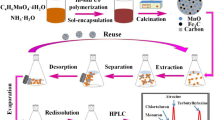
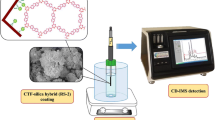
Abstract
Based on covalent organic framework (COF) 1,3,5-tris-(4-formylphenyl)benzene-benzidine (TFPB-BD) in situ grown on Fe3O4 hollow microspheres and combined with gas chromatography-flame thermionic detector, a rapid and simple stir bar sorptive-dispersive microextraction method was developed for the determination of five triazole pesticides (paclobutrazol, hexaconazole, flusilazole, propiconazole, and tebuconazole). The synthesized TFPB-BD/Fe3O4 microspheres were characterized by transmission electron microscope, vibrating sample magnetometer, and thermogravimetric analysis, which showed that the material has strong magnetism and higher load capacity of COF. Under optimal conditions, the extraction equilibrium could be achieved within 9 min with detection limits of 0.17–1.48 μg L−1 (S/N = 3) and a linear range of 5–1000 μg L−1. The developed method was applied to the determination of trace triazole pesticides in apples, pears, and cabbages with recoveries from 81 to 117%.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triazole pesticides, a series of organic heterocyclic compounds, are easy to residue in fruits and vegetables because of their stable structure and rarely photodegradation under environmental conditions [1,2,3,4]. Long-term exposure will cause reproductive toxicity, hepatotoxicity, and developmental toxicity [5,6,7]. Therefore, a simple, rapid, and efficient method for determination of triazole pesticides in fruits and vegetables is critical to control food safety and protect human health.
There are several analytical methods used for the detection of triazole pesticides such as colorimetric sensing [8, 9], fluorescence sensing [6], and chromatography [10]. Among them, chromatography is widely used for the analysis of various triazole pesticides, and the sensitivity and accuracy of determination are depend on efficient sample pretreatment [11]. Common pretreatment methods include liquid–liquid extraction (LLE) [12], liquid–liquid microextraction (LLME) [13], solid-phase microextraction (SPME) [1], magnetic solid-phase extraction (MSPE) [14], and stir bar sorptive extraction (SBSE) [15]. LLE needs to consume a large amount of organic solvents [16, 17]. Although LLME consumes less organic solvent, phase separation by centrifugation is still complicated to realize [18, 19]. SPME and SBSE use solid adsorbents that are easy to separate, but the contact area between adsorbents and samples is limited, which leads to longer extraction time [15, 20]. With the development of nanotechnology, MSPE has become a hot technology due to its low organic solvent consumption, fully contact with samples, and short extraction time [21, 22]. Nevertheless, in the process of MSPE extraction, the additional magnetic field is needed to collect the adsorbents in the both extraction and desorption process [23], which makes the operation a little complicated and may cause operation error.
Stir bar sorptive-dispersive microextraction (SBSDME) combines the advantages of SBSE and MSPE [24,25,26]. With the vigorous stirring of the magnetic stir bar, the mass transfer rate is faster than that of the conventional MSPE, and the adsorbents are adsorbed on the stirring bar without external magnetic field [27]. Furthermore, the magnetic adsorbent is easy to recycle and has strong reusability, and it can adsorb different substances by adjusting its properties under different synthesis conditions. Benedé et al. [28] used CoFe2O4@oleic as an adsorbent in SBSDME to realize sensitive detection of eight lipophilic UV filters in water samples with the detection limits (LODs) in the low ng L − 1 range. Grau et al. [29] used CoFe2O4@Strata-X™-AW in SBSDME to selective determine triphenyl and diphenyl phosphate in urine of nail polish users, and the method showed enrichment factors of 17 and 30. Madej et al. [30] synthesized magnetically modified graphene (G-Fe3O4) for the effective extraction of seven pesticides in aqueous solution by SBSDME, and the LODs were below 14 ng mL−1.
Magnetic covalent organic frameworks (MCOFs) not only have high saturation magnetization, but also have the advantages of COF, such as ordered crystal structure, large specific surface area and chemical stability, which make them potential magnetic adsorbents [31, 32]. Gao et al. [33] used in situ growth method to synthesize the stable core–shell structured MCOF Fe3O4@TbBd for selective enrichment of peptides. Our group synthesized MCOF of 1,3,5-tris-(4-formylphenyl) benzene-Benzidine/Fe3O4 (TpBD/Fe3O4) by a facile coprecipitation method and used it for MSPE of 15 phthalate esters in beverages [34].
At present, most in situ growth methods of MCOFs are based on Fe3O4 solid microspheres/nanoparticles, so the COF layer was only limited to the surface [35]. Fe3O4 hollow microspheres have unsealed and loose structure, its strong magnetism is conducive to rapid magnetic separation, and its hollow structure improves the load capacity. Fe3O4 hollow microspheres can grow more COF than Fe3O4 solid microspheres, thus providing more adsorption sites [36].
Herein, COF TFPB-BD was in situ grown on Fe3O4 hollow microspheres (TFPB-BD/Fe3O4) as the adsorbent of SBSDME for rapid extraction of triazole pesticides. Combined with gas chromatography-flame thermionic detector (GC-FTD), triazole pesticides in fruits and vegetables (apples, pears, and cabbages) were conveniently extracted and determined.
Materials and methods
Materials and reagents
All the reagents used were at least analytical grade. 1,3,5-tris-(4-formylphenyl) benzene (TFPB) was purchased from Tongchuangyuan Pharmaceutical Technology Co., Ltd. (Sichuan, China). Benzidine (BD) and mesitylene were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Iron chloride hexahydrate (FeCl3·6H2O), 1,6-hexanediamine, anhydrous sodium acetate, glycol, methanol (MeOH), N,N-dimethylformamide (DMF), 1,4-dioxane, and glacial acetic acid were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Five triazole pesticides standard solutions with a concentration of 1000 mg L−1 were obtained from Tanmo Quality Inspection Technology Co., Ltd. (Jiangsu, China), including paclobutrazol, hexaconazole, flusilazole, propiconazole, and tebuconazole. The standard solutions were diluted with HPLC grade methanol to prepare stock solution and intermediate solution, and were stored at − 20 ℃ and 4 ℃ respectively. Fruits and vegetable samples (apples, pears, and cabbages) were randomly obtained from local supermarkets (Wuxi, China) and stored at 4 ℃ before analysis.
Instrumentation and analytical conditions
The morphology and element distribution of the TFPB-BD/Fe3O4 were obtained by JEM 2100F transmission electron microscope (TEM, JEOL, Japan), high angle annular dark field scanning transmission electron microscopy (HAADF-STEM), and energy dispersive X-ray spectroscopy (EDX) (FEI, USA). Fourier transform infrared spectroscopy (FT-IR) was obtained on Nicolet IS10 spectrometer (Nicolet, USA). The magnetic hysteresis loops of the Fe3O4 and TFPB-BD/Fe3O4 were measured by LakeShore7404 vibrating sample magnetometer (VSM, LakeShore, USA). The N2 adsorption experiment was carried out on Autosorb-iQ analyzer (Quantachrome, USA). The thermogravimetric analysis (TGA) was conducted on STA 449 F5 Jupiter thermal gravimetric analyzer (NETZSCH, Germany). The SBSDME experiment was carried out on KX-85-2AS intelligent constant temperature magnetic stirrer (Zhida Shengtong Scientific Instrument Co., Ltd., China). The magnetic bar was used as the stir bar (20 mm in length and 3 mm in diameter, Shenzhen Lala Magnetic Materials Co., Ltd., China).
Data collection and analysis were carried out on Shimadzu GC system (2030, Shimadzu, Japan). Capillary column HP-5 (30 m × 0.25 mm × 0.25 μm, Agilent, USA) was used for quantitative determination in the experiment. The injection was accomplished in splitless mode, the inlet temperature was 280 ℃, and the injection volume was 1 μL. The air flow rate was 145 mL min−1, the H2 flow rate was 2 mL min−1, and the carrier gas (N2) flow rate was 30 mL min−1. The column temperature program used for the separation was as follows: the initial temperature maintained at 80 ℃ (held for 2 min), then 20 ℃ min−1 to 260 ℃, and finally 10 ℃ min−1 to 280 ℃ (held for 1 min). The temperature of flame ionization detector (FTD) was 300 ℃.
Synthesis of the TFPB-BD/Fe3O4
Amine-functionalized Fe3O4 hollow microspheres (Fe3O4-NH2) were synthesized by one-pot method (Text S1) according to the reported method [36]. The synthesis of the TFPB-BD/Fe3O4 (Optimization was provided in Text S2) was carried out as follows: 150 mg Fe3O4-NH2 was dispersed in 10 mL 1,4-dioxane, then 10 mg TFPB and 150 μL acetic acid were added. The product TFPB/Fe3O4 was washed with DMF and 1,4-dioxane and then dried under vacuum. Then, 50 mg TFPB/Fe3O4 was dissolved in 4.5 mL mesitylene/1,4-dioxane (1:1, V:V) solution; 39.06 mg TFPB, 27.63 mg BD, and 0.5 mL 9 mol L−1 acetic acid solution were added, and the reaction was carried out at room temperature for 48 h. The TFPB-BD/Fe3O4 was separated by a magnet, washed with DMF and ethanol, and dried under vacuum overnight.
SBSDME procedure
Sixteen milligrams of TFPB-BD/Fe3O4 composite was added in 10 mL spiked solution or sample solution. A magnetic bar (20 mm in length and 3 mm in diameter) was placed in the beaker and the SBSDME procedure was carried out. At the stirring rate of 600 rpm, the TFPB-BD/Fe3O4 was dispersed in the solution for extraction for 9 min, and then the stirring stopped; the TFPB-BD/Fe3O4 was adsorbed back to the stirring bar. After extraction, the magnetic bar was taken out and washed twice with water, then 500 μL MeOH was used for ultrasonic-assisted elution for 6 min. The eluent was filtered with a 0.22-μm filter for GC-FTD analysis.
Analysis of real samples
In order to evaluate the feasibility of SBSDME method in real samples, the quantitative determination of triazole pesticides was carried out in apples, pears, and cabbages. The sample preparation process was as follows: the fruit and vegetable samples were washed and used for homogenizing treatment. Then, 1.0-g sample was diluted with 10 mL pure water, added 0.1 g NaCl, and adjusted pH to 7 with 0.1 M HCl and 0.1 M NaOH solution. The SBSDME procedure was performed according to the method described in the “SBSDME procedure” section.
Results and discussion
The design of TFPB-BD/Fe3O4 for SBSDME of triazole pesticides
Triazole pesticides are nitrogenous heterocyclic compounds containing phenyl and triazolyl groups with the molecular sizes between 10.1 and 13.0 Å (Fig. S4). And COF TFPB-BD has a large π conjugated system with the pore size of 32 Å, which is larger than the molecular sizes of triazole pesticises [37]. Triazole pesticides may be extracted from the sample solution onto COF TFPB-BD due to the action of the pore size, π–π interaction, or hydrogen bond, etc. Therefore, we used COF TFPB-BD as an adsorbent to be magnetized. Firstly, the monomer TFPB was bonded to the amine-functionalized Fe3O4 hollow microspheres by the aldehyde group of TFPB reacted with free -NH2 groups. The TFPB-BD/Fe3O4 was synthesized by the condensation with the other monomer BD on TFPB/Fe3O4 via Schiff base reaction (Fig. 1a). Then, the prepared TFPB-BD/Fe3O4 composite was added to the sample solution, and SBSDME process was carried out with a magnetic bar (Fig. 1b). At a high stirring rate, the TFPB-BD/Fe3O4 was dispersed in the solution for extraction, and when the stirring stopped, the magnetic material was attracted back to the stirring bar. In this way, triazole pesticides were adsorbed to the TFPB-BD/Fe3O4 composite. After extraction, the stirring bar with TFPB-BD/Fe3O4 was taken out and eluted with MeOH. The eluent was filtered and quantitatively analyzed by GC-FTD.
Characterization of the TFPB-BD/Fe3O4
The HAADF-STEM and EDX elemental mapping data (Fig. 2a) show that the TFPB-BD (containing elements C, N, O) are uniformly bonded on the Fe3O4 hollow microspheres (containing elements Fe, O), and the thickness of COF TFPB-BD is about 1 μm. The distribution of element Fe is a hollow ring, and the elements C, N, and O are uniformly distributed in the whole sphere.
Thermogravimetric analysis (TGA) was used to compare the proportion of COF in MCOF grown in situ on Fe3O4 solid microspheres (Fe3O4@TFPB-BD) (Fe3O4@TFPB-BD was synthesized according to the reported method) [38] and hollow microspheres (TFPB-BD/Fe3O4) (Fig. 2b). For the two materials, there are obvious weight reduction curves in the range of 500–680 ℃, indicating the weight reduction of COF shell. The residual mass of TFPB-BD/Fe3O4 was 54.4%, while the residual mass of Fe3O4@TFPB-BD was 75.6%, indicating that the proportion of COF in TFPB-BD/Fe3O4 is higher. Thus, the synthesis of MCOF with Fe3O4 hollow microspheres can increase the load capacity of COF. Other characterizations of TFPB-BD/Fe3O4 were provided in the Electronic Supplementary Material Text S3.
Optimization of the SBSDME parameters
In order to enhance the SBSDME extraction effect, the extraction and elution conditions were optimized. For extraction conditions, we investigated the effects of the amount of adsorbent, stirring rate, pH, salt concentration, and extraction time. The amount of the TFPB-BD/Fe3O4 was optimized in the range of 4–20 mg (Fig. 3a), and the quantitative extraction has been reached at 16 mg. Stirring during the extraction process can disperse the magnetic adsorbent and accelerate the diffusion of the target analyte from the sample to the adsorbents (Fig. 3b). The adsorption was balanced when the stirring rate reaches 600 rpm. Other optimizations of the SBSDME parameters were provided in the Electronic supplementary material Text S4.
Method validation and adsorption mechanism exploration
The analytical performance of the TFPB-BD/Fe3O4-SBSDME-GC-FTD method for triazole pesticides was studied under optimized conditions (Table S1). The linear range of five triazole pesticides was 5–1000 μg L−1 with R2 > 0.9949. The LODs (S/N = 3) and the quantification limits (LOQs) (S/N = 10) were 0.17–1.48 μg L−1 and 0.57–4.93 μg L−1, respectively. Additionally, the intra-day and inter-day precision were evaluated by measuring the standard solution of 50 μg L−1. As shown in Table S1, the intra-day RSDs (n = 6) and inter-day RSDs (n = 6) of five triazole pesticides were less than 9.9% and 11.5%, respectively. In order to evaluate the properties of the adsorbent materials, the reusability of the TFPB-BD/Fe3O4 was investigated (Fig. 4). After 20 adsorption/desorption cycles, the extraction efficiency of the TFPB-BD/Fe3O4 can maintain above 84.5%. These results show that the TFPB-BD/Fe3O4-SBSDME-GC-FTD method has good performance in the extraction of triazole pesticides. In order to further study the adsorption mechanism, XPS experiments of TFPB-BD/Fe3O4 before and after adsorption of triazole pesticides were observed (Fig. S5). The peak value of C = C at 284.43 eV before adsorption shifts to 284.51 eV after adsorption, which indicates the π–π interaction between TFPB-BD/Fe3O4 and triazole pesticides [39].
Compared with other triazole pesticides extraction methods reported in the literature (Table 1), this method has a shorter extraction time (9 min), and its linear range, LODs, and RSDs are similar to or better than other work. Most of the methods to detect triazole pesticides in fruits and vegetables need to consume a large amount of organic solvents (10–20 mL) to extract and purify [40,41,42,43,44]. The sample preparation of the TFPB-BD/Fe3O4-SBSDME-GC-FTD method for fruits and vegetables only needs homogenization and dilution. The extraction process does not require organic solvent consumption, and only 0.5 mL organic solvent is needed in the elution process, which is more environmentally friendly.
Application in real samples
In order to evaluate the accuracy and practicability of the TFPB-BD/Fe3O4-SBSDME-GC-FTD method, the triazole pesticides in fruits and vegetables (apples, pears, and cabbages) were detected. As shown in Fig. 5, paclobutrazol and flusilazole were detected in apple, pear, and cabbage samples (Table 2). And the contents of paclobutrazol in apples, pears, and cabbages were 0.42 mg kg−1, 0.39 mg kg−1, and 0.22 mg kg−1. The contents of flusilazole were all below the LOQ.
The accuracy of the developed method was verified by a recovery study with spiking triazole pesticides at three concentration levels (0.1 mg kg−1, 0.5 mg kg L−1, and 1.0 mg kg L−1). The calculation of recoveries showed that the sample matrix has little effect on the extraction efficiency of the method. As shown in Table 2, the recoveries of five triazole pesticides were 81–117%, and the RSDs < 11.3%, which showed that the method could be applied to the analysis of triazole pesticides in fruits and vegetables.
Conclusion
We explored a COF magnetization method based on Fe3O4 hollow microspheres to synthesize the TFPB-BD/Fe3O4, which was used as the adsorbent of SBSDME to extract triazole pesticides from fruits and vegetables. This method had the advantages of simple pretreatment, less organic solvent consumption, and short treatment time. Overall, it provided an example for the application of magnetic COFs combined with SBSDME method in the separation and enrichment of pesticides in complex matrix foods. Furthermore, other aromatic compounds close to the molecular sizes of triazole pesticides may cause interference. In future study, we will improve the selectivity of COF by functionalizing it (modifying functional groups, preparing molecular imprinting, etc.). And a small amount of organic solvent is still needed for desorption. In order to achieve environmentally friendly purposes, a step without solvent consumption also needs to be developed.
Data availability
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
References
Majdafshar M, Piryaei M, Abolghasemi MM, Rafiee E (2019) Polyoxometalate-based ionic liquid coating for solid phase microextraction of triazole pesticides in water samples. Sep Sci Technol 54(10):1553–1559. https://doi.org/10.1080/01496395.2019.1572625
Necibi M, Saadaoui H, Atayat A, Mzoughi N (2020) Determination of triazole pesticides in the surface water of the Medjerda River. Tunisia Analytical Letters 54(4):742–759. https://doi.org/10.1080/00032719.2020.1780250
Zhao F, She Y, Zhang C, Cao X, Wang S, Zheng L, Jin M, Shao H, Jin F, Wang J (2017) Selective solid-phase extraction based on molecularly imprinted technology for the simultaneous determination of 20 triazole pesticides in cucumber samples using high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 1064:143–150. https://doi.org/10.1016/j.jchromb.2017.08.022
Saadaoui H, Boujelbane F, Serairi R, Ncir S, Mzoughi N (2012)Transformation pathways and toxicity assessments of two triazole pesticides elimination by gamma irradiation in aqueous solution. Sep Purif Technol 276.https://doi.org/10.1016/j.seppur.2021.119381
De Rop J, Senaeve D, Jacxsens L, Houbraken M, van Klaveren J, Spanoghe P (2019) Cumulative probabilistic risk assessment of triazole pesticides in Belgium from 2011–2014. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 36(6):911–921. https://doi.org/10.1080/19440049.2019.1606943
Omeroglu I, Tumay SO, Makhseed S, Husain A, Durmus M (2021) A highly sensitive “ON-OFF-ON” dual optical sensor for the detection of Cu(II) ion and triazole pesticides based on novel BODIPY-substituted cavitand. Dalton Trans 50(19):6437–6443. https://doi.org/10.1039/d1dt00792k
Yang R, Wu J, Lu G, Huang X (2021) Efficient capture of carbamate and triazole pesticides in environmental waters by functional groups-rich monolithic fibers prior to chromatographic quantification. Microchem J 171.https://doi.org/10.1016/j.microc.2021.106833
Koushkestani M, Abbasi-Moayed S, Ghasemi F, Mahdavi V, Hormozi-Nezhad MR (2021) Simultaneous detection and identification of thiometon, phosalone, and prothioconazole pesticides using a nanoplasmonic sensor array. Food Chem Toxicol 151:112109. https://doi.org/10.1016/j.jct.2021.112109
Kalantari K, Fahimi-Kashani N, Hormozi-Nezhad MR (2022) Development of a colorimetric sensor array based on monometallic and bimetallic nanoparticles for discrimination of triazole fungicides. Anal Bioanal Chem 414:5297–5308. https://doi.org/10.1007/s00216-021-03272-0
Farajzadeha MA, Mogaddama MRA, Ghorbanpour H (2014) Development of a new microextraction method based on elevated temperature dispersive liquid-liquid microextraction for determination of triazole pesticides residues in honey by gas chromatography-nitrogen phosphorus detection. J Chromatogr A 1347:8–16. https://doi.org/10.1016/j.chroma.2014.04.067
Liu G, Tian M, Lu M, Shi W, Li L, Gao Y, Li T, Xu D (2021) Preparation of magnetic MOFs for use as a solid-phase extraction absorbent for rapid adsorption of triazole pesticide residues in fruits juices and vegetables. J Chromatogr B Analyt Technol Biomed Life Sci 1166:122500. https://doi.org/10.1016/j.jchromb.2020.122500
Da Silva Souza NR, Navickiene S (2019) Multiresidue determination of carbamate, organophosphate, neonicotinoid and triazole pesticides in roasted coffee using ultrasonic solvent extraction and liquid chromatography-tandem mass spectrometry. J AOAC Int 102(1):33–37. https://doi.org/10.5740/jaoacint.18-0294
Dmitrienko SG, Apyari VV, Tolmacheva VV, Gorbunova MV (2020) Dispersive liquid-liquid microextraction of organic compounds: an overview of reviews. J Anal Chem 75(10):1237–1251. https://doi.org/10.1134/S1061934820100056
Liu G, Li L, Huang X, Zheng S, Xu D, Xu X, Zhang Y, Lin H (2018) Determination of triazole pesticides in aqueous solution based on magnetic graphene oxide functionalized MOF-199 as solid phase extraction sorbents. Microporous Mesoporous Mater 270:258–264. https://doi.org/10.1016/j.micromeso.2018.05.023
Farajzadeh MA, Djozan D, Nouri N, Bamorowat M, Shalamzari MS (2010) Coupling stir bar sorptive extraction-dispersive liquid-liquid microextraction for preconcentration of triazole pesticides from aqueous samples followed by GC-FID and GC-MS determinations. J Sep Sci 33(12):1816–1828. https://doi.org/10.1002/jssc.201000088
Farajzadeh MA, Sheykhizadeh S, Khorram P (2013) Extraction and preconcentration of some triazole pesticides in grape juice by salting out homogeneous liquid–liquid extraction in a narrow-bore tube prior to their determination by gas chromatography–flame ionization detection. Food Anal Methods 7(6):1229–1237. https://doi.org/10.1007/s12161-013-9737-y
Jin BH, Xie LQ, Guo YF, Pang GF (2012) Multi-residue detection of pesticides in juice and fruit wine: a review of extraction and detection methods. Food Res Int 133:109141. https://doi.org/10.1016/j.foodres.2011.12.003
Dmitrienko SG, Apyari VV, Tolmacheva VV, Gorbunova MV (2021) liquid-liquid extraction of organic compounds into a single drop of the extractant: overview of reviews. J Anal Chem 76(8):907–919. https://doi.org/10.1134/S1061934821080049
Jalili V, Kakavandi MG, Ghiasvand A, Barkhordari A (2022) Microextraction techniques for sampling and determination of polychlorinated biphenyls: a comprehensive review. Microchem J 179:107442. https://doi.org/10.1016/j.microc.2022.107442
Abolghasemi MM, Habibiyan R, Jaymand M, Piryaei M (2018) A star-shaped polythiophene dendrimer coating for solid-phase microextraction of triazole agrochemicals. Microchim Acta 185(3):179. https://doi.org/10.1007/s00604-017-2639-8
Zhao JL, Huang PF, Wang XM, Yang J, Zhou Z, Du XZ, Lu XQ (2022) Efficient adsorption removal of organic nitrogen pesticides: insight into a new hollow NiO/Co@C magnetic nanocomposites derived from metal-organic framework. Sep Purif Technol 287:120608. https://doi.org/10.1016/j.foodres.2020.109141
Ren K, Zhang W, Cao S, Wang G, Zhou Z (2018) Carbon-based Fe3O4 nanocomposites derived from waste pomelo peels for magnetic solid-phase extraction of 11 triazole fungicides in fruit samples. Nanomaterials (Basel) 8(5):302. https://doi.org/10.3390/nano8050302
Bagheri AR, Aramesh N, Chen J, Liu W, Shen W, Tang S, Lee KH (2022) Polyoxometalate-based materials in extraction, and electrochemical and optical detection methods: a review. Anal Chim Acta 1209:339509. https://doi.org/10.1016/j.aca.2022.339509
Benedé LJ, Chisvert A, Giokas DL, Salvador A (2014) Development of stir bar sorptive-dispersive microextraction mediated by magnetic nanoparticles and its analytical application to the determination of hydrophobic organic compounds in aqueous media. J Chromatogr A 1362:25–33. https://doi.org/10.1016/j.chroma.2014.08.024
Benedé JL, Chisvert A, Moyano C, Giokas DL, Salvador A (2018) Expanding the application of stir bar sorptive-dispersive microextraction approach to solid matrices: determination of ultraviolet filters in coastal sand samples. J Chromatogr A 1564:25–33. https://doi.org/10.1016/j.chroma.2018.06.003
He M, Wang Y, Zhang Q, Zang L, ChenHu B (2021) Stir bar sorptive extraction and its application. J Chromatogr A 1637:461810. https://doi.org/10.1016/j.chroma.2020.461810
Vallez-Gomis V, Grau J, Benede JL, Giokas DL, Chisvert A, Salvador A (2021) Fundamentals and applications of stir bar sorptive dispersive microextraction: a tutorial review. Anal Chim Acta 1153:338271. https://doi.org/10.1016/j.aca.2021.338271
Benedé JL, Chisvert A, Giokas DL, Salvador A (2016) Determination of ultraviolet filters in bathing waters by stir bar sorptive-dispersive microextraction coupled to thermal desorption-gas chromatography-mass spectrometry. Talanta 147:246–252. https://doi.org/10.1016/j.talanta.2015.09.054
Grau J, Benede JL, Serrano J, Segura A, Chisvert A (2019) Stir bar sorptive-dispersive microextraction for trace determination of triphenyl and diphenyl phosphate in urine of nail polish users. J Chromatogr A 1593:9–16. https://doi.org/10.1016/j.chroma.2019.02.014
Madej K, Jonda A, Borcuch A, Piekoszewski W, Chmielarz L, Gil B (2019) A novel stir bar sorptive-dispersive microextraction in combination with magnetically modified graphene for isolation of seven pesticides from water samples. Microchem J 147:962–971. https://doi.org/10.1016/j.microc.2019.04.002
He M, Liang Q, Tang L, Liu Z, Shao B, He Q, Wu T, Luo S, Pan Y, Zhao C, Niu C, Hu Y (2021) Advances of covalent organic frameworks based on magnetism: classification, synthesis, properties, applications. Coord Chem Rev 449.https://doi.org/10.1016/j.ccr.2021.214219
Qian HL, Wang Y, Yan XP (2022) Covalent organic frameworks for environmental analysis. TrAC Trends Anal Chem 147.https://doi.org/10.1016/j.trac.2021.116516
Gao C, Lin G, Lei Z, Zheng Q, Lin J, Lin Z (2017) Facile synthesis of core-shell structured magnetic covalent organic framework composite nanospheres for selective enrichment of peptides with simultaneous exclusion of proteins. J Mater Chem B 5(36):7496–7503. https://doi.org/10.1039/c7tb01807j
Pang YH, Yue Q, Huang YY, Yang C, Shen XF (2020) Facile magnetization of covalent organic framework for solid-phase extraction of 15 phthalate esters in beverage samples. Talanta 206:120194. https://doi.org/10.1016/j.talanta.2019.120194
Sargazi M, Kaykhaii M (2022) Magnetic covalent organic frameworks-fundamentals and applications in analytical chemistry. Crit Rev Anal Chem 52:1–27. https://doi.org/10.1080/10408347.2022.2107872
Wang L, Bao J, Wang L, Zhang F, Li Y (2006) One-pot synthesis and bioapplication of amine-functionalized magnetite nanoparticles and hollow nanospheres. Chemistry 12(24):6341–6347. https://doi.org/10.1002/chem.200501334
Guo JX, Qian HL, Zhao X, Yang C, Yan XP (2019) In situ room-temperature fabrication of a covalent organic framework and its bonded fiber for solid-phase microextraction of polychlorinated biphenyls in aquatic products. J Mater Chem A 7(21):13249–13255. https://doi.org/10.1039/c9ta02974e
Li Y, Yang CX, Yan XP (2017) Controllable preparation of core–shell magnetic covalent-organic framework nanospheres for efficient adsorption and removal of bisphenols in aqueous solution. Chem Commun 53:2511–2514. https://doi.org/10.1039/c6cc10188g
Cui YY, Ren HB, Yang CX, Yan XP (2019) Facile synthesis of hydroxyl enriched microporous organic networks for enhanced adsorption and removal of tetrabromobisphenol A from aqueous solution. Chem Eng J 373:606–615. https://doi.org/10.1016/j.cej.2019.05.082
Dos Santos EO, Gonzales JO, Ores JC, Marube LC, Caldas SS, Furlong EB, Primel EG (2019) Sand as a solid support in ultrasound-assisted MSPD: a simple, green and low-cost method for multiresidue pesticide determination in fruits and vegetables. Food Chem 297:124926. https://doi.org/10.1016/j.foodchem.2019.05.200
Güdücü HE, İnam R, Aboul-Enein HY (2011) Determination of organophosphorus and triazole pesticides by gas chromatography and application to vegetable and commercial samples. J Liq Chromatogr Relat Technol 34(19):2473–2483. https://doi.org/10.1080/10826076.2011.591027
Hergueta-Castillo ME, Lopez-Rodriguez E, Lopez-Ruiz R, Romero-Gonzalez R, GarridoFrenich A (2022) Targeted and untargeted analysis of triazole fungicides and their metabolites in fruits and vegetables by UHPLC-orbitrap-MS(2). Food Chem 368:130860. https://doi.org/10.1016/j.foodchem.2021.130860
Liu J, Ji C, Liu X, Li X, Wu H, Zeng D (2021) Fe3O4 nanoparticles as matrix solid-phase dispersion extraction adsorbents for the analysis of thirty pesticides in vegetables by ultrahigh-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 1165:122532. https://doi.org/10.1016/j.jchromb.2021.122532
Shuang Y, Zhang T, Zhong H, Li L (2021) Simultaneous enantiomeric determination of multiple triazole fungicides in fruits and vegetables by chiral liquid chromatography/tandem mass spectrometry on a bridged bis(beta-cyclodextrin)-bonded chiral stationary phase. Food Chem 345:128842. https://doi.org/10.1016/j.foodchem.2020.128842
Farajzadeh MA, Bahram M, Jafary F, Bamorowat M (2011) Combination of extraction by silylated vessel-dispersive liquid–liquid microextraction as a high-enrichment factor technique: optimization and application in preconcentration of some triazole pesticides from aqueous samples followed by GC-FID determination. Chromatographia 73(3–4):393–401. https://doi.org/10.1007/s10337-010-1895-0
Funding
This work was supported by the National Natural Science Foundation of China (22276077, 21976070, 22076067) and the Fundamental Research Funds for the Central Universities (JUSRP22003).
Author information
Authors and Affiliations
Contributions
Yu-Xin Wang: conceptualization, methodology, formal analysis, investigation, writing original draft. Xiao-Fang Shen: conceptualization, resources, writing review and editing, funding acquisition. Yong-Wei Feng: conceptualization, methodology, investigation. Yue-Hong Pang: methodology, formal analysis, validation, investigation, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, YX., Shen, XF., Feng, YW. et al. Covalent organic framework in situ grown on Fe3O4 hollow microspheres for stir bar sorptive-dispersive microextraction of triazole pesticides. Microchim Acta 190, 34 (2023). https://doi.org/10.1007/s00604-022-05613-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05613-x