Abstract
The amphi-Adriatic region, and especially the Western Balkan Peninsula, belongs to the most important biodiversity hotspots in the temperate region. Nevertheless, detailed phylogeographic and molecular systematic studies in the Western Balkan are rare due to sporadic sampling in regions, where access has been, until recently, restricted by war. The Cardamine maritima group, which is the focus of this study, comprises not only the currently recognised species C. maritima and C. monteluccii, but also other taxa, which have been rendered to synonymy by most of the national floras and checklists. Molecular data acquired by the amplified fragment length polymorphism method showed a clear pattern within the group. Italian populations of C. monteluccii are well separated from Balkan taxa. In a step forward from previous taxonomic confusion surrounding Balkan populations, the present study confirms that five allopatric units—each with a clearly delimited and a rather restricted distribution range—can be easily recognised here. They correspond to C. fialae, C. serbica, C. rupestris, and two genetically distant and allopatric units within C. maritima. While individual taxa gained high bootstrap support in the neighbour-joining tree, there is low support for the internal nodes and it is hard to infer any relationships among taxa based on this information. The majority of Balkan populations of the C. maritima group exhibit features of genetic variability that enable us to hypothesise that these populations are relic ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Balkan Peninsula, with its environmental stability and topographic and climatic diversity, is a fascinating region for phylogenetic and phylogeographic studies, because it harbours high floral and faunal diversity (Turrill 1929; Polunin 1997; Kryštufek and Reed 2004), served as a refugium during the Pleistocene (Comes and Kadereit 1998; Hewitt 2000; Hampe et al. 2003; Petit et al. 2003; Eastwood 2004; Tzedakis 2004; Médail and Diadema 2008), and has received very little attention until now. Examples of a few previous studies include examinations of genetic diversity within and between species of Balkan beech populations (Gömöry et al. 1999) using isozymes; the Balkan-endemic Martino’s vole (Kryštufek et al. 2007), isophyllous species of Campanula (Park et al. 2006) and the genus Heliosperma (Frajman and Oxelman 2007) all by employing DNA sequence data.
Climatic fluctuations of the late Pliocene and Pleistocene and subsequent changes in Adriatic sea level led to the formation of landbridges in the northern part of the sea (Colantoni et al. 1979; Voges 1995), enabling trans-Adriatic exchange, while in warmer periods the northern Adriatic basin was flooded and acted as a barrier, supporting vicariance events in a previously continuous distribution area. Park et al. (2006) hypothesised that repeated cycles of isolation of populations during climatically more favourable periods reinforced speciation of Campanula taxa on the east and west Adriatic coasts. Similarly, isolation of populations on a small geographical scale during interglacial periods, with secondary contacts during glacial maxima, shaped the present patterns of genetic variation in Martino’s vole (Dinaromys bogdanovi, Kryštufek et al. 2007) and the Heliosperma pusillum group (Frajman and Oxelman 2007) within the western Balkan Peninsula. It can be expected that similar evolutionary patterns exist in other species.
The amphi-Adriatic region, and especially the Western Balkan Peninsula, is important for better understanding the diversity and history of the Mediterranean flora. Detailed studies of genetic variation of taxa in this region are also important for establishing conservation priorities. Nevertheless, such small-scale phylogeographic and other molecular studies in the Western Balkan are rare due to sporadic sampling in regions where access has been, until recently, restricted by war (but see Park et al. 2006; Kryštufek et al. 2007; Frajman and Oxelman 2007).
The genus Cardamine is represented in Europe by approximately 54 species (Lihová and Marhold 2006). Our current study concentrates on the least-known European species group of the genus, which comprises the Western Balkan C. maritima DC. (Candolle 1821) (including C. fialae Fritsch) and the Apennine C. monteluccii Br.-Cat. & Gubell. (Brilli-Cattarini and Gubellini 1986) in the circumscription as given in the Flora Europaea (Jones and Akeroyd 1993) and Atlas Florae Europaeae (Jalas and Suominen 1994). Although only these two species are currently generally accepted, another four taxa from the Western Balkan Peninsula were described as being part of this group, namely C. fialae Fritsch (Fritsch 1897), C. maritima var. maglicensis Rohlena (Rohlena 1906), C. serbica Pančić (Pančić 1884), and C. maritima “proles” rupestris O. E. Schulz (Schulz 1903). Jones and Akeroyd (1993) and Jalas and Suominen (1994) doubted the status of C. fialae, considering it to be “perhaps a subspecies of C. maritima.”
Data from Brilli-Cattarini and Gubellini (1986) and our preliminary results (J. Kučera et al., unpublished data) have shown that the taxa of this group are diploid. Populations included here are annual or biennial, and they occupy calcareous rocks and sliderocks. Both autogamy and allogamy were proven in a preliminary study of the breeding systems of C. maritima, C. monteluccii, and C. rupestris (J. Kučera, unpublished data).
Results of the study of hexaploid C. asarifolia L. by Lihová et al. (2006), which was based on ITS and CHS sequences and which also included some taxa treated here as C. maritima, C. rupestris (O.E. Schulz) K. Maly, C. fialae, C. serbica, and C. monteluccii, indicate that the C. maritima group is monophyletic. The cpDNA sequences (trnL-trnF), however, are not as conclusive (Lihová et al. 2006). This study also indicated this group’s basal position among European Cardamine diploids, as well as its considerable genetic variation.
More than a century ago, Fritsch (1895) and also Schulz (1903) hypothesised that C. maritima is a hybrid species, which arose from the hybridisation of C. glauca DC. and C. graeca L. According to Schulz (1903, p. 580), C. maritima “proles” rupestris is morphologically closer to C. glauca, while C. maritima “proles” serbica (Pančić) O. E. Schulz more closely resembles C. graeca. C. rupestris is sometimes considered to be a hybrid between C. glauca and C. maritima (Beck 1903; Hayek 1927; Rohlena 1942). Indeed, both C. glauca and C. graeca—like C. maritima—were shown to occupy a basal position with respect to other European diploid Cardamine taxa (Lihová et al. 2006).
The distribution areas of Cardamine maritima and related taxa on the Western Balkan Peninsula largely coincide with major refugia of Mediterranean plants during glacial periods (Médail and Diadema 2008). Refugial populations usually harbour higher allelic diversity compared with those that experienced serious bottlenecks (Widmer and Lexer 2001). It is therefore expected that the genetic variation and consequently also the taxonomic structure of the group of C. maritima may differ from those of other taxa of the genus Cardamine distributed in the areas seriously influenced by Pleistocene glaciations (Lihová et al. 2003; Kučera et al. 2006; Perný et al. 2005a, b, c).
The main questions addressed by this study are as follows: (1) What are the patterns of genetic variation among populations of C. monteluccii, broadly conceived C. maritima, and the related taxa C. glauca and C. graeca? (2) Is there any support for the currently recognised or previously described taxa; should they be taxonomically recognised? (3) Is there any genetic evidence among the studied taxa, supporting small-scale (mainly altitudinal) distribution shifts influenced by glaciation events as opposed to the large-scale latitudinal shifts? In contrast to species in northern European areas, species in southern European regions survived Pleistocene glaciations without large geographical displacements, but rather by ascending or descending mountains, as exemplified in Armeria (Gutiérrez Larena et al. 2002). The hypothesised long-term in situ survival in mountains of the Balkan Peninsula without the need for large-scale geographical migrations and gene flow should result in isolated and highly divergent genetic entities, which should coincide with mountain ranges. A high number of fragments should be found exclusively in separated mountain ranges.
Amplified fragment length polymorphism (AFLP, Vos et al. 1995) is a standard marker for assessing inter- (Prohens et al. 2006; Guo et al. 2005; Nguyen et al. 2004) and intraspecific (Pilon et al. 2007; Marghali et al. 2005; Juan et al. 2004) genetic variation and differentiation. Despite some concerns that AFLP fragments of the same length seen in two species might not be homologous and/or some fragments might not be independent, it was suggested that this method can be used for phylogenetic reconstructions, especially for species groups that have diverged recently (e.g. Bussell et al. 2005; Koopman 2005). Although a dominant marker, AFLP is powerful in addressing questions like those in our study (e.g. Prohens et al. 2006; Lara-Cabrera and Spooner 2004) and was also successfully applied to other Cardamine species complexes—both diploid and polyploid (Lihová et al. 2003, 2004a, b; Perný et al. 2005a, b).
Materials and methods
Plant material
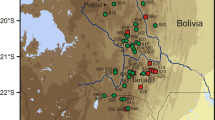
Plant material was collected with the aim of getting representative samples of C. maritima and related taxa from both the Apennine and Balkan Peninsulas. Material includes samples from populations previously classified as C. maritima, C. rupestris, C. monteluccii, C. fialae, C. maritima var. maglicensis, and C. serbica, including type localities of the latter three taxa. In addition, samples from two populations of C. graeca (from the Apennine and Balkan Peninsulas) and from five populations of C. glauca were included, as these taxa were hypothesised to be parental species of C. maritima and, like this species, were shown to be in a basal position with respect to other European diploid Cardamine taxa (Lihová et al. 2006). Samples included silica-gel-dried material from 1 to 5 individual plants per population. Details of the localities are given in Table 1, and their locations are shown in Fig. 1a, b.
a Map showing the distribution of the sampled material of C. monteluccii (circle with cross), C. maritima populations from Croatia (square with cross), C. maritima populations from Montenegro (square), C. fialae (circle with dot), C. rupestris (triangle), and C. serbica (circle). b Map showing the distribution of the sampled material of C. graeca (square) and C. glauca (triangle)
Amplified fragment length polymorphism
For extracting genomic DNA, we used silica-gel-dried leaves. DNA was extracted using CTAB isolation buffer (Doyle and Doyle 1987). Isolated genomic DNA was checked on an agarose gel and quantified with a UV 160A Spectrophotometer. The AFLP procedure was performed according to a standard protocol (Applied Biosystems 1996) with minor modifications. The genomic DNA was cut with MseI and EcoRI restriction endonucleases and ligated with two double MseI and EcoRI adaptors for 2 h at 37°C in a thermal cycler (GeneAmp® PCR System 9700, PE Applied Biosystems). The product was diluted with TE0.1 buffer. For pre-selective amplification in a thermal cycler, the following program was used: an initial hold at 72°C for 2 min; 20 cycles of 94°C for 1 s, 56°C for 30 s, and 72°C for 2 min; a final hold at 60°C for 30 min; and cooling to 4°C. The product of pre-selective amplification was checked on an agarose gel and diluted with TE0.1 buffer. For selective amplification, EcoRI-AAG (HEX)/MseI-CTG, EcoRI-ATC (6-FAM)/MseI-CAG, and EcoRI-AGC (NED)/MseI-CTG selective primer combinations (VBC Genomics, Vienna) were used, together with the following selective amplification program in a thermal cycler: an initial cycle of 94°C for 2 min, 65°C for 30 s, and 72°C for 2 min; eight cycles of 94°C for 1 s, 64°C for 30 s (decreasing by 1°C in each cycle from 64°C to 57°C), and 72°C for 2 min; 23 cycles of 94°C for 1 s, 56°C for 30 s, and 72°C for 2 min; a final hold at 60°C for 30 min; and cooling to 4°C. The products were loaded on 5% polyacrylamide gels with the size standard GeneScan 500 ROX on an automated sequencer (ABI 377, PE Applied Biosystems). AFLP fragments were analysed by GeneScan® (PE Applied Biosystems) and the Genographer program (version 1.6.0, ©Montana State University, 1999; http://hordeum.msu.montana.edu/genographer/).
Data analysis
Koopman (2005) tested the congruence of AFLP and ITS topologies; through the study of the example genus Lactuca s.l. as well as based on data from the literature survey of the wider spectrum of species and genera, he demonstrated that AFLP-based relationships among genotypes in plants that are 10–30 (or possibly up to 35) ITS-nucleotides apart are usually recovered with good bootstrap support. Therefore, AFLP markers in plants are likely to be phylogenetically informative at this level of ITS sequence divergence. The amount of sequence divergence among plants of the C. maritima group ascertained by Lihová et al. (2006) in the internal transcribed spacer region (ITS) was up to 21 bp, while the divergence was up to 54 bp when plants of the C. maritima group, C. graeca, and C. glauca were included. This indicates that, at least for the evaluation of the relationships within the C. maritima group, AFLP data represent an adequate tool. To avoid potential bias caused by the inclusion of C. graeca and C. glauca, some analyses were performed both with and without these taxa.
The presence or absence of fragments ranging from 75 to 500 bp was scored for each sample and transferred into a binary matrix. The secondary matrix was computed with Jaccard’s coefficient, and principal coordinate analysis (PCoA) was subsequently performed with the SYN-TAX 2000 package (Podani 2001). The neighbour-joining tree was computed using Nei and Li (1979) genetic distance in the PAUP* program (version 4.0b10, Swofford 2003). The tree was rooted at the midpoint. Group support was assessed with the same program by repeated bootstrap analyses with 5,000 replications.
To estimate the pattern of variation of the material studied by clustering individuals into groups, Bayesian analysis of “population” structure was carried out using the program BAPS 3.2 (Corander et al. 2006). In this analysis, both the frequencies of AFLP fragments and the number of genetically diverged groups are treated as random variables. Stochastic optimisation is used to infer the posterior mode of the genetic structure. It searches for the number of clusters with the highest natural logarithm of the marginal likelihood of the data and gives a clustering of individuals for the best solution.
For each previously recognised taxon or grouping resulting from the Bayesian analysis of population structure, as well as for each population, the following parameters were calculated: total number of fragments (bands) per taxon or population; average, minimum, and maximum number of fragments per individual; and number of exclusive fragments (present in a given taxon only, but not necessarily in all its samples). In addition, for each previously recognised taxon or grouping resulting from the Bayesian analysis of population structure, the proportion of polymorphic fragments was calculated.
To avoid subjective definitions of rare markers [e.g., markers present in <10% of the investigated individuals (Stehlik et al. 2002) or in less than a certain number of individuals (Tribsch et al. 2002)], we followed Schönswetter and Tribsch (2005) in calculating “frequency-downweighted marker values” (DW) equivalent to range-downweighted species values in historical biogeographical research (Crisp et al. 2001). These values were calculated for each previously recognised taxon or grouping resulting from the Bayesian analysis of population structure, as well as for each population.
DW, the number of fragments, the number of exclusive fragments, and the proportion of polymorphic fragments depend on the number of sampled individuals in each group (population or species). Usually, when the groups are represented by different numbers of individuals in the dataset, individuals are randomly deleted to obtain equal numbers of individuals (e.g. by Schönswetter and Tribsch 2005). However, if several plants are deleted from the sample, much of the information is wasted by this approach, and there is also a greater danger of obtaining incorrect results just by chance. Therefore, we repeated the resampling of the whole dataset using our own scripts with Scilab software (http://www.scilab.org) to achieve the same sample size in each taxon/group or population. Replications were allowed in the resampling (i.e. one individual may occur more than one time in the resulting dataset). Hence, our approach is a special case of bootstrap analysis, and it is possible to estimate sample characteristics based on the repeated resampling. We selected nine individuals per taxon or group as defined by NJ and Bayesian clustering in each step and two individuals per population in each step. (The resampling has been done separately for taxa and populations.) Thousand replications were used for calculating the number of fragments and the proportion of polymorphic fragments, whereas 10,000 replications were used for calculating DW and the number of exclusive fragments, because these characteristics are influenced not only by the selection of individuals within the respective group but also by the selection of individuals within the other groups. Minimum and maximum or average values based on corresponding resamplings are presented.
Additional genetic diversity parameters were calculated using POPGENE 1.32 (Yeh et al. 1997). For each taxon (or group of populations from a particular area) and population, the following parameters were calculated: HNei—Nei’s average gene diversity corrected for small sample size (Nei 1978; see also Bonin et al. 2007: Box 2; Kosman 2003) and Shannon’s diversity index (Lewontin 1972). AFLP fragment sharing among particular pairs or groups of taxa and populations was evaluated as well.
Differentiation between populations and the geographic structure of genetic variation was studied by analysis of molecular variance (AMOVA). Original binary matrices were divided into separate matrices for each taxon. Molecular variance was calculated from a matrix of squared Euclidean distances using the program ARLEQUIN (version 2.000, Schneider et al. 2000). Total genetic variation was partitioned into levels: among individuals within populations, among populations, and among groups or regions. Thousand permutations were run to obtain test statistics.
Results
NJ tree
A mid-point-rooted neighbour-joining tree (Fig. 2) of the C. maritima group (without samples of C. glauca and C. graeca) shows C. monteluccii, C. fialae, and C. rupestris in clusters with 99 or 100% bootstrap support. Cardamine maritima from Croatia and from Montenegro form clusters with 96 and 88% support, respectively. These clusters appear on different parts of the tree. Cardamine serbica and C. maritima var. maglicensis appear in one cluster with 100% support, with C. serbica nested within C. maritima var. maglicensis. Other internal nodes on the tree have only low support, except for the cluster containing C. serbica and C. maritima var. maglicensis (from here on referred to as C. serbica + C.* maglicensis), and C. rupestris, which has 84% support. When C. glauca and C. graeca are included in the tree (Fig. 3), all the abovementioned clades have 86–100% support, but there is no support for the clade C. serbica + C.* maglicensis and C. rupestris. Both C. graeca and C. glauca have 100% support.
Neighbour-joining analysis of AFLP data of the Cardamine maritima group. Bootstrap support ≥50% is shown above branches. For population codes, see Table 1
Neighbour-joining analysis of AFLP data of the Cardamine maritima group, C. glauca, and C. graeca. Bootstrap support ≥50% is shown above branches. For population codes, see Table 1
Principal coordinates analysis
On the ordination diagram of the principal coordinates analysis (PCoA) based on AFLP data (Fig. 4), individuals of most of the previously recognised taxa formed their own groupings, which clearly differentiated from other taxa. Similar to the NJ tree, the sole exceptions were the division of C. maritima into two isolated groupings, reflecting their geographical isolation (Croatia vs. Montenegro), and C. maritima var. maglicensis and C. serbica, which formed one grouping. Samples were divided mainly along the first principal coordinate, where C. fialae, C. maritima from Croatia and Montenegro, and C. monteluccii appear on the left side of the diagram, while C. glauca, C. graeca, C. serbica + C.* maglicensis, and C. rupestris are on the right side of the diagram. Cardamine glauca and C. serbica + C.* maglicensis appear on extreme ends of the second principal coordinate.
Principal coordinate analysis of AFLP data (based on Jaccard’s coefficient) of C. graeca (balloon), C. monteluccii (cube), C. rupestris (club), C. fialae (pyramid), C. maglicensis (star), C. serbica (flag, marked by arrow), C. maritima—Montenegro (diamond), C. maritima—Croatia (cross), and C. glauca (cylinder). The first three coordinates explain 15.22, 12.99, and 10.16% of the total variation, respectively
BAPS
The optimal partition with the highest log marginal likelihood (−5,417.9) produced by BAPS consisted of eight clusters that corresponded to the groups in the NJ and PCoA analyses (Figs. 2, 3, 4).
AFLP fragments and their sharing
The AFLP analysis of C. maritima and related taxa, 110 plant individuals in total, resulted in 232 scorable fragments or bands. Fourteen fragments were distributed to a single individual and one fragment was monomorphic. (The number of monomorphic fragments increased to six when C. glauca and C. graeca were not included in the analysis.) The average total number of fragments per species was highest for C. maritima from Croatia and from Montenegro (68.80 and 50.95 fragments) and lowest for C. graeca, C. serbica + C.* maglicensis, and C. rupestris (27.97, 33.92, and 34.51 fragments, respectively; Table 2). The average number of fragments per individual was higher in populations of C. maritima from Montenegro and Croatia, C. fialae, and C. monteluccii, ranging from 36.12 to 41.17 fragments. Lower values were found in C. graeca (24.43), C. glauca (26.34), C. rupestris (28.36), and C. serbica + C.* maglicensis (29.13).
The highest number of exclusive fragments per species (or group of populations) was observed in C. glauca (33.10) and C. maritima from Croatia (26.30). The lowest number was found in C. fialae (13.54). When the number of exclusive fragments was calculated for populations, a tendency toward higher values was found in C. glauca, C. graeca, both entities of C. maritima, and C. fialae. Lower values were found in C. rupestris and C. serbica + C.* maglicensis; no exclusive fragments were found for populations of C. monteluccii.
The frequency-downweighted marker values (DW) calculated for species were high in C. maritima from Croatia and in C. glauca (38.01 and 35.50); intermediate for C. maritima from Montenegro, C. fialae, and C. monteluccii (26.50, 25.64, and 25.59, respectively); and low for C. graeca, C. rupestris, and C. serbica + C.* maglicensis (22.53, 21.54, and 21.01, respectively; Table 2). When DW values were calculated for populations, there were no conspicuous differences among taxa, except for higher values for C. graeca (Table 3).
With respect to the shared fragments calculated from the whole data set (without resampling), the least similar taxa were C. glauca, C. graeca, C. rupestris, and C. serbica + C.* maglicensis; they shared only one or two exclusive fragments with other taxa. The remaining taxa shared more fragments. Cardamine maritima from Croatia, the taxon sharing the most fragments with other taxa, shared five exclusive fragments with C. fialae, three with C. monteluccii, and four with C. maritima from Montenegro.
Analyses of molecular variance and genetic diversity
The highest genetic variation appeared among groups that resulted from the NJ and BAPS analyses. In such cases, the variation among groups was as high as 77.04% of the total variation (Table 4, a). Separating C. maritima var. maglicensis and C. serbica into two groups decreased the variation among groups to 76.57% (Table 4, b). Collecting populations of C. maritima from Croatia and from Montenegro into one group decreased this value even more to 66.11% of the total variation (Table 4, c). Upon the division of populations by geographical regions into two groups, Apennine and Balkan ones, genetic differentiation among the groups was only 22.70% of the total variation, while variation among populations in such cases accounted for 68.69% of the total (Table 4, d).
In analysing populations of C. maritima only, 57.71% of total genetic variation was accounted for among groups when populations of C. maritima from Croatia were separated from those from Montenegro (Table 4, e).
Analysis of the genetic diversity of populations and individual taxa (Table 3) showed that in three populations all individuals shared the same AFLP phenotype (C. monteluccii—Tuscany 2, C.* maglicensis—Maglić 3, and C. serbica—Tara). The lowest genetic diversity (low H Nei and H Sh) appeared in populations of C. monteluccii and C. serbica + C.* maglicensis. On the other hand, the highest genetic diversity was found in C. fialae and C. maritima (populations from both Croatia and Montenegro).
Discussion
Taxonomical implications
Originally, six taxa were described within the C. maritima group from the Balkan and Apennine Peninsulas, namely C. monteluccii, C. maritima, C. fialae, C. maritima var. maglicensis, C. serbica, and C. maritima “proles” rupestris. According to Flora Europea (Jones and Akeroyd 1993), Atlas Florae Europeae (Jalas and Suominen 1994), and most recent national floras from this area (Nikolić 1997; Trinaistić 1976; Josifović 1972; Pignatti 1982), only C. maritima, C. monteluccii, and C. fialae are recognised; the rest of the names are considered to be synonyms of C. maritima.
Molecular data presented in this study showed a clear pattern within the Cardamine maritima group. Italian populations of C. monteluccii are well separated from Balkan taxa. In a step forward from taxonomic confusion surrounding Balkan populations, our study confirms that five allopatric units—each with a clearly delimited and a rather restricted distribution range—can be easily recognised here. Molecular analyses showed that C. maritima var. maglicensis from the Maglić Mts. (Montenegro) and C. serbica from the Tara Mts. (Serbia) represent, genetically, the same taxon. The validly published name C. serbica (Pančić 1884) should be applied here; the name C. maritima var. maglicensis (Rohlena 1906) should be rendered into synonymy. While the names C. serbica (with C. maritima var. maglicensis included in synonymy), C. rupestris, and C. fialae refer to clearly defined Balkan taxa, the name C. maritima applies to two genetically and geographically different entities. Because the populations from the type locality of C. maritima were not included in this study for technical reasons, the application of this name will require further attention, including the consideration of morphological aspects.
Our preliminary results (Kučera et al., unpublished data) show that the taxa supported by molecular markers are also differentiated morphologically. This is the subject of an ongoing study, which will also include populations from the type locality of C. maritima.
Relationships among taxa
There is low support for the internal nodes on the neighbour-joining trees, and it is hard to infer any relationships among taxa based on this information. In contrast to the situation for the internal nodes, individual taxa in all cases (except C. serbica and C. maritima var. maglicensis) gained high bootstrap support.
The NJ tree (Fig. 2), and in part the band-sharing data, indicate that populations of C. fialae, C. monteluccii, and two entities of C. maritima may be genetically closer to each other than to C. rupestris and C. serbica, which mostly occur in areas more distant from the coast. Moreover, C. maritima from Croatia shares the highest number of fragments with other taxa. There are two possible explanations for this pattern. The first scenario suggests that a formerly widespread taxon was isolated in refugial areas, where it evolved in situ into the taxa restricted to particular mountain ranges. This was followed by secondary contacts among taxa on both sides of the Adriatic Sea, involving all taxa but C. rupestris and C. serbica. During glacial periods, the level of the Mediterranean Sea was 100–200 m lower than that at present; many islands were interconnected or united to the mainland; and a wide landbridge filled the gap between the eastern and western Adriatic coasts (Colantoni et al. 1979, Voges 1995, Dawson 1992), thus providing opportunities for dispersal. Among the studied taxa, Croatian C. maritima is the most widespread, and it naturally has the greatest possibility of forming secondary contacts with other taxa. This is similar to the situation in rock partridges and Campanula (Randi et al. 2003; Park et al. 2006). Partridges in the Apennines and Albania-Greece are thought to have been connected by gene flow through a late Pleistocene Adriatic landbridge (Randi et al. 2003). Similarly, climatic fluctuations of the late Pliocene and Pleistocene have been invoked for trans-Adriatic exchange in Campanula (Park et al. 2006). The alternative scenario suggests that C. rupestris and C. serbica originated considerably earlier than taxa occurring on the Adriatic coast, resulting in the somewhat isolated position of these taxa. There is no visible support in our data for this alternative. Naturally, a combination of these scenarios is also possible. A more detailed study using chloroplast and nuclear DNA sequences, including single- or low-copy genes, may elucidate these relationships.
Although the PCoA diagram supports genetic homogeneity of the taxa (and two entities of C. maritima) recognised in this paper, their positions on the diagram are likely influenced not only by the actual relationships among taxa, but also by the number of fragments.
There is no evidence in our data (especially with respect to fragment sharing), which would support a hybrid or hybridogenous origin of C. maritima, C. rupestris, or C. serbica. Nevertheless, taking into account relationships depicted on the NJ tree that also included C. glauca and C. graeca, an ancient hybrid origin of C. rupestris and C. serbica involving C. glauca cannot be firmly excluded either (Fig. 3). Further studies using appropriate molecular markers may shed more light on this problem.
Influence of the glacial events
Detection of highly variable populations and populations containing rare fragments enable postulating hypotheses of long-term survival of these populations. The value of DW is expected to be high in populations isolated for a long period, in which rare markers should accumulate due to mutations, whereas newly established populations are expected to exhibit low values. The number of unique fragments also supports the long-term survival of populations in their present day locations.
According to Médail and Diadema (2008), the Velebit Mts., S. Bosnia/Biokovo, and Montenegro are among the fifty major refugia of Mediterranean plants. This region coincides with the distribution areas of both entities of C. maritima, C. rupestris, and partly also C. fialae and C. serbica and suggests that populations of those taxa may have survived glaciation events in their current localities and were subject to altitudinal rather than latitudinal vegetation movements.
Cardamine serbica populations have low values of both indices of genetic diversity, DW as well as unique fragments, when all the populations are considered together. Nevertheless, in two of four analysed populations, the number of unique fragments and DW values are slightly higher. As both these populations occur in mountain gorges, which represented the refugial biotopes (Thompson 2005), we can speculate that the populations are relic ones. This is particularly true for the upper part of the Piva river valley (population Maglić 2). We can also speculate that, during their evolutionary history, populations of C. serbica experienced a serious bottleneck, which may account for their generally low genetic diversity.
Populations of both entities of Cardamine maritima, occurring on the open mountain slopes and gorges oriented towards the sea, are genetically more variable. This is particularly true for the Croatian populations from Biokovo and Velebit. This variability is apparent when considering individual populations, as well as when calculating corresponding values for C. maritima from Croatia as a whole and for C. maritima from Montenegro as a whole. One can speculate that the higher values for C. maritima from Croatia are influenced by the number of populations analysed, but the values also reflect the considerably larger distribution area of this entity, compared to the other taxa studied here (except for C. monteluccii). Higher diversity in this case might be caused, not only by the relic status of these populations, but also by the possibility of making secondary contacts, especially during the glacial periods. Compared to C. maritima, populations of C. serbica were most likely isolated from other species of the complex for a much longer time.
Although the populations of C. fialae occur in hills that are some distance from the coast and in different ecological conditions (they grow shaded in the forest rather than on sunny slopes of the sea coast as C. maritima), C. fialae populations exhibit considerable genetic variability; it is likely that their distribution area was not considerably influenced by the glacial events. A peculiar feature of this taxon is a low number of exclusive fragments for the species (although this is not completely true on the population level).
Somewhat in-between with respect to genetic variation are populations of Cardamine rupestris. They occupy an area, which is distant from the coast and grow shaded in the forest in hilly ranges, but not to the same extent as C. serbica, and we can expect some secondary contacts of these populations with other taxa of the group.
Both diversity indices—the low obtained values of DW, as well as the number of unique fragments—fail to indicate the relict origin of the analysed populations of C. monteluccii. Postglacial re-colonisation from adjacent regions is a more plausible scenario, but more robust sampling (including the southern part of the distribution area) is desirable for elucidating the phylogeography of this taxon.
Similarly, both diversity index and DW of Cardamine graeca accessions (species widespread across the Mediterranean region) suggest a decrease in genetic variation within the analysed populations. On the other hand, the number of exclusive fragments was significantly higher. Long-term spatial separation and the at least partial autogamy found in C. graeca (Kučera, unpubl.) may be responsible for the presence of the detected exclusive fragments.
The large genetic variation of five populations of Cardamine glauca from Montenegro suggests the refugial character of the sampled area (gorge of the river Piva and mountains in Kotor Bay), though the still unclear taxonomy (diversification within the taxon and the presence of further hidden, not-yet-recognised entities, Lakušić, personal communication) of this polymorphic taxon may underlie the increased genetic richness of the populations.
From the evidence discussed above, we can conclude that the majority of Balkan populations exhibit features of genetic variability that enable us to hypothesise that they belong to relic populations. In spite of the possibility of secondary contacts among taxa (or groups of populations) during glaciation periods, it is very likely that the overall distribution areas of these taxa were not influenced by the glacial events. This is in sharp contrast to the phylogeographic history of many other European species of the genus Cardamine, particularly polyploid ones, which show patterns of genetic variation indicating that their evolutionary history and distribution areas were considerably influenced by the glacial events (e.g., Franzke and Hurka 2000).
Conservation measures
The results of our analysis show that apart from currently recognised taxa, namely C. maritima and C. monteluccii, there are several units, which are strongly genetically differentiated. Some of them, namely C. fialae and C. serbica, occupy only restricted distribution areas, and appropriate conservation measures for their protection should be taken. This is in accordance with the opinion of Moritz (1994), who stressed the need to recognise not only conservation units based on predominantly morphological criteria, but also those concerned with historical population structure and DNA phylogeny. Nevertheless, our ongoing studies are also likely to reveal morphological differences among at least some of the abovementioned units, and they can be treated as species in the traditional sense.
References
Applied Biosystems (1996) AFLPTM plant mapping protocol. PE Applied Biosystems, Foster City
Beck GV (1903) Flora Bosne, Hercegovine i Novopazarskog Sandžaka I. Zemaljska Štamparija, Sarajevo
Bonin A, Ehrich D, Manel S (2007) Statistical analysis of amplified fragment length polymorphism data: a toolbox for molecular ecologists and evolutionists. Molec Ecol 16:3737–3758
Brilli-Cattarini AJB, Gubellini L (1986) Una nuova specie di Cardamine (Cruciferae) dalla Penisola Italiana e Sicilia. Webbia 39:397–407
Bussell JD, Waycott M, Chappill JA (2005) Arbitrarily amplified DNA markers as characters for phylogenetic inference. Perspect Pl Ecol Evol Syst 7:3–26
Candolle AP (1821) Regni vegetabilis systema naturale 2. Treutel et Würz, Paris
Colantoni P, Gallignani P, Lenaz R (1979) Late Pleistocene and Holocene evolution of the North Adriatic continental shelf (Italy). Mar Geol 33:M41–M50
Comes PH, Kadereit JW (1998) The effects of quaternary climatic changes on plant distribution and evolution. Trends Pl Sci 3:432–438
Corander J, Marttinen P, Sirén J, Tang J (2006) BAPS: Bayesian analysis of population structure, Manual v. 4.1. Available at: http://www.rni.helsinki.fi/-jic/bapspage.html
Crisp MD, Laffan S, Linder HP, Monro A (2001) Endemism in the Australian flora. J Biogeogr 28:183–198
Dawson AG (1992) Ice age Earth: Late Quaternary geology and climate. Routledge, London
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull Bot Soc Amer 19:11–15
Eastwood WJ (2004) East Mediterranean vegetation and climatic change. In: Griffiths HI, Kryštufek B, Reed JM (eds) Balkan biodiversity—pattern and process in the European hotspot. Kluwer, Dordrecht, pp 25–48
Frajman B, Oxelman B (2007) Reticulate phylogenetics and phytogeographical structure of Heliosperma (Sileneae, Caryophyllaceae) inferred from chloroplast and nuclear DNA sequences. Molec Phylogenet Evol 43:140–155
Franzke A, Hurka H (2000) Molecular systematics and biogeography of the Cardamine pratensis complex (Brassicaceae). Pl Syst Evol 224:213–234
Fritsch C (1895) Beiträge zur Flora der Balkanhalbinsel mit besonderer Berücksichtigung von Serbien II. Verh K K Zool Bot Ges Wien 44:301–327
Fritsch C (1897) Ueber eine neue Cardamine aus der Hercegovina. Österr Bot Z 47:44–46
Gömöry D, Paule L, Brus R, Zhelev P, Tomović Z, Gračan J (1999) Genetic differentiation and phylogeny of beech on the Balkan Peninsula. J Evol Biol 12:746–754
Guo Y-P, Saukel J, Mittermayr R, Ehrendorfer F (2005) AFLP analyses demonstrate genetic divergence, hybridization, and multiple polyploidization in the evolution of Achillea (Asteraceae-Anthemideae). New Phytol 166:273–290
Gutiérrez Larena B, Fuertez Aquilar J, Feliner GN (2002) Glacial-induced altitudinal migrations in Armeria (Plumbaginaceae) inferred from patterns of chloroplast DNA haplotype sharing. Molec Ecol 11:1965–1974
Hampe A, Arroyo P, Jordano P, Petit J (2003) Rangewide phylogeography of a bird-dispersed Eurasian shrub: contrasting Mediterranean and temperate glacial refugia. Molec Ecol 12:3415–3426
Hayek A (1927) Prodromus florae peninsulae Balcanicae 1. Verlag des Repertorium, Dahlem bei Berlin
Hewitt GM (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913
Jalas J, Suominen J (1994) Atlas Florae Europaeae 10. The Committee for Mapping the Flora of Europe and Societas Biologica Fennica Vanamo, Helsinki
Jones BMG, Akeroyd JR (1993) 40. Cardamine L. In: Tutin TG, Burges NA, Chater AO, Edmondson JR, Heywood VH, Moore DM, Valentine DH, Walters SM, Webb DA (eds) Flora Europaea 1, 2nd edn. Cambridge University Press, Cambridge, pp 346–351
Josifović M (ed) (1972) Flora SR Srbije. Srpska Akademija Nauka u Umetnosti, Beograd
Juan A, Crespo MB, Cowan RS, Lexer C, Fay MF (2004) Patterns of variability and gene flow in Medicago citrina, an endangered endemic of island in the western Mediterranean, as revealed by amplified fragment length polymorphism (AFLP). Molec Ecol 13:2679–2690
Koopman WJM (2005) Phylogenetic signal in AFLP data sets. Syst Biol 54:197–217
Kosman E (2003) Nei’s gene diversity and the index of average differences are identical measures of diversity within populations. Pl Pathol 52:533–535
Kryštufek B, Buzan EV, Hutchinson WF, Hänfling B (2007) Phylogeography of the rare Balkan endemic Martino’s vole, Dinaromys bogdanovi, reveals strong differentation within the western Balkan Peninsula. Molec Ecol 16:1221–1232
Kryštufek B, Reed JM (2004) Pattern and process in Balkan biodiversity—an overview. In: Griffiths HI, Kryštufek B, Reed JM (eds) Balkan biodiversity—pattern and process in the European hotspot. Kluwer, Dordrecht, pp 1–8
Kučera J, Lihová J, Marhold K (2006) Taxonomy and phylogeography of Cardamine impatiens and C. pectinata (Brassicaceae). Bot J Linn Soc 152:169–195
Lara-Cabrera SI, Spooner DM (2004) Taxonomy of North and Central American diploid wild potato (Solanum sect. Petota) species: AFLP data. Pl Syst Evol 248:129–142
Lewontin RC (1972) The apportionment of human diversity. Evol Biol 6:381–398
Lihová J, Marhold K (2006) Phylogenetic and diversity patterns in Cardamine (Brassicaceae)—a genus with conspicuous polyploid and reticulate evolution. In: Sharma AK, Sharma A (eds) Plant genome: biodiversity and evolution, vol. 1C: phanerogams (Angiosperms–Dicotyledons). Science Publishers, Inc., Enfield, pp 149–186
Lihová J, Tribsch A, Marhold K (2003) The Cardamine pratensis (Brassicaceae) group in the Iberian Peninsula: taxonomy, polyploidy and distribution. Taxon 52:783–802
Lihová J, Marhold K, Tribsch A, Stuessy TF (2004a) Morphometric and AFLP reevaluation of tetraploid Cardamine amara (Brassicaceae) in the Mediterranean. Syst Bot 29:134–146
Lihová J, Tribsch A, Stuessy TF (2004b) Cardamine apennina: a new endemic diploid species of the C. pratensis group (Brassicaceae) from Italy. Pl Syst Evol 245:69–92
Lihová J, Shimizu KK, Marhold K (2006) Allopolyploid origin of Cardamine asarifolia (Brassicaceae): incongruence between plastid and nuclear ribosomal DNA sequences solved by a single-copy nuclear gene. Molec Phylogenet Evol 39:759–786
Marghali S, Panaud O, Lamy F, Ghariani S, Sarr A, Marrakchi M, Trifi-Farah N (2005) Exploration of intra- and inter-population genetic diversity in Hedysarum coronarium L. by AFLP markers. Genet Resour Crop Evol 52:277–284
Médail F, Diadema K (2008) Importance of refugia in shaping plant diversity in the Mediterranean Basin. J Biogeogr (in press)
Moritz C (1994) Defining ‘evolutionary significant units’ for conservation. Trends Ecol Evol 9:373–375
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nei M, Li W-H (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Nguyen TT, Taylor PW, Redden RJ, Ford R (2004) Genetic diversity estimates in Cicer using AFLP analysis. Pl Breed (New York) 123:173–179
Nikolić T (ed) (1997) Flora Croatica. Index Florae Croaticae. Pars 2. Nat Croat 6(Suppl 1):1–232
Pančić J (1884) Dodatak flory Kneževine Srbije. Izdane i štampa kraľ.-srp. Državne štampariji, Beograd
Park J-M, Kovačić S, Liber Z, Eddie WMM, Schneeweiss GM (2006) Phylogeny and biogeography of isophyllous species of Campanula (Campanulaceae) in the Mediterranean area. Syst Bot 31:862–880
Perný M, Tribsch A, Stuessy TF, Marhold K (2005a) Taxonomy and cytogeography of Cardamine raphanifolia and C. gallaecica (Brassicaceae) in the Iberian Peninsula. Pl Syst Evol 254:69–91
Perný M, Tribsch A, Anchev ME (2005b) Infraspecific differentiation in diploid Cardamine acris (Brassicaceae) from Balkan Peninsula: morphological and molecular evidence. Folia Geobot 39:405–429
Perný M, Tribsch A, Stuessy T, Marhold K (2005c) Allopolyploid origin of Cardamine silana (Brassicaceae) from Calabria (southern Italy): karyological, morphological and molecular evidence. Bot J Linn Soc 148:101–116
Petit RJ, Aguinagalde I, de Beaulieu J-L, Bittkau C, Brewer S, Cheddadi R, Ennos R, Fineschi S, Grivet D, Lascoux M, Mohanty A, Müller-Starck G, Demesure-Musch B, Palme A, Martin JP, Rendell S, Vendramin GG (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300:1563–1565
Pignatti S (ed) (1982) Flora d’Italia 1. Edagricole, Bologna
Pilon Y, Qamaruz-Zaman F, Fay MF, Hendoux F, Piquot Y (2007) Genetic diversity and ecological differentation in the endangered fen orchid (Liparis loeselii). Conserv Genet 8:177–184
Podani J (2001) Syn-Tax 2000, Computer programs for data analysis in ecology and systematics, user′s manual. Scentia Publishing, Budapest
Polunin O (1997) Flowers of Greece and the Balkans. Oxford University Press, Oxford
Prohens J, Anderson GJ, Blanca JM, Cañizares J, Zuriaga E, Nuez F (2006) The implications of AFLP data for the systematics of the wild species of Solanum section Basarthum. Syst Bot 31:208–216
Randi E, Tabarroni C, Rimondi S, Lucchini V, Sfougaris A (2003) Phylogeography of the rock partridge (Alectoris graeca). Molec Ecol 12:2201–2214
Rohlena J (1906) Beitrag zur Flora von Montenegro. Repert Nov Spec Regni Veg 3(36/37):145–149
Rohlena J (1942) Conspectus florae Montenegrinae. Preslia 21/22:1–775
Schneider S, Roessli D, Excoffier L (2000) Arlequin, vers. 2.000: a software for population genetics data analysis. Genetics and Biometry Laboratory, University of Geneva, Geneva
Schönswetter P, Tribsch A (2005) Vicariance and dispersal in the alpine perennial Bupleurum stellatum L. (Apiaceae). Taxon 54:715–732
Schulz OE (1903) Monographie der Gattung Cardamine. Bot Jahrb Syst 29:280–623
Stehlik I, Schneller JJ, Bachmann K (2002) Immigration and in situ glacial survival of the low-alpine Erinus alpinus (Scrophulariaceae). Biol J Linn Soc 77:87–103
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (* and other methods), version 4.0b10. Sinauer Associates, Sunderland
Thompson JD (2005) Plant evolution in the Mediterranean. Oxford University Press, Oxford
Tribsch A, Schönswetter P, Stuessy TF (2002) Saponaria pumila (Caryophyllaceae) and the ice-age in the Eastern Alps. Amer J Bot 89:2024–2033
Trinaistić I (1976) Analitička flora Jugoslavije 2. Institut za Botaniku, Sveučilišta u Zagrebu, Zagreb
Turrill WB (1929) The plant life of the Balkan Peninsula: a phytogeographical study. Clarendon Press, Oxford
Tzedakis PC (2004) The Balkans as prime glacial refugial territory of European temperate trees. In: Griffiths HI, Kryštufek B, Reed JM (eds) Balkan biodiversity-pattern and process in the European hotspot. Kluwer, Dordrecht, pp 49–68
Voges A (ed) (1995) International Quaternary map of Europe 1: 2 500 000, B 10 Bern. Bundesanstalt für Geowissenschaften und Rohstoffe/UNESCO, Hannover
Vos P, Hogers R, Bleeker R, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucl Acids Res 23:4407–4414
Widmer A, Lexer Ch (2001) Glacial refugia: sanctuaries for allelic richness, but not for gene diversity. Trends Ecol Evol 16:267–269
Yeh FC, Yang R-C, Boyle TJ, Ye Z-H, Mao JX (1997) POPGENE, the user-friendly shareware for population genetic analysis. Molecular Biology and Biotechnology Centre, University of Alberta, Canada
Acknowledgments
We acknowledge the financial support provided by the Grant Agency VEGA, Bratislava, Slovak Republic (project no. 6055, to Judita Lihová), by the Slovak Research and Development Agency (project no. RPEU-0003-06, to Karol Marhold), by the Ministry of Education, Youth and Sports of the Czech Republic (grant no. 0021620828, to Karol Marhold), and by the Spanish Ministerio de Educación y Ciencia (Juan de la Cierva fellowship, to Karin Tremetsberger). Thanks are also due to J. Lihová (Bratislava, Slovakia) for her useful help and comments on the manuscript, F. Selvi (University of Firenze, Italy), and S. Redžić (from University of Sarajevo, Bosnia and Hercegovina) for their assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kučera, J., Tremetsberger, K., Vojta, J. et al. Molecular study of the Cardamine maritima group (Brassicaceae) from the Balkan and Apennine Peninsulas based on amplified fragment length polymorphism. Plant Syst Evol 275, 193–207 (2008). https://doi.org/10.1007/s00606-008-0061-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-008-0061-8