary adenoma [4, 5] treated by stent placement and stent-assisted coiling placement.
After the analysis of the morphology of the fistula, we believe that the complication derived from both stent-related problems (the use of a coronary stent, stent oversize and stiffness, which may result in arterial rupture) and patient-related problems (calcification, plaque rupture and tortuousness of ICA due to age). As one might conclude in this case, identification of appropriate candidates for treatment remains a challenge. Collaterally, would the authors systematically advise a brain CT angiogram as a pre-treatment work-up tool in older patients to find out ICA calcifications?
Additionally, an accurate measure of the size of the ICA is essential, because this allows a correct choice of the size of the stent to be deployed. It is known that a high-pressure inflation is often performed in coronary stent placement to optimize the stent apposition to the arterial wall. However, in the case of intracranial vessels, the potential risk of rupture from high-pressure inflations may be greater due to a lack of surrounding supportive tissue in the subarachnoid space. But, considering the risk of vessel-wall rupture, the maximal pressure in the balloon may not be as important as the rate of inflation. The question of what constitutes a safe inflation pressure and rate in the intracranial vessels is an important one. Equally, with balloon-expandable stents, as used in this patient, adequate predilatation of the vessel may be crucial. Predilatation prepares a route of atraumatic passage for the balloon-mounted stent and reduces the resistance to expansion of the stent during inflation. Vascular biomechanics are important to define differences between cerebral arteries and extracranial vessels and partly explain the technical challenges facing cerebral artery revascularization compared with revascularization of coronary arteries. In terms of a safe high-flow fistula embolisation, it may be important to consider the use of a balloon that could be insufflated inside the stent to prevent coils from herniating through open cells of the stent into the parent artery. These are issues that are open to discussion.
Finally, the authors could have commented on the newly available covered, or self-expanding stents as a conceptual idea to surmount these complications in the future, and perhaps compare this case with their center’s experience with the new intracranial devices. We agree that covered stents may be technically difficult to deliver, especially in elderly patients with tortuous vessels, as it was the case here.
Overall, this paper raises the question of the intracranial use of coronary stent. Stents are very useful devices that allow initial reconstruction of the damaged segment of the ICA and then controlled coil deposition into the cavernous sinus in CCF. Balloon-expandable coronary stents used “off-label” or with modified coronary devices in approval studies showed a wide range of complication rates and variable short-term follow-up results and raised doubts concerning the clinical effectiveness of the treatment [6–10].
References
1. Weber W, Henkes H, Berg-Dammer E, Esser J, Kuhne D. (2001) Cure of a direct carotid cavernous fistula by endovascular stent deployment. Cerebrovasc Dis 12:272–275
2. Kim SH, Qureshi AI, Boulos AS et al (2003) Intracranial stent placement for the treatment of a carotid-cavernous fistula associated with intracranial angioplasty: case report. J Neurosurg 98:1116–119
3. Mollers MO, Reith W.(2004) Intracranial arteriovenous fistula caused by endovascular stent-grafting and dilatation. Neuroradiology 46: 323–325
4. Kocer N, Kizilkilic O, Albayram S, Adelatli I, Kantarci F, Islak C (2002) Treatment of iatrogenic internal carotid artery laceration and carotid cavernous fistula with endovascular stent-graft placement. AJNR Am J Neuroradiol 23:442–446
5. Vannien RL, Manninen HI, Rinne J (2003) Intrasellar latrogenic carotid pseudoaneurysm: endovascular treatment with a polytetrafluoroethylene-covered stent. Cardiovasc Intervent Radiol 26:298–301
6. SSYLVIA Study Investigators (2004) Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke 35:1388–1392
7. du Mesnil de Rochemont, Turowski B, Buchkremer M, Sitzer M, Zanella FE, Berkefeld J (2004) Recurrent symptomatic high-grade intracranial stenoses: safety and efficacy of undersized stents—initial experience. Radiology 231:45–49
8. Weber W, Mayer TE, Henkes H, Kis B, Hamann GF, Schulte-Altedornenburg G, Brückmann H, Kuehne D (2005) Stentangioplasty of intracranial vertebral and basilar artery stenoses in symptomatic patients. Eur J Radiol 55:231–236
9. Yu W, Smith WS, Singh V, Ko NU, Cullen SP, Dowd CF, Halbach VV, Higashida RT (2005) Long-term outcome of endovascular stenting for symptomatic basilar artery stenosis. Neurology 64:1055–1057
10. Chow MM, Masaryk TJ, Woo HH, Mayberg MR, Rasmussen PA (2005) Stent-assisted angioplasty of intracranial vertebrobasilar atherosclerosis: midterm analysis of clinical and radiologic predictors of neurological morbidity and mortality. AJNR Am J Neuroradiol 26(4):869–874
Oscar L. Alves
Portugal
Rights and permissions
About this article
Cite this article
Yoon, W.K., Kim, Y.W., Kim, S.R. et al. Transarterial coil embolization of a carotid-cavernous fistula which occurred during stent angioplasty. Acta Neurochir 151, 849–854 (2009). https://doi.org/10.1007/s00701-009-0351-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-009-0351-0