Abstract
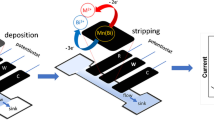
A disposable paper-based bipolar electrochemical biosensor is reported for determination of glucose. The closed bipolar electrochemical cell is fabricated on a small part of paper using a laser printing-based process for paper hydrophobization. The bipolar and driving electrodes are provided by pressing the writing pencil HB on the paper. The mechanism of sensing of glucose is oxidation of the analyte in the sensing cell using glucose oxidase followed by reduction of the produced H2O2 by application of an external potential (10.0 V). This causes the oxidation of K4Fe(CN)6 in the presence of Fe(II) ions and subsequent formation of Prussian Blue (PB) particles in the reporting cell. The intensity of the blue color in the reporting cell is used as a visual and colorimetric signal that can be digitally read using a scanner of digital camera. The parameters affecting the performance of the device were optimized using experimental design and chemometrics modeling. The P-BPE represents a very wide response range that extends from 0.1 mmol.L−1 to 4.0 mol.L−1 in the case of hydrogen peroxide, and from 0.1 to 50 mmol.L−1 in the case of glucose. The limit of detections for hydrogen peroxide and glucose are 4.9 μmol.L−1 and 70 μmol.L−1 respectively.

Analyte solution (H2O2) and deionized water is injected to the sensing and the reporting cells respectively. By applying of an external potential, H2O2 reduction and potassium ferrocyanide (K4Fe(CN)6) oxidation is performed. This provides the appropriate condition for Prussian blue (PB) production (dark blue) in the reporting cell.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bipolar electrochemical assays had gained lots of attention. An electronic conductor in contact with a conductive electrolyte which acts as mixed electrodes (one side as an anode and the other side as a cathode) is called a bipolar electrode (BPE) [1]. By applying a high electric field to the driving electrode (DE) in touch with the electrolyte, a linear potential drop among the two DEs and also the two end sides of BPE would be developed. This makes it feasible to have both reduction and oxidation reactions simultaneously but on the two end sides of BPE. The favorable outcome has risen in this field because of elimination of power supply/electrode connection. This would lead to indirect control of the polarization of many electrodes by single power supply and availability of wide ranges of BPE size. Since BPE had no connection with readout systems, the Faradic current cannot be measured by conventional methods. Generally, the cell where analyte reaction takes place is called sensing cell and then the front side cell is called reporting cell, where the latter is used for detection of Faraday current. Various reporting methods had been introduced until now to circumvent the current measurement impediment [2,3,4,5].
Paper-based BPE (P-BPE) microfluidic assays have attracted a lot of attention [6,7,8,9]. This is due to their low preparation cost, self-pumping property of paper, being portable and needing very low volumes of reagents as well. The dominant reporting method in the introduced P-BPEs is electrochemiluminescence (ECL) [10]. There are some reports on the glucose and H2O2 determination by P-BPE using ECL as the reporting method [7, 11]. ECL limits the infield application of this type of paper-based sensor as they need expensive photon counting compartment (e.g., photomultiplier; PMT). Developing a colorimetric method instead of ECL can be a promising feature since the analyte quantification would be done by a digital camera, a smart phone or a scanner.
The coloration of an electroactive species upon a redox reaction is termed electrochromism [12]. Prussian blue (PB), a hexacyanoferrate compound [13], as one of the most used compounds in electrochromism, has widely used in different research fields especially for biosensor construction [14]. Besides its electro-catalytic properties, substantial changes in its optical properties upon electrochemical reactions had been used many times [15]. In bipolar electrochemistry field, there are just a few reports on using the electrochromisity of PB. Li, Wang and co-workers prepared an electrodeposited PB on an indium tin oxide (ITO) BPE [16, 17]. They used this BPE for electrochromic studying of the catalytic activities of the catalysts and signaling of the carcinoembryonic antigen (CEA). The authors designed a fluorescence switch system based on the spectral overlap between the absorption band of electrodeposited PB on ITO BPE and the emission spectrum of Au nanoclusters [18]. Yu et al. established a bio-molecular keypad lock on the basis of a closed bipolar electrode using electrodeposited PB on ITO as a reporting section. The color change of PB to Prussian white and vice versa was the main factor in this keypad lock design [19]. In all of these reported PB applications in bipolar systems, use of the spectrometers and presence of the transparent BPE are required. Besides that, a pre-step of PB electrodeposition is needed for construction of the devices working upon electrochromisity.
In the present study, the electrodeposition of PB on the paper fibers has been introduced for the first time which has been used directly as a colorimetric method for the reporting cell of P-BPE. It uses smartphone mobile camera or digital scanner as an alternative to PMT in ECL or any spectrometer for analyte quantification. Also, the application of the paper substrate would eliminate the need for the transparent bipolar electrode. Therefore, it increases the variety of choices as a BPE along with reducing the costs. Applicability of this colorimetric method on the P-BPE for H2O2 and glucose determination has been investigated.
Material and methods
Reagents and materials
S&S (Schleicher & Schull) Nr 2040 B filter paper (58 × 60) was used for device fabrication. Pencil core HB Panter 0.5 × 60 mm was obtained from a local shop. Reagents including KCl, KNO3, K4Fe(CN)6, KSCN, FeSO4, H2O2, NaH2PO4, Na2HPO4, H3PO4, NaCl, K2CrO4, NaBH4, AgNO3, Na3C6H5O7 were all obtained from Merck (www.merck.com). D-Glucose, H3BO3, and CH3COOH were from BDH (www.bdhme.com) while universal pH indicator was obtained from Hopkin & Williams Ltd. (www.chemicalscout.com). Glucose oxidase (GOx), from Aspergillus niger, and uric acid were purchased from Sigma-Aldrich (www.sigmaaldrich.com). Ascorbic acid was from Riedel-deHaën (www.riedeldehaen.com). Vacuum blood collection tube GD060SGC was obtained from Gongdong (www.chinagongdong.com).
Instrumentation
HP LaserJet 1320 Printer, CanoScan LIDE 700F scanner, a MEGATEK power supply, and Memmert oven were used in device fabrication and analysis while a stencil was prepared by wire-cutting Machine. Energy dispersive spectroscopy (EDS) and tscanning electron characterization of the P-BPE device were performed by TESCAN Vega3 scanning electron microscope (SEM). Behdad centrifuge model 3480 was used in the step of real sample preparation.
Device fabrication
Fabrication of the bipolar paper-based device was started by drawing the closed version of the previously reported pattern [11] in AutoCAD software. It was printed by LaserJet printer and then was heated in an oven of 200 °C for hydrophobization of the printed pattern. The BPE, DEs dimensions and channel pattern (bottom image in Scheme S1) used here was the same as reported in [11] but in the closed cell format (top image in Scheme S1). To prevent direct contact between power supply alligator clips and the electrolyte solution, the DEs were drawn on the hydrophobized paper. Printing of DEs and BPE on the P-BPE was done by pressing pencil HB core using a stencil attached to the paper by the help of a magnet [20]. On the left side of Scheme 1, the process of preparing the P-BPE is shown.
Paper-based bipolar electrode (P-BPE) platform procedure
Hydrogen peroxide determination
As it is shown on the right side of Scheme 1, P-BPE assays were prepared by injecting 5.0 μL aqueous solution of 0.38 mol.L−1 KCl to both reporting and sensing cells and then drying for 2.0 min in 60 °C. In the next step, 5.0 μL of the 0.2 mol.L−1 universal buffer (mixture of boric acid, phosphoric acid, and acetic acid) with pH of 3.9 and 5.4 were injected to the reporting and sensing cells, respectively. After dryness of the buffer, by keeping the device in an oven of 60 °C, a 5.0 μL aliquot of a mixture solution containing 6.5 mmol.L−1 FeSO4 and 36.5 mmol.L−1 K4Fe(CN)6 was injected to the reporting cell and let it to dry.
The fabricated P-BPE device was used for the determination of hydrogen peroxide. To do so, a 5.0 μL portion of deionized water was injected into the reporting cell while 5.0 μL of H2O2 standard solutions of variable concentrations were injected to the sensing cell. DEs were then connected to the power supply (DEs in the reporting cell and sensing cell were attached to negative and positive poles of power supply respectively) adjusted on 10.0 V for 3.0 min. Then, the image of the devices was captured by a smartphone camera or by a digital scanner. The blank assay was done by injecting 5.0 μL portion of deionized water to the sensing cell instead of H2O2 solution.
Glucose determination
The reporting cell of the P-BPE device was prepared as before but a simple modification was done on the sensing cell. After injecting 5.0 μL solution of 0.38 mol.L−1 KCl in the sensing cell, a 5.0 μL aliquot of GOx aqueous solution of 3.0 U.μL−1 (in 0.2 mol.L−1 phosphate buffer of pH = 7), was added and let it to dry in the room temperature. The P-BPE is now ready for analysis of glucose. To do so, a 5.0 μL portion of glucose standard solutions with a specified concentration was added to the sensing cell and the DE electrodes were connected to the power supply of 10.0 V potential difference. The changes in the color intensity of the reporting cell were measured after 3.0 min. It should be noted that for each standard solution of glucose, a separate P-BPE was used.
For determination of glucose in real samples, the blood samples were collected in the vacuum tubes containing clot activator which accelerates clotting of the blood. After 15.0 min, the test tubes were placed in the centrifuge. The serum was separated from the clotted blood with centrifuge at 2500 rpm for 15 min. Then, 5.0 μL of the serum sample was injected to the sensing cell of P-BPE device. The used P-BPE device was prepared as same as explained in the above paragraph. After applying a potential difference of 10 V for 3.0 min, the images were captured. The images of the blank assays were captured by injecting 5.0 μL portion of serum sample to the P-BPE device with the sensing cell in the absence of GOx.
Data acquisition and colorimetric analysis
Image analysis was performed by calculating the difference between color intensities of the reporting cells of the blank and sample P-BPE devices. In the blank assay, deionized water was injected into both sensing and reporting cells. We used the Red-Green-Blue (RGB) color space for colorimetric determinations [21]. In this color space, a single color would be formed by a mixture of three values (0–255) for each channel of the red, green, and blue. A calibration plot obtained from RGB values in various analyte standards would allow the measurement of its concentration in the unknown samples.
A lab-made software in MATLAB (Mathworks Inc., www.mathworks.com) was used for colorimetric analyses. Briefly, the blank and sample assay images in the jpg format were loaded in the software. Only the images of reporting cell were selected for further analysis. A reference pixel was selected inside each of the reporting cell by clicking on the scanned image of the P-BPE device. By choosing enough tolerance level (about 80) around the chosen pixel intensity, all of the similar pixels in the selected part of the image (reporting cells) are found. The average number of pixels of a particular type in each chosen colorimetric space is calculated. The software is able to export the difference between the calculated average pixel intensities of blank and sample images. Software with the same function was also written for smartphone usage which the reference pixel was chosen by touching the appropriate part of the image.
Results and discussion
Fabrication of hydrophobic patterns
Here, we used the laser printing method for fabrication of hydrophilic channels on the paper. To remove contaminations from the filter paper, it was washed before printing [22]. Printing the pattern (Scheme S1) and heating for hydrophobization of paper substrate had been introduced before [20]. This was done by double side printing of the pattern on paper and then heating for 4–5 min on a hot plate at 200 °C. Here, a modification of this procedure was done by increasing the time of heating to 45.0 min in the oven with 200 °C, so one side printing was enough for paper hydrophobization. This eliminated the difficulties related to the superimposition of patterns in both sides of the paper. SEM images of the surface of the printed paper before and after heating are shown in Fig. 1. As can be seen, ink particles have been melted and penetrated into the paper pores. The cross-section SEM image of filter paper, taken after heating of the printed paper, confirms blocking of paper pores compared with its cross-section image taken before printing. As it is discussed in the supplementary material section, the solutions are not diffused from sensing cell to the reporting cell.
Device fabrication and characterization
The P-BPE device was fabricated in a closed cell format using a hydrophobic barrier of 1 mm width, which separated the anodic and cathodic cells. A rectangular BPE sizing (3 × 1) mm was drawn in the middle of the device and on the hydrophobic barrier. Considering 1 mm width of the hydrophobic barrier, the size of BPE, which is exposed to each one of the anodic and cathodic poles, is (1 × 1) mm. The sizes of DEs are (4 × 5) mm. The distance between DE and hydrophobic barrier in each half-cell is 6.5 mm. This design of the P-BPE device is the same as reported before [11], so it would be possible to make a better comparison between the two reporting methods.
Reproducibility of the electrode drawing was investigated in two writing force conditions of soft tracing and hard tracing manner. In each case, the effect of the number of times that the pencil should be used to draw on the paper was checked. As can be seen in Table S2, increasing the drawing times result in improved conductivity, which can be attributed to deposition of more graphite on the paper. For saving time and having appropriate conductivity, further electrode preparations were performed by applying 10 times hard drawing.
To test whether the fabricated P-BPE works properly, water electrolysis was investigated using universal pH indicators [23]. Fig. S2 of the supporting information shows the response of the assay with the applied voltage of 40.0 V. Water electrolysis was recognized from changes in the color of pH indicator in half cells of the P-BPE. By decreasing the voltage down to 10.0 V, the color change was observed. At lower applied potential no color changes were observed, revealing water was not electrolyzed at applied potentials ≤10.0 V.
For characterization of the printed BPEs and DEs, EDS analysis was performed. As it is shown in Fig. S4 of the supporting information, the paper and pencil core is composed of 46% and 95% carbon element, respectively. The pencil coated paper has around 71% of carbon element, which provides enough conductivity for the printed electrodes.
Prussian blue (PB) as a reporting colorimetric reagent
The reaction between ferric and ferrocyanide ions or ferrous and ferricyanide ions would lead to formation of PB. A soluble form of PB (KFeIII [FeII(CN)6]) is formed when ferrocyanide or ferricyanide ions are in excess. In the presence of an excess of a ferrous or ferric salt, insoluble PB (FeIII4[FeII(CN)6]3) would be formed [24]. Accordingly, the mixture of K4Fe(CN)6/FeSO4 was injected to the reporting cell, in which oxidation of FeII in the K4Fe(CN)6 or FeSO4 would create the appropriate condition for formation of PB (Fig. 2c). The amount of the formed deep blue color (as a result of PB formation) is proportional to Faradic current in BPE electrode.
The images of P-BPE in the absence and presence of the analyte (here hydrogen peroxide or glucose) are given in Fig. 2. As it can be seen, K4Fe(CN)6/FeSO4 mixture had a light blue color corresponding to the following reaction [25]:
However, PB has a deep blue color which is totally distinguishable from the initial light blue color. Fortunately, this difference in color supervised by image analysis allows colorimetric detection of hydrogen peroxide.
SEM images for investigation of PB formation around BPE were obtained and results are reported in Fig. S5 of the supporting information. The existence of PB particles on the paper fibers is obvious by comparing the top row of images in Fig. S5 with the bottom one. PB particles are in the size range of 100–500 nm. The EDS chemical mapping (Fig. S6) provides evidence for the formation of PB (see supporting information for more details).
To investigate which species (K4Fe(CN)6 or FeSO4) is oxidized at the BPE, instead of K4Fe(CN)6/FeSO4 mixture, a mixture containing 1:1 volume ratio from 0.01 mol.L−1 FeSO4 and 0.01 mol.L−1 KSCN was injected in the reporting cell. Other steps were the same as K4Fe(CN)6/FeSO4 case. Formation of the red color, which should be the result of the formation of Fe(SCN)2+ complex, was not observed, indicating that in that voltage FeSO4 was not oxidized.
Electrolyte conductivity
Electrolyte conductivity was optimized by changing the KCl concentration. Various concentrations of different supporting electrolytes were injected to both sensing and reporting cells and the P-BPE was connected to the power supply with different voltages to study the behavior of P-BPE device. In the case of 3.0 mol.L−1 KCl and applied voltage of 50.0 V, sparking in the middle of BPE was seen. The sparking on the P-BPE sometimes was so intense that a burned hole was formed in the middle of BPE. Further analysis revealed that the appropriate electrolyte conductivity without paper burning is obtained under two different conditions; (i) low KCl concentrations (lower than 1.0 mol.L−1) with high applied potential (around 50.0 V) or (ii) low applied voltages (lower than 20.0 V) and high KCl concentration (around 3.0 mol.L−1). To prevent water oxidation, the latter condition was used. Further investigations for using KNO3 as a supporting electrolyte showed that using 0.5 mol.L−1 solution of KNO3 at applied voltage of 50.0 V and KNO3 solution of 3.0 mol.L−1 at applied voltages <20.0 V would be applicable. However, since in the case of KCl, a wider concentration range of electrolyte was available, it was chosen as the supporting electrolyte.
Optimization and model fitting
The following parameters were optimized by experimental design: (a) concentration of K4Fe(CN)6; (b) concentration of FeSO4; (c) concentration of KCl; (d) the pH of sensing cell and (e) the pH of reporting cell. It should be noted that the chemical reactions in the sensing and reporting cells are different (reduction of H2O2 and oxidation of Fe2+ take place in sensing and reporting cells, respectively). Therefore, they are affected by pH in different manner and hence they were optimized separately. Respective text and Figures on optimizations are given in the electronic supporting material. In short, the following experimental conditions were found to give the best results: (a) concentration of K4Fe(CN)6: 0.073 mol.L−1; (b) concentration of FeSO4: 0.013 mol.L−1; (c) concentration of KCl: 0.38 mol.L−1; (d) pH of sensing cell: 5.4 and (e) pH of reporting cell: 3.9. Since mixtures of 1:1 (V/V) K4Fe(CN)6 and FeSO4 were injected to the reporting cell, the final concentrations of FeSO4 and K4Fe(CN)6 in the mixture were 6.5 mmol.L−1 and 36.5 mmol.L−1, respectively.
For measuring color intensity in RGB space, one can choose either individual color elements of R, G, and B or their combinations (e.g., R + B, R + G, B + G or R + B + G). Using the response variables given in Table S4, the color space of GB was chosen since it represented larger variations (higher sensitivity) compared to other color spaces.
In addition to the chemical factors, two other physical factors of time and applied potential can affect the behavior of the designed P-BPE. Low potentials may not provide sufficient driving force for either reducing hydrogen peroxide in the sensing cell or oxidizing Fe(II) in the sensing cell whereas higher potentials may lead to unwanted reactions (e.g. oxidation or reduction of water).
Regarding the required time to record the response after application of potential, it should be as short as possible, ideally. Considering no interaction between these parameters, they were optimized by one at a time method, of course in the optimized values of the already studied chemical parameters. Applied voltages in the range of of 2.0 to 10.0 V were investigated. No reaction color change was observed at voltages<2.0 V, and at voltages>10.0 V, water electrolysis took place as it was explained previously. As can be seen in Table S5, the P-BPE device response increases by increasing the potential. However, by increasing the time of applied potential (10.0 V), the device’s response first increases till 3.0 min and then decreases. The times shorter than 3.0 min are not enough for completion of oxidation/reduction reactions at BPEs whereas at longer times the device is being dried by vaporization of water in the air. According to these data, application of 10.0 V voltage to BPEs for 3.0 min was used for future analyses.
Determination of H2O2 and glucose
As a proof of applicability of the reported method, the designed P-BPE was then applied for sensing of H2O2, as one of the very important chemical species produced from enzymatic reactions. As it is shown in Fig. 3a, the P-BPE device’s response (∆GB) shows dependency on the concentration of hydrogen peroxide in a very wide concentration range. The scanned images of the paper-based devices after addition of various concentrations of H2O2 solutions are represented in Fig. 3c-h. The obvious dependency between ∆GB and H2O2 concentration was then transformed to the linear regression by plotting ∆GB versus Log [concentration H2O2]. The calibration graph is then linear in the concentration range of 1.0 × 10−5 - 4.0 mol.L−1 with the limit of detection (LOD) of 4.9 μmol.L−1. LOD was calculated by summing up “3.3× standard deviation of blank” with the average value of the blank signal, and then dividing by the slope of the calibration plot. As can be seen in Table 1, by using a colorimetric detection method which does not need any expensive readout devices, acceptable results have been achieved.
Changes in the ΔGB values of the reporting cell over concentration of standard solutions of (a) H2O2 and (b) glucose. Insets are linear regression between ΔGB values and logarithmic of concentration. The error bars in calibration plots correspond to standard deviations of replicated analysis (n = 3). c-h: Scanned images of sensors after addition of (c) 0 mol.L−1, d 0.01 mol.L−1, e 0.05 mol.L−1, f 0.5 mol.L−1, g 2.0, and (h) 4.0 mol.L−1 H2O2 solutions to the sensing cell
Detection of H2O2 is often the basis of fabrication of many biosensors. Here, we investigated the possibility of using our P-BPE device as a glucose biosensor. The procedure of glucose determination is given in the material and methods section. As it is shown in Fig. 3b, there is a linear correlation between the ΔGB values and the logarithm value of the concentration of glucose.
Effect of the interfering species
Possible electroactive interferences in blood are ascorbic acid (AA) and uric acid (UA). While the normal physiological level of glucose is 3–8 mmol.L−1 [27], the concentrations of AA and UA in blood serum are 0.1 mmol.L−1 and 0.3 mmol.L−1 [28], respectively or 0.02 mmol.L−1 for UA according to [29, 30]. For studying the effect of the presence of these interfering species on glucose detection, the solutions of 3.0 mmol.L−1 glucose in the presence of 0.1 mmol.L−1 AA or 0.3 mmol.L−1 UA were injected to the sensing cell of the d P-BPE. After 3.0 min applying of the 10.0 V potential difference, the images of the P-BPE devices were analyzed and the ΔGB values were put in the linear regression equation of glucose calibration plot. The calculated concentrations were then compared with the case of glucose 3.0 mmol.L−1 in the absence of interferences. The relative errors of glucose determination in the presence of AA and UA where 2.0% and 4.2% respectively, suggesting that AA and UA had negligible interferences on glucose detection.
It should be noted that the possible interferences (those are electroactive at the applied potential) can be managed using a two-measurement protocol. One measurement is done with the P-BPE having GOx (assay #1) and another is done with that without GOx (assay #2). Interfering species would react in both assays whereas glucose gives response in just assay #1. Taking a difference between the color intensities of two assays would eliminate the colorimetric response attributed to the interferences.
Analysis of real samples
The biosensor was used for determination of glucose in human serum samples. Two samples were prepared from a local pathobiology laboratory. Then, the glucose levels in the samples were measured using our P-BPE biosensor. The results (in Table 2) were compared with the test results by Auto Analyzer Hitachi RA-XT in the local laboratory. As seen, the results obtained with the P-BPE are in good agreement with those measured in the laboratory. Student t-test confirmed that there was not a statistically significant difference in the concentration values obtained by P-BPE biosensor and Auto Analyzer with 95% confidence level.
Conclusions
A colorimetric method as a reporting technique in paper-based bipolar electrochemistry is presented. The basis of this method is the formation of Prussian Blue by oxidation of K4Fe(CN)6 in the presence of FeSO4 in the reporting cell. Combining paper substrate as a very cheap substrate and its simple fabrication by one-side laser printing with a colorimetric detection method made the designed P-BPE as a practical tool. Applicability of this method is proved by measurement of hydrogen peroxide and glucose as a model analyte. Bipolar and driving electrodes are all prepared by drawing a pencil on the paper. An interesting advantage of this P-BPE device is its very wide dynamic range. In comparison with previously reported BPEs, our biosensor is far from needing expensive photon counter in ECL or spectrophotometer in ITO-based sensors. So, our portable biosensor is a candidate for in-filed applications.
References
Mavré FO, Anand RK, Laws DR et al (2010) Bipolar electrodes: a useful tool for concentration, separation, and detection of Analytes in microelectrochemical systems. Anal Chem 82:8766–8774. https://doi.org/10.1021/ac101262v
Chow KF, Chang BY, Zaccheo BA, Mavré F, Crooks RM (2010) A sensing platform based on electrodissolution of a Ag bipolar electrode. J Am Chem Soc 132:9228–9229. https://doi.org/10.1021/ja103715u
Arora A, Eijkel JCT, Morf WE, Manz A (2001) A wireless electrochemiluminescence detector applied to direct and indirect detection for electrophoresis on a microfabricated glass device. Anal Chem 73:3282–3288. https://doi.org/10.1021/ac0100300
Shayan M, Kiani A (2015) Digital versatile disc bipolar electrode: a fast and low-cost approach for visual sensing of analytes and electrocatalysts screening. Anal Chim Acta 888:52–58. https://doi.org/10.1016/j.aca.2015.07.014
Klett O, Nyholm L (2003) Separation high voltage field driven on-chip amperometric detection in capillary electrophoresis. Anal Chem 75:1245–1250. https://doi.org/10.1021/ac020660h
Liu H, Zhou X, Liu W, Yang X, Xing D (2016) Paper-based bipolar electrode Electrochemiluminescence switch for label-free and sensitive genetic detection of pathogenic bacteria. Anal Chem 88:10191–10197. https://doi.org/10.1021/acs.analchem.6b02772
Chen L, Zhang C, Xing D (2016) Paper-based bipolar electrode-electrochemiluminescence (BPE-ECL) device with battery energy supply and smartphone read-out: a handheld ECL system for biochemical analysis at the point-of-care level. Sensors Actuators B Chem 237:308–317. https://doi.org/10.1016/j.snb.2016.06.105
Feng Q-M, Pan J-B, Zhang H-R, Xu JJ, Chen HY (2014) Disposable paper-based bipolar electrode for sensitive electrochemiluminescence detection of a cancer biomarker. Chem Commun 50:2–4. https://doi.org/10.1039/c4cc03102d
Rafatmah E, Hemmateenejad B (2019) Fabrication of the first disposable three-dimensional paper-based concentration cell as Ammonia sensor with a new method for paper Hydrophobization by laser patterned Parafilm®. Electroanalysis 31:632–638. https://doi.org/10.1002/elan.201800289
Lu W-X, Bao N, Ding S-N (2016) A bipolar electrochemiluminescence sensing platform based on pencil core and paper reservoirs. RSC Adv 6:25388–25392. https://doi.org/10.1039/C6RA01460G
Liu R, Zhang C, Liu M (2015) Open bipolar electrode-electrochemiluminescence imaging sensing using paper-based microfluidics. Sensors Actuators B Chem 216:255–262. https://doi.org/10.1016/j.snb.2015.04.014
Monk PMS, Mortimer RJ, Rosseinsky DR et al (1995) Electrochromism: fundamentals and applications, 1st edn. VCH Verlagsgesellschaft mbH, Weinheim
Xu Y, Zheng S, Tang H, Guo X, Xue H, Pang H (2017) Prussian blue and its derivatives as electrode materials for electrochemical energy storage. Energy Storage Mater 9:11–30. https://doi.org/10.1016/j.ensm.2017.06.002
Karyakin AA (2017) Advances of Prussian blue and its analogues in (bio)sensors. Curr Opin Electrochem 5:92–98. https://doi.org/10.1016/j.coelec.2017.07.006
Koncki R, Lenarczuk T, Radomska A, Gląb S (2001) Optical biosensors based on Prussian blue films. Analyst 126:1080–1085. https://doi.org/10.1039/b103044m
Zhai Q, Zhang X, Xia Y, Li J, Wang E (2016) Electrochromic sensing platform based on steric hindrance effects for CEA detection. Analyst 141:3985–3988. https://doi.org/10.1039/c6an00675b
Zhang X, Shang C, Gu W, Xia Y, Li J, Wang E (2016) A renewable display platform based on the bipolar electrochromic electrode. ChemElectroChem 3:383–386. https://doi.org/10.1002/celc.201500282
Xing H, Zhang X, Zhai Q, Li J, Wang E (2017) Bipolar electrode based reversible fluorescence switch using Prussian blue/Au nanoclusters nanocomposite film. Anal Chem 89:3867–3872. https://doi.org/10.1021/acs.analchem.7b00246
Yu X, Liang J, Yang T, Gong M, Xi D, Liu H (2018) A resettable and reprogrammable keypad lock based on electrochromic Prussian blue films and biocatalysis of immobilized glucose oxidase in a bipolar electrode system. Biosens Bioelectron 99:163–169. https://doi.org/10.1016/j.bios.2017.07.054
Dey R, Kar S, Joshi S, Maiti TK, Chakraborty S (2015) Ultra-low-cost ‘paper-and-pencil’ device for electrically controlled micromixing of analytes. Microfluid Nanofluid 19:375–383. https://doi.org/10.1007/s10404-015-1567-3
Plataniotis KN, Venetsanopoulos AN (2000) Color image processing and applications, 1st edn. Springer-Verlag Berlin Heidelberg, Berlin
Taghizadeh-Behbahani M, Hemmateenejad B, Shamsipur M (2017) Colorimetric determination of acidity constant using a paper-based microfluidic analytical device. Chem Pap 72:1–9. https://doi.org/10.1007/s11696-017-0357-7
Scida K, Fosdick SE, Renault C et al (2013) Paper-Based Bipolar Electrochem. J Electrochem Sci Technol 4:146–152. https://doi.org/10.5229/JECST.2013.4.4.146
Coleby LJM (1939) A history of Prussian blue. Ann Sci 4:206–211. https://doi.org/10.1080/00033793900201211
Farkas J, Farkas B (1988) Technology and biochemistry of wine: volume 2. Taylor & Francis Ltd, London
Liu M, Liu R, Wang D, Liu C, Zhang C (2016) A low-cost, ultraflexible cloth-based microfluidic device for wireless electrochemiluminescence application. Lab Chip 16:2860–2870. https://doi.org/10.1039/C6LC00289G
Lee YJ, Park JY (2011) A coral-like macroporous gold-platinum hybrid 3D electrode for enzyme-free glucose detection. Sensors Actuators B Chem 155:134–139. https://doi.org/10.1016/j.snb.2010.11.037
Han L, Zhang S, Han L, Yang DP, Hou C, Liu A (2014) Porous gold cluster film prepared from Au@BSA microspheres for electrochemical nonenzymatic glucose sensor. Electrochim Acta 138:109–114. https://doi.org/10.1016/j.electacta.2014.06.095
Zhong GX, Zhang WX, Sun YM, Wei YQ, Lei Y, Peng HP, Liu AL, Chen YZ, Lin XH (2015) A nonenzymatic amperometric glucose sensor based on three dimensional nanostructure gold electrode. Sensors Actuators B Chem 212:72–77. https://doi.org/10.1016/j.snb.2015.02.003
Shu H, Cao L, Chang G, He H, Zhang Y, He Y (2014) Direct electrodeposition of gold nanostructures onto glassy carbon electrodes for non-enzymatic detection of glucose. Electrochim Acta 132:524–532. https://doi.org/10.1016/j.electacta.2014.04.031
Acknowledgments
Financial support of this work by Shiraz University Research Council is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2.41 MB)
Rights and permissions
About this article
Cite this article
Rafatmah, E., Hemmateenejad, B. Colorimetric and visual determination of hydrogen peroxide and glucose by applying paper-based closed bipolar electrochemistry. Microchim Acta 186, 684 (2019). https://doi.org/10.1007/s00604-019-3793-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3793-y