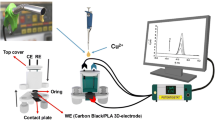
Abstract
Low oxidation stability is the main drawback of biodiesels and biokerosenes that is overcome by using antioxidants, which can be combined due to synergistic effects. This paper demonstrates that 3D-printed electrochemical devices can be applied to biofuel electroanalysis, including the monitoring of oxidation stability by quantifying the antioxidant content in biofuels. Fabrication requires 3D-printed acrylic templates at which a polylactic acid (PLA) filament with conducting carbon-black filling sensors is extruded by a 3D pen. The antioxidants butyl hydroxyanisole (BHA) and tert-butylhydroquinone (TBHQ) are the most employed additives in biodiesel production, and thus, their electrochemical behavior was investigated; 2,6-ditertbutylphenol (2,6-DTBP) was included in this investigation because it is commonly added to biokerosenes. The electrochemical surface treatment of the 3D-printed electrodes improved the current responses of all antioxidants; however, the electrochemical oxidation of TBHQ was clearly more affected by an electrocatalytic action shifting its oxidation towards less positive potentials (~200 mV), which resulted in a better separation of TBHQ and BHA oxidation peaks (+0.4 and +0.6 V vs Ag|AgCl, respectively). The oxidation of 2,6-DTBP occurred at more positive potentials (+1.2 V vs Ag|AgCl). The simultaneous determination of TBHQ and BHA by differential-pulse voltammetry resulted in linear responses in the range 0.5 and 175 μmol L−1 with limits of detection and quantification of 0.15 μmol L−1 and 0.5 μmol L−1, respectively. The presence of Fe3+, Cu2+, Pb2+, Mn2+, Cd2+, and Zn2+, even in high concentrations, did not interfere in the determination of TBHQ and BHA. The determination of 2,6-DTBP in biokerosene was achieved by cyclic voltammetry. All relative standard deviations (RSD) were lower than 6.0 %, indicating adequate precision of the methods. Spiked biofuel samples were analyzed (after dilution in electrolyte) and recovery values between 85 and 120% were obtained, which indicates absence of sample matrix effects.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel is a renewable and biodegradable energy source that has been incorporated in the marked mixed with petroleum-derived diesel [1, 2]. The most common route is catalyzed transesterification reactions of vegetable oils, recycled oils, or animal fats leading to the formation of a mixture of fatty acid esters (biodiesel itself) and glycerol [3, 4]. The obtained biofuel is free of sulfur and aromatics and presents lower particulate emissions (HC, CO, and CO2) than diesel oil [5, 6]. On the other hand, biodiesel is more prone to auto-oxidation than fossil fuels [5, 7] due to the presence of high levels of unsaturated fatty acids originated from the vegetable oil sources (commonly about 8% of linolenic acid) that are transferred to the methyl or ethyl esters produced after transesterification. Oxidation reactions start from these unsaturated fatty acids [8, 9] that under storage conditions, such as exposure to air (oxygen and moisture), light, high temperatures, and contaminants, are accelerated explaining the higher instability of biodiesels [1].
The addition of antioxidants is extremely necessary to retard the oxidation process and increase the oxidative stability of biodiesel [7, 10,11,12]. Antioxidants are compounds that delay, control, or inhibit autoxidation processes of substrates and decrease the formation of unwanted secondary products [11]. In this sense, antioxidants have been widely used to control the biodiesel degradation process, and the phenolic antioxidants are commonly used because of their low cost and high antioxidant activity [10, 13]. tert-Butylhydroquinone (TBHQ), butyl hydroxyanisole (BHA), butyl hydroxytoluene (BHT), pyrogallol (PY), and propyl gallate (PG) are the most commonly used antioxidants in biofuels [10, 13]. TBHQ exhibits good synergism with BHT or BHA and not with PG [11]. The structures of these phenolic compounds (BHA and TBHQ in Fig. 1) are responsible for their antioxidant activity, allowing the donation of protons to free radicals, preventing the oxidative process [14, 15]. Similarly, other biofuels such as biokerosene require the addition of antioxidants; however, other phenolic compounds have been considered such as 2,6-ditertbutylphenol (2,6-DTBP) [16] (its structure is presented in Fig. 1).
To evaluate the antioxidant efficiency on the oxidative stability of biodiesel or biokerosene, accelerated oxidation methods are often employed, such as the Rancimat method, which is based on the indirect determination of oxidation products [17]. The induction period of the Rancimat method is determined by the sudden increase in conductivity of water absorbing oxidation products (volatile organic acids generated during the accelerated oxidation). The European standard, EN 14112, recommends the Rancimat induction period method as the official method to determine oxidation stability of biodiesel [1, 9, 17]. Usually, the longer the induction period, the better the oxidation stability [3, 17]. Currently, Brazilian, American, and European regulatory agencies establish 12 h of induction period, which requires hundreds of parts per millions of antioxidants to achieve this value. Studies have shown that the synergistic effect of a combined formulation containing tert-butylhydroquinone (TBHQ) and butylated hydroxyanisole (BHA) (500 mg L−1 for each antioxidant) provided greater oxidative stability for biodiesel reaching induction periods of 12 h [1, 12]. Hence, the oxidative stability of biodiesel can be predicted by determining the concentration of antioxidants in biodiesel.
Consequently, the addition of antioxidants at appropriate concentrations protects the product during storage [1]. Different analytical methods have been used for the sensitive and accurate detection of these compounds, such as high-performance liquid and gas chromatography and voltammetric methods [14]. The advantages of electroanalytical methods in relation to chromatography include the use of miniaturized sensors operated by affordable and portable instrument for in loco analysis while accuracy, sensitivity, and reproducibility are not compromised. However, most electroanalytical methods reported so far use commercial substrates (glassy carbon, boron-doped diamond, and gold) [5, 10], which have relatively higher costs when compared to 3D-printed electrodes, which makes large-scale manufacturing unfeasible for routine applications.
Compact and portable electroanalytical devices, including cells and electrodes, have been enabled by 3D printing [18, 19]. Extrusion-based 3D printers [20] and their combination with 3D pens [21, 22] have been highlighted employing conductive filaments overall made of polylactic acid (PLA) filled with carbon black or graphene as conducting agents [23]. This association combines highly precise prototyping and reduction amount of waste compared with traditional subtractive prototyping (in this case, polymeric materials). Post-treatment of 3D-printed surfaces has demonstrated to improve the electrochemical activity of these electrodes probably making more available the conductive sites [24]. The success of 3D-printed sensors has reached different areas, including biological [25], food [26], and environmental [31], due to its special advantages such as rapid prototyping, design freedom, low energy demand, and large-scale production. Their application to biofuel electroanalysis is still emerging.
In this context, we demonstrate that 3D-printed electrodes can detect antioxidants commonly added to biofuels, including biodiesel and biokerosene. The electrochemistry of BHA, TBHQ, and 2,6-DTBP (see Fig. 1) was investigated and the effect of surface treatment provided dramatic enhancement of peak resolution between BHA and TBHQ. The sensor was compatible to varied amount of ethanol, which was required in the electrolyte to dilute biodiesel or biokerosene.
Experimental procedures
Instrumentation, electrochemical cell, and electrodes
The voltammetric measurements were performed using an μ-AUTOLAB type III potentiostat/galvanostat (Metrohm Autolab BV, Utrecht, the Netherlands) coupled to a computer. The NOVA 1.11.0 software for windows 8 was used to control the instrument. Data were treated using the ultimate software for graphing and analysis OriginPro8.5 (OriginLab, Northampton, MA, USA). A beaker of 10 mL was used as electrochemical cell. The reference and auxiliary electrodes were an Ag|AgCl (saturated KCl) and a platinum wire, respectively.
A Photon digital UV light processing 3D printer was obtained from ANYCUBIC Co. Ltd. (Shenzhen, China) with UV curable acrylic resin (UV-LED, λ = 405 nm, XY resolution of 47 μm, rated power 40W, software: ANYCUBIC Photon (Slicer) used to print a customized cylinder (3.5 cm length × 3.8 mm diameter)). The template model of substrate was designed using Blender software 2.81a and the STL file was generated by ChituboxTM (Shenzhen, China). A 3D pen purchased from Sanmersen (Shenzhen, China) was used to print the conductive part of the electrode. The conductive filament was made of PLA containing carbon black, named as CB/PLA in the text (Proto-Pasta® obtained from ProtoPlant Inc., Vancouver, Canada).
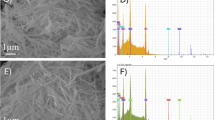
The electrochemical measures were made using a laboratory-made working electrode which will be described further in the text. Images of the 3D-printed electrode surface (CB/PLA) were performed by scanning electron microscopy (SEM) using a Vega 3-Tescan instrument (TESCAN, Brno-Kohoutovice, Czech Republic) operating at 20 kV.
Reagents and solutions
All solutions were prepared with high-purity deionized water (R≥18MΩcm) obtained from a Direct-Q3 water purification system (Millipore, Bedford, MA, USA). Concentrated perchloric acid (70% w/v), acetic acid (99.7% w/v), and nitric acid (65% w/v) from Vetec (Rio de Janeiro, Brazil), ethanol (99.8% v/v), sodium hydroxide (98% w/w), sulfuric acid (98% w/v), and concentrated hydrochloric acid (37% w/v) from Synth (Sao Paulo, Brazil) were diluted in an appropriate concentration to study the composition of the supporting electrolyte. The antioxidants tert-butylhydroquinone (TBHQ) (99% w/w) and 2,6-ditertbutylphenol (2,6-DTBP) (99% w/w) from Sigma-Aldrich (Steinheim, Germany), butyl hydroxyanisole (BHA) (98.5% w/w) from Synth (Diadema, Brazil), and hexaammineruthenium (III) chloride (98.0%) from Sigma-Aldrich (St. Louis, USA) were used. The standard stock solutions of TBHQ, BHA, and 2,6-DTBP were prepared in ethanol (99.8% v/v). Aqueous standard solutions of Cu (II), Fe (III), Pb (II), Mn (II), Zn (II), and Cd (II) (all at 1000 mg L−1) were purchased from Quimlab (Jacarei, Brazil).
Working standard solutions were prepared in pure ethanol immediately before use by appropriate dilution of the stock solution in the supporting electrolyte. Two methyl biodiesel samples were analyzed, one produced from soybean oil in the laboratory according to a procedure described in the literature [27], and another sample donated by a biodiesel power plant (Caramuru, Itumbiara, Brazil) identified as commercial biodiesel. These biofuel samples were free from synthetic antioxidants according to previous analyses. Biokerosene was produced in the laboratory according to a previous protocol reported in the literature and fortified with 2,6-DTBP [28].
Production of 3D-printed electrodes
Fig. S1 illustrates the 3D pen used in this work and its different parts, including filament input at which the conductive CB/PLA filament is introduced and unload and load filament buttons to control its introduction. The pen has a speed controller and an integrated heating nozzle from which the molten filament is released. On the right of Fig. S1, the 3D pen application of the CB/PLA filament within the 3D-printed customized cylinder is highlighted. Hollow cylinders (3.5 cm length × 3.8 mm diameter) were 3D-printed (25 units in a row) employing acrylic resin. From one side, a copper wire was introduced concentrically into the cylinder in such a way that one side served as electrical contact with potentiostat and the other side to connect with the CB/PLA material to serve as sensor. Then, the 3D pen was used to apply the conductive CB/PLA filament covering the copper wire inside the cylinder (on the right of Fig. S1). These steps (insertion of copper wire and application of the molten filament) are made one-by-one manually and takes around 2 min for each electrode.
After fabrication, the electrode was polished in a sandpaper (600 grit followed by 1200 grit) moistened with ultrapure water until a homogeneous surface was verified. Next, an electrochemical treatment procedure of the 3D-printed electrodes was performed before subsequent experiments by amperometry in NaOH (0.5 mol L−1) solution, with application of a potential of +1.4 V per 200 s, followed of a potential of −1.0 V for the same time. This protocol was optimized in a previous work that showed the great exposure of carbon black conducting sites [29].
Electrochemical measurements
The electrochemical measures were executed without removing dissolved oxygen and at laboratory room temperature (around 25 °C). The polishing process and electrochemical activation employing 0.5 mol L−1 NaOH were sequentially performed at the beginning of each working day.
The supporting electrolyte solutions evaluated by cyclic voltammetry (keeping 1 mmol L−1 of each antioxidant) were the following: 0.05 mol L−1 H2SO4, 0.1 mol L−1 HCl, 0.1 mol L−1 HClO4, 0.1 mol L−1 HNO3, and 0.1 mol L−1 CH3COOH. The 0.1 mol L−1 HClO4 solution was selected as the supporting electrolyte for the analysis of biodiesel or biokerosene containing ethanol.
Differential-pulse voltammetry (DPV) was evaluated in the presence of 50 μmol L−1 of TBHQ or BHA in 0.1 mol L−1 HClO4 as the supporting electrolyte.
Biofuel preparation for electroanalysis
The biofuel samples (biodiesel or biokerosene) were prepared separately in a dilution ratio corresponding to 1:10 biofuel / ethanol (v/v); then, an aliquot of 20 μL (around 0.0018 g) of the diluted biofuel was added to a 0.1 mol L−1 HClO4 solution. The standard addition method was selected to the simultaneous determination of BHA and TBHQ in biodiesel and 2,6-DTBP in biokerosene.
Results and discussion
Voltammetry of the antioxidants on the 3D-printed CB/PLA
The electrochemical profiles of three different antioxidants were investigated using the 3D-printed CB/PLA electrodes. Fig. 2 shows the voltammetric profile of TBHQ, BHA, and 2,6-DTBP in 0.1 mol L−1 HClO4 (red lines) and the respective blanks (dashed lines). Considering that the surface electrochemical treatment of such a 3D-printed CB/PLA surface may improve their electrochemical activity, Fig. 2 also shows the voltammetric profiles obtained using the treated electrodes (blue lines).
Cyclic voltammetric recordings for 1 mmol L−1 of each (A) TBHQ, (B) BHA, and (C) 2,6-DTBP in 0.1 mol L−1 HClO4 containing 10% (v/v) ethanol before (red solid line) and after (blue solid line) chemical/electrochemical treatment of electrode. The dashed lines correspond to the respective blanks. Other conditions: scan rate: 50 mV s−1 and step potential: 5 mV
The electrochemical oxidation of the three antioxidants is feasible on the 3D-printed CB/PLA electrode with oxidation peaks at +0.65 V for TBHQ, +0.75 V for BHA, and +1.25 V for 2,6-DTBP. The first two electrochemical oxidations occur through the formation of quinone derivatives [1, 12], which explain the facilitated oxidation processes in comparison with 2,6-DTBP whose oxidation process occurs via a radical formation [24]. All the electrochemical processes are pH-dependent and involve the transfer of two electrons as well documented in the literature [1, 12, 24]. Interestingly, all the electrochemical oxidation processes were facilitated on the electrochemically treated CB/PLA electrode (blue lines) probably by the formation of oxygenated species and higher exposure of carbon black sites, as shown in the SEM images (Fig. S2A and B). Electrochemical impedance spectroscopy (EIS) measurements confirm this observation, where on the treated surface, a smaller charge transfer resistance (26.8 Ω) was obtained (Figure S2C). These results are in agreement with the documentation in the previous work [29]. The oxidation of TBHQ and 2,6-DTBP was anticipated in 200 mV, higher electrochemical reversibility and higher currents were verified (lower separation of oxidation and reduction peaks of each process), and the appearance of a reduction process in the reverse scan for 2,6-DTBP which was not evident on the non-treated electrode was noticed. It is also noteworthy that the electrochemical surface treatment provided the enhancement of electron transfer for all antioxidants and higher separation between the oxidation peaks of TBHQ and BHA due to the potential displacement occurred for TBHQ, which will benefit the simultaneous determination of TBHQ and BHA. To confirm this statement, Fig. 3 shows the cyclic voltammograms obtained for the three antioxidants in the same scan (equimolar mixture of TBHQ, BHA, and 2,6-DTBP) on the 3D-printed CB/PLA before and after the electrochemical surface treatment.
Cyclic voltammetric recordings for 1 mmol L−1 of each TBHQ, BHA, and 2,6-DTBP in supporting electrolyte of 0.1 mol L−1 containing 10% (v/v) ethanol before (red solid line) and after (blue solid line) chemical/electrochemical treatment of electrode. The dashed lines correspond to the respective blanks. Conditions: scan rate: 50 mV s−1 and step potential: 5 mV
The cyclic voltammogram for the three antioxidants on the untreated CB/PLA electrode in the same scan presents overlapped responses for TBHQ and BHA while the third antioxidant can be discriminated. On the other hand, the same voltammetric recording obtained on the electrochemically treated CB/PLA electrode provided a higher separation between the TBHQ and BHA oxidation peaks, which is mandatory for the simultaneous determination of both antioxidants that can be found in biodiesel together. The effect of the electrochemical surface treatment shifted the oxidation peaks of TBHQ and 2,6-DTBP that indicate a common effect for these two antioxidants, which can be related to a surface interaction between the generated oxygenated groups at the CB/PLA electrode and these antioxidants. A previous work evaluated in details the surface changes of 3D-printed CB/PLA electrodes after the same electrochemical protocol in NaOH solution using X-ray spectroscopy [30], and the authors revealed an increase in the of C–C groups that indicated the formation of graphitic groups after the treatment probably due to the consumption of PLA that exposed more carbon black sites. Such a surface modification contributed to the improved electrochemical oxidation of the antioxidants; however, further investigation is required to understand and prove this hypothesis.
The cyclic voltammetry of the three antioxidants was also investigated in the different acidic solutions used as supporting electrolytes as shown in Fig. S3. Well-separated peaks for TBHQ, BHA, and 2,6-DTBP and higher currents were observed in HClO4 and for this reason, this solution was selected in further experiments. TBHQ and BHA can be found concomitantly in biodiesel due to the synergistic effect of the antioxidants on the oxidation stability, while 2,6-DTBP is added to biokerosene used as fuel aviation. Hence, in the next section, the simultaneous determination of TBHQ and BHA in biodiesel is focused.
Simultaneous voltammetric determination of TBHQ and BHA in biodiesel
Preliminary experiments using DPV and square-wave voltammetry (SWV) were performed to compare both techniques regarding analytical response and stability (repetitive measures). The DPV technique was more stable (more repetitive current responses) than SWV in the same electrolyte used for the voltammetric evaluation of TBHQ and BHA, and thus, it was selected in further experiments. The DPV parameters were assessed in 0.1 mol L−1 HClO4 in 10% (v/v) ethanol for a constant concentration of 50 μmol L−1 of TBHQ and 50 μmol L−1 of BHA. Table S1 summarizes the assessed DPV parameters, the studied range, and the selected values for subsequent studies. This selection considered the highest current response, signal stability (repeatability for triplicate measurements), and the resolution/separation of TBHQ and BHA peaks after baseline treatment as shown in supporting information (Figs. S4, S5, S6, and S7).
Under the selected parameters listed in Table S1, the sensor was evaluated for the simultaneous determination of TBHQ and BHA. The linear ranges between the peak currents and the concentration levels of TBHQ and BHA were obtained from 0.5 to 175 μmol L−1 with good correlation coefficients, r=0.993 and r=0.995, respectively (Fig. 4). The linear regression equations were Ipa (μA) = (0.0285 ± 0.0008) [TBHQ] (μΜ) + 0.17 ± 0.04 and Ipa (μA) = (0.095 ± 0.003) [BHA] (μΜ) + 0.08 ± 0.03.
(A) DPV responses (n=3) of TBHQ and BHA (from 0.5 to 175 μmol L−1) and (B) respective calibration curves. Optimized conditions in Table S1
From these curves, the values of limit of detection (LOD) and quantification (LOQ) of these antioxidants and sensitivity (slopes) were obtained and are shown in Table 1. LOD and LOQ values were calculated according to the IUPAC definition as follows: LOD = 3σ/s and LOQ = 10σ/s, in which σ indicates the standard deviation of baseline noise while s corresponds to the sensitivity of the calibration curve (slopes).
A series of repetitive electrochemical determinations were performed to evaluate the precision of this method. The repeatability study was conducted with three different levels of concentration of TBHQ and BHA (10, 50, and 100 μmol L−1) using the same 3D-printed electrode. Satisfactory results were obtained for the simultaneous determination of TBHQ and BHA since all relative standard deviation (RSD) values were lower than 6.07%, indicating good precision. The values of the inter-day variation were calculated by the RSD values of slopes from two analytical curves obtained in two different days. The electrode fabrication was evaluated by the inter-electrode variation and the values of 1.95% for TBHQ and 2.03% for BHA proved the high reproducibility of the protocol.
Interference study
The metals, such as copper, iron, manganese, and nickel, affect tremendously the biodiesel oxidative stability as the metallic species acts as catalyzers of oxidation processes, leading to biodiesel degradation [2, 3]. Considering these negative effects, maximum limits for some contaminants, including water, methanol, sulfate, chloride, sodium, and iron, are established by regulatory agencies worldwide (e.g., in Europe, the USA, and Brazil). The Brazilian agency ANP presents a resolution (number 842, of May 14th, 202) that define a strict upper limit for some metals (Fe3+, Cu2+, Pb2+, Mn2+, and Cr2+) in the biofuel (1.0 mg kg−1). Such high amounts of metals in biodiesel may occur due to the corrosion of metallic parts (engines or tanks) in contact with the biofuel [13,14,15].
The metallic species Fe3+, Cu2+, Pb2+, Mn2+, Cd2+, and Zn2+ were kept at 10 mg L−1 as interferents on the detection of TBHQ and BHA at 200 μmol L−1. All measurements were performed in triplicates. DPV recordings for these experiments are presented in Fig. S8. Low deviations (lower than 3%) were obtained (see Table S2 with the data extracted from Fig. S8) for both TBHQ and BHA even after the addition of each interferent. All metallic species did not provide an electrochemical response concomitant with TBHQ and BHA oxidation peaks; hence, no interference from Fe3+, Cu2+, Pb2+, Mn2+, Zn2+, and Cd2+ was observed.
Application in real samples
After the analytical features were obtained, the DPV method using the manufactured CB/PLA electrode was applied for the simultaneous determination of TBHQ and BHA in samples of soybean biodiesel and a commercial biodiesel sample.
The accuracy of the method was evaluated by addition-recovery tests, which means that the samples were spiked with known amounts of both antioxidants in three levels, TBHQ (1.7, 5.0, and 8.3 mg L−1) and BHA (1.8, 5.4, and 9.0 mg L−1), which correspond to 10, 30, and 50 μmol L−1 in the electrochemical cell after dilution. The content of both antioxidants in biodiesel was estimated to achieve 12 h of induction period measured by the Rancimat method.
The standard addition curves showed good linearity (r > 0.99); Fig. 5 and Table 2 summarize the recovery values for the analysis of spiked samples. Satisfactory recovery values were verified (between 85 and 120%), according to the acceptance criteria for recovery, established by the National Institute of Metrology, Quality and Technology (INMETRO) [31] of Brazil, showing acceptable accuracy in the level of studied concentrations and no interference problems from the sample matrix (soybean biodiesel and biodiesel plant samples) under the optimized conditions. Therefore, the acceptable recovery values indicated that the proposed sensor can be used to quantify TBHQ and BHA at low concentration levels using the 3D printed CB/PLA electrode.
DPV recordings (n=3) for TBHQ and BHA detection in commercial biodiesel sample spiked with a standard solution resulting (A), (B), and (C) and in soybean biodiesel sample (D), (E), and (F) in the final concentrations in the cell of 10 (A and D), 30 (B and E), and 50 (C and F) μmol L−1 followed by three additions of standard solutions. Respective calibration curves are presented beside each DPV scans. In all plots, the 1st scan shows blanks; 2nd scan corresponds to sample; 3rd, 4th, and 5th scans show the simultaneous addition of standard solutions of TBHQ and BHA (each added amount can be followed in the respective curves). Optimized conditions in Table S1
The procedure developed for the simultaneous determination of TBHQ and BHA in biodiesel using the CB/PLA electrode was compared with other procedures available in the literature (Table 3). The evaluated parameters, including type of working electrode, technique, LOD, linear range, and sensibility, denote that the proposed method provides equivalent or better results to those already existing in the literature. Exception to a DPV method using a mercury electrode (HDME) [32] that obtained lower LOD than CB/PLA. However, the HDME electrode is not ecologically friendly and poses a health risk to the analyst. It is possible to note that several works used commercial electrodes (glassy carbon, gold, and boron-doped diamond) that have a relatively high cost when compared to the 3D-printed electrode employed in this work, some of them prepared after multi-steps that increase the time of preparation and error sources that affect reproducibility [1]. On the other hand, a commercial platinum electrode, ready-to-use but with a high cost, showed lower sensitivity and higher LOD when compared to the analysis of the 3D-printed electrode proposed in this work [33].
Voltammetric determination of 2,6-DTBP in biokerosene
Preliminary experiments using DPV and SWV exploring the 3D-printed electrode revealed electrode fouling for repetitive scans in the presence of 2,6-DTBP which can be explained by an adsorption process of the oxidation products which follows a different mechanism in comparison with BHA and TBHQ. On the other hand, the cyclic voltammetric technique, which was used for preliminary studies, showed acceptable results for the quantification of 2,6-DTBP in 0.1 mol L−1 HClO4 in 10% (v/v). Opposite to BHA and TBHQ, the antioxidant 2,6-DTBP requires a higher ethanol amount in the electrolyte to its complete dissolution, and thus, the effect of ethanol on the performance of the 3D-printed device for electroanalysis in solutions containing a higher ethanolic amount was investigated. The higher the ethanol amount, the more stable response was obtained, and the addition of 80% (v/v) ethanol keeping 0.1 mol L−1 HClO4 provided the best condition. The 3D-printed electrode stood for a whole day under the exposure to this solution; however, the electrode was replaced for the next day of measurements because the electrode became brittle. This can be considered a disadvantage regarding commercial electrodes (e.g., GCE or BDDE), as they can be used for more days (although they require a new surface treatment). On the other hand, conventional SPEs cannot be used for a single use in solutions containing higher ethanol content, with exception to SPEs designed to be compatible with organic solvents [16]. Fig. S9 shows cyclic voltammograms for blank and continuous addition of 2,6-DTBP standard solutions, and a linear range was verified between 0.18 and 2.0 mmol L−1 (r = 0.999). Table S3 shows the analytical features obtained from this calibration curve, including linear range, LOD, LOQ, and repeatability (two different levels). RSD values lower than 5.2% attested the precision of the method.
Fig. 6 shows cyclic voltammograms for the analysis of biokerosene samples spiked with two amounts of 2,6-DTBP. Recoveries values between 85 and 90% were obtained (see Table S4), indicating adequate accuracy of the method. Pure biokerosene samples presented concentration levels below LOD because they were lab-produced. Hence, the 3D-printed CB/PLA sensor can also be used to quantify 2,6-DTBP in biokerosene samples after a similar sample preparation step (sample dilution in electrolyte) using cyclic voltammetry. Further experiments are required to provide lower LOD values if required.
(A) Cyclic voltammetric recordings (n=3) for 2,6-DTBP determination in biokerosene sample fortified with a standard solution resulting (A) and (B) in the final concentrations in the cell of 0.38 (A) and 0.48 (B) mmol L−1 followed by three additions of standard solutions. Respective standard addition curves side to side to its voltammogram. The 1st scans show the blanks (supporting electrolyte); 3rd, 4th, and 5th scans: addition of standard solutions of 2,6-DTBP (values can be seen in the respective curves). Supporting electrolyte: 80% (v/v) ethanol containing 0.1 mol L−1 HClO4
Conclusion
This work has showed that the environmentally-friendly composite made of carbon black-integrated PLA can be used to fabricate 3D-printed electrochemical devices for biofuel analysis. The electrochemical surface treatment improved the electrochemical responses for the three antioxidants with potential anticipation for TBHQ and 2,6-DTBP, which enhanced peak resolution between BHA and TBHQ. The fabricated electrode was successfully employed for the simultaneous quantification of TBHQ and BHA via DPV in lab-made and commercial biodiesel samples. Accuracy, precision, selectivity, and sensitivity were investigated and the sensor is well compared with previous electrodes reported for the detection of BHA or TBHQ. Additionally, the voltammetric quantification of 2,6-DTBP in biokerosene samples was accomplished using the 3D-printed electrode in a supporting electrolyte solution containing 80% (v/v) ethanol. Therefore, it is possible to conclude that the use of such a source of disposable electrodes for biofuel quality control makes the method cheaper and portable, allowing field analysis. It is noteworthy that the CB/PLA electrode presented an analytical performance comparable to conventional electrodes for the simultaneous determination of TBHQ and BHA. In this sense, the proposed electrode proved to be efficient for fuel electroanalysis in the field. Importantly, such electrodes can be used in solutions with higher ethanol content for 1 day, which is not possible using conventional SPEs; however, conventional working electrodes can stand longer in such electrolyte composition. On the other hand, the low cost of the proposed electrodes allows their replacement and use as a disposable source of electrochemical sensors.
References
Caramit RP, De Freitas Andrade AG, Gomes De Souza JB et al (2013) A new voltammetric method for the simultaneous determination of the antioxidants TBHQ and BHA in biodiesel using multi-walled carbon nanotube screen-printed electrodes. Fuel 105:306–313. https://doi.org/10.1016/j.fuel.2012.06.062
Fernandes DM, Squissato AL, Lima AF et al (2019) Corrosive character of Moringa oleifera Lam biodiesel exposed to carbon steel under simulated storage conditions. Renew Energy 139:1263–1271. https://doi.org/10.1016/j.renene.2019.03.034
Knothe G, Steidley KR (2018) The effect of metals and metal oxides on biodiesel oxidative stability from promotion to inhibition. Fuel Process Technol 177:75–80. https://doi.org/10.1016/j.fuproc.2018.04.009
Tormin TF, Gimenes DT, Silva LG et al (2010) Direct amperometric determination of tert-butylhydroquinone in biodiesel. Talanta 82:1599–1603. https://doi.org/10.1016/j.talanta.2010.07.011
Goulart LA, Teixeira ARL, Ramalho DA et al (2014) Development of an analytical method for the determination of tert-butylhydroquinone in soybean biodiesel. Fuel 115:126–131. https://doi.org/10.1016/j.fuel.2013.06.050
Shan R, Lu L, Shi Y et al (2018) Catalysts from renewable resources for biodiesel production. Energy Convers Manag 178:277–289. https://doi.org/10.1016/j.enconman.2018.10.032
Tormin TF, Cunha RR, Richter EM, Munoz RAA (2012) Fast simultaneous determination of BHA and TBHQ antioxidants in biodiesel by batch injection analysis using pulsed-amperometric detection. Talanta 99:527–531. https://doi.org/10.1016/j.talanta.2012.06.024
Domingos AK, Saad EB, Vechiatto WWD et al (2007) The influence of BHA, BHT and TBHQ on the oxidation stability of soybean oil ethyl esters (biodiesel). J Braz Chem Soc 18:416–423. https://doi.org/10.1590/S0103-50532007000200026
Squissato AL, Neri TS, Coelho NMM et al (2018) In situ electrochemical determination of free Cu(II) ions in biodiesel using screen-printed electrodes: Direct correlation with oxidation stability. Fuel 234:1452–1458. https://doi.org/10.1016/j.fuel.2018.08.027
Squissato AL, Richter EM, Munoz RAA (2019) Voltammetric determination of copper and tert-butylhydroquinone in biodiesel: a rapid quality control protocol. Talanta 201:433–440. https://doi.org/10.1016/j.talanta.2019.04.030
Varatharajan K, Pushparani DS (2018) Screening of antioxidant additives for biodiesel fuels. Renew Sustain Energy Rev 82:2017–2028. https://doi.org/10.1016/j.rser.2017.07.020
van der Westhuizen I, Focke WW (2018) Stabilizing sunflower biodiesel with synthetic antioxidant blends. Fuel 219:126–131. https://doi.org/10.1016/j.fuel.2018.01.086
Tomášková M, Chýlková J, Šelešovská R, Janíková L (2017) Voltammetric method for rapid determination of propyl gallate and its application for monitoring of biofuels quality. Monatsh Chem 148:457–461. https://doi.org/10.1007/s00706-016-1860-1
Nunes Angelis P, de Cássia Mendonça J, Rianne da Rocha L et al (2020) Feasibility of a nano-carbon black paste electrode for simultaneous voltammetric determination of antioxidants in food samples and biodiesel in the presence of surfactant. Electroanalysis 32:1198–1207. https://doi.org/10.1002/elan.201900479
Rezende MJC, de Lima AL, Silva BV, et al. (2021) Biodiesel: an overview II. J Braz Chem Soc 32:1301–1344 https://doi.org/10.21577/0103-5053.20210046
Almeida ES, Silva LAJ, Sousa RMF et al (2016) Organic-resistant screen-printed graphitic electrodes: application to on-site monitoring of liquid fuels. Anal Chim Acta 934:1–8. https://doi.org/10.1016/j.aca.2016.05.055
Zhou J, Xiong Y, Liu X (2017) Evaluation of the oxidation stability of biodiesel stabilized with antioxidants using the Rancimat and PDSC methods. Fuel 188:61–68. https://doi.org/10.1016/j.fuel.2016.10.026
Silva AL, Salvador GM da S, Castro SVF, et al. (2021) A 3D printer guide for the development and application of electrochemical cells and devices. Front Chem 9https://doi.org/10.3389/fchem.2021.684256
Cardoso RM, Mendonça DMH, Silva WP et al (2018) 3D printing for electroanalysis: from multiuse electrochemical cells to sensors. Anal Chim Acta 1033:49–57. https://doi.org/10.1016/j.aca.2018.06.021
Cardoso RM, Kalinke C, Rocha RG et al (2020) Additive-manufactured (3D-printed) electrochemical sensors: a critical review. Anal Chim Acta 1118:73–91. https://doi.org/10.1016/j.aca.2020.03.028
Cardoso RM, Castro SVF, Stefano JS, Muñoz RAA (2020) Drawing electrochemical sensors using a 3D printing pen. J Braz Chem Soc 31:1764–1770 https://doi.org/10.21577/0103-5053.20200129
João AF, Castro SVF, Cardoso RM, et al. (2020) 3D printing pen using conductive filaments to fabricate affordable electrochemical sensors for trace metal monitoring. J Electroanal Chem 876https://doi.org/10.1016/j.jelechem.2020.114701
Hamzah HH, Shafiee SA, Abdalla A, Patel BA (2018) 3D printable conductive materials for the fabrication of electrochemical sensors: a mini review. Electrochem Commun 96:27–31. https://doi.org/10.1016/j.elecom.2018.09.006
Rocha DP, Rocha RG, Castro SVF, et al (2021) Posttreatment of 3D-printed surfaces for electrochemical applications: a critical review on proposed protocols. ElectrochemSci Adv 1–15https://doi.org/10.1002/elsa.202100136
Silva VAOP, Fernandes-Junior WS, Rocha DP et al (2020) 3D-printed reduced graphene oxide/polylactic acid electrodes: a new prototyped platform for sensing and biosensing applications. Biosens Bioelectron 170:112684. https://doi.org/10.1016/j.bios.2020.112684
Katic V, Dos Santos PL, Dos Santos MF et al (2019) 3D printed graphene electrodes modified with Prussian blue: emerging electrochemical sensing platform for peroxide detection. ACS Appl Mater Interfaces 11:35068–35078. https://doi.org/10.1021/acsami.9b09305
Serqueira DS, Fernandes DM, Cunha RR et al (2014) Influence of blending soybean, sunflower, colza, corn, cottonseed, and residual cooking oil methyl biodiesels on the oxidation stability. Fuel 118:16–20. https://doi.org/10.1016/j.fuel.2013.10.028
Llamas A, García-Martínez MJ, Al-Lal AM et al (2012) Biokerosene from coconut and palm kernel oils: production and properties of their blends with fossil kerosene. Fuel 102:483–490. https://doi.org/10.1016/j.fuel.2012.06.108
Rocha DP, Squissato AL, da Silva SM et al (2020) Improved electrochemical detection of metals in biological samples using 3D-printed electrode: chemical/electrochemical treatment exposes carbon-black conductive sites. Electrochim Acta 335:1–11. https://doi.org/10.1016/j.electacta.2020.135688
Ataide VN, Rocha DP, de Siervo A et al (2021) Additively manufactured carbon/black-integrated polylactic acid 3Dprintedsensor for simultaneous quantification of uric acid and zinc in sweat. Microchim Acta 188:1–11. https://doi.org/10.1007/s00604-021-05007-5
Instituto Nacional de Metrologia Q e T (INMETRO) (2020) ORIENTAÇÃO SOBRE VALIDAÇÃO DE MÉTODOS ANALÍTICOS. DOQ-CGCRE-008 1–30, http://www.inmetro.gov.br/Sidoq/pesquisa_link.asp?seq_tipo_documento=5&cod_uo_numeracao=00774&num_documento=008. Accessed Jan 2022
De Araujo TA, Barbosa AMJ, Viana LH, Ferreira VS (2011) Electroanalytical determination of TBHQ, a synthetic antioxidant, in soybean biodiesel samples. Fuel 90:707–712. https://doi.org/10.1016/j.fuel.2010.09.022
Schaumlöffel de S L, Dambros JWV, Bolognese Fernandes PR et al (2019) Direct and simultaneous determination of four phenolic antioxidants in biodiesel using differential pulse voltammetry assisted by artificial neural networks and variable selection by decision trees. Fuel 236:803–810. https://doi.org/10.1016/j.fuel.2018.09.048
Hoffmann Da Rocha AA, Casagrande M, De Souza Schaumlöffel L et al (2017) Simultaneous voltammetric determination of tert-butylhydroquinone and propyl gallate in biodiesel-ethanol at a Pt ultramicroelectrode. Energy Fuels 31:7076–7081. https://doi.org/10.1021/acs.energyfuels.7b00204
Funding
The authors are grateful to the Brazilian agencies CAPES (001), CNPq (427731/2018-6, 307271/2017-0, 163330/2020-4, and TWAS/CNPq 137634/2017-0), INCTBio (CNPq grant no. 465389/2014–7), and FAPEMIG (RED-00042-16, PPM-00640-16 and APQ-03141-18) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Inoque, N.I.G., João, A.F., de Faria, L.V. et al. Electrochemical determination of several biofuel antioxidants in biodiesel and biokerosene using polylactic acid loaded with carbon black within 3D-printed devices. Microchim Acta 189, 57 (2022). https://doi.org/10.1007/s00604-021-05152-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05152-x