Abstract
CdTe quantum dots (QDs) were integrated with polyethyleneimine-coated carbon dots (PEI-CDs) to form a dually emitting probe for heparin. The red fluorescence of the CdTe QDs is quenched by the PEI-CDs due to electrostatic interactions. In the presence of heparin, the blue fluorescence of PEI-CDs remains unaffected, while its quenching effect on the fluorescence of CdTe QDs is strongly reduced. A ratiometric fluorometric assay was worked out. The ratio of the fluorescences at 595 and 436 nm serves as the analytical signal. Response is linear in the concentration range of 50–600 ng·mL−1 (0.1–1.2 U·mL−1) of heparin. The limit of detection is 20 ng·mL−1 (0.04 U·mL−1). This makes the method a valuable tool for heparin monitoring during postoperative and long-term care. This assay is relatively free from the interference by other analogues which commonly co-exist with heparin in samples, and it is more robust than single-wavelength based assays.

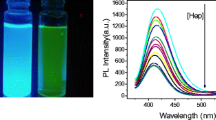
In the presence of heparin, the fluorescence of polyethyleneimine-coated carbon dots (PEI-CDs) at 436 nm remains unaffected, while its quenching effect on the fluorescence of CdTe at 595 nm is strongly reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heparin, a chain of polysaccharide sulfate, is produced and stored in mast cells in animal tissues. As a naturally occurring biomolecule, it has the wide medical applications. Heparin can accelerate the interaction between antithrombin and protease factor Xa, so it is used as an anticoagulant drug in open-heart surgery and emergency deep venous thrombosis treatment [1]. Heparin also can inhibit the growth and replication of the human immunodeficiency virus and inhibit angiogenesis and tumor growth [2]. The recommended heparin therapeutic standard is 2–8 U·mL−1 for cardiovascular surgery and 0.2–1.2 U·mL−1 for postoperative and long-term care [3]. The higher dosage and prolonged use often induce adverse effects, such as hemorrhage, heparin-induced thrombocytopenia, and osteoporosis [4]. Based on the clinical importance, it is essential to develop a rapid and accurate method for the monitoring of heparin level [5, 6].
Up to now, numerous methods have been developed for the detection of heparin. Activated coagulation time [7], activated clotting time [8], surface-enhanced Raman scattering (SERS) [9], and electrochemical methods [10] were reported. Although colorimetric approaches based on conjugated oligoelectrolyte/graphene oxide [11], supermolecule [12] and aniline dye [13] have been applied to the heparin assay, the sensitivities are not very good.
Fluorescence analysis is widely applied in the detection of heparin due to its advantages of high sensitivity, low cost and rapid. A heparin assay using a complex of multiple-adenosine (A20) and coralyne was developed [3]. Likely, an adenosine-based molecular beacon (MB) was reported for heparin assay [14]. Based on the interaction between heparin and CuInS2, a turn off nanosensor was established [15]. Carbon dots [16, 17] and water-soluble silicon nanoparticles [18] were also successfully applied to heparin detection. These methods offer reasonable sensitivities, but heparin quantification based on single-wavelength fluorescence signal may be affected by various factors, probe concentration, instrumental efficiency, excitation intensity, and measurement condition, for example. On the contrary, ratiometric fluorescence assay uses the intensity ratio of two different emission wavelengths instead of the absolute intensity of one wavelength to measure, and provides a self-calibration, thus makes the analysis more accurate [5, 19,20,21].
Polyethyleneimine (PEI), containing primary, secondary, and tertiary amino groups, has been used in various researches because of the high positive charge, good water solubility and biocompatibility [22]. It, including its complexes functionalized as templating, stabilizing and filming with other organic or inorganic materials, exhibits outstanding adsorption capacity on CO2, heavy metals or dyes [23,24,25] and interacts with biomolecules [26, 27]. Some other researchers also reported the methods of heparin detection using PEI modified materials [28, 29].
Semiconductor quantum dots and carbon dots (CDs) are the most widely used fluorescent nanoparticles in biochemical analysis due to their adjustable surficial groups, excellent water solubility, high photostability and tunable excitation wavelength [30, 31]. Based on CdTe QDs quantum dots and polyethyleneimine-coated carbon dots (PEI-CDs), this paper proposes a novel ratiometric fluorescence method for the detection of heparin (Scheme 1). As a fluorescence switch, the positive charge PEI-CDs can quench the fluorescence of CdTe QDs through electrostatic attraction, while itself fluorescence remains unchanged. In the presence of heparin, PEI-CDs interact with anionic heparin, leading its desorption from CdTe QDs surface and recovering the fluorescence of CdTe QDs. Taking the constant fluorescence intensity of PEI-CDs as reference, heparin can be detected through measuring the fluorescence turn-on process of CdTe QDs.
Experimental
Materials and chemicals
Heparin (185 U/mg), bovine serum albumin (BSA) and adenosine triphosphate (ATP) were purchased from Sangon biotech Co., Ltd. (Shanghai, China, https://www.sangon.com/). PEI (Mw 10,000, 99%), chondroitin 4-sulfate (Chs) and hyaluronic acid (HA) were supplied by Aladdin Reagent Co., Ltd. (Shanghai, China, http://www.aladdin-e.com/). Cadmium chloride (CdCl2), tellurium (Te) powder, melamine, sodium citrate, dextran (Dex), 3-mercaptopropionic acid (3-MPA), sodium borohydride, NaHCO3, NaHPO4, NaAc and Na2SO4 were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China, http://www.sinoreagent.com/). All chemicals and reagents were analytical grade. The water was purified using a Millipore Milli-Q system and has a resistivity greater than 18 MΩ·cm.
Instruments
Fluorescence measurement was performed on a LS-55 luminescence spectrometer (Perkin-Elmer, USA, http://www.perkinelmer.com.cn/). Fourier-transform infrared (FT-IR) spectra were recorded using a FT-IR spectrophotometer (Perkin Elmer, USA). Transmission electron microscopy images were obtained using a Tecnai G2 F20 microscope (FEI, USA, https://www.fei.com/home/). Zeta potential was measured using a Malvern Zetasizer Nano ZS90 (Malvern, UK, http://malvern.cnpowder.com.cn/). All the experiments were carried out at room temperature.
Preparation of PEI-CDs and CdTe QDs
PEI-CDs were obtained by the modified method of preparation of PEI–GO composite [32]. Firstly, a microwave-assisted hydrothermal treatment was applied to prepare CDs according to the reported method [33]. In a typical synthesis, 0.58 g of trisodium citrate dihydrate and 0.12 g of melamine were added into 25 mL of H2O. The mixture solution was placed in microwave reactor at 180 °C and kept for 6 h. Then, 80 μL of 25% (wt) PEI aqueous solution was added to above solution under sonicating and continued stirring at 60 °C for 12 h to form a PEI-CDs homogeneous solution. The obtained PEI-CDs solution was cooled and stored at 4 °C.
According to the reported method [34], CdTe QDs were prepared. Briefly, 126 mL of 3-MPA and 0.228 g of CdCl2·2.5 H2O were added into 40 mL of water, and NaOH solution of 2.0 mol·mL−1 was dropwise added with stirring to adjust the pH to 10.5. The mixture was heated under N2 atmosphere to 100 °C, and 10 mL of the NaHTe solution was added to prepare stable, water-soluble 3-MPA-capped CdTe QDs. After three absolute ethyl alcohol precipitation and drying in vacuum oven, the purified CdTe QDs were obtained.
Fluorescence experiment
Fluorescence measurement was performed at an excitation wavelength of 360 nm and both 10 nm of excitation and emission slit widths. The stock solutions of CdTe QDs (0.2 mg·mL−1) and PEI-CDs (22.5 nmol·L−1) were prepared with the dilution of Tris buffer solution (pH 7.5, 20 mmol·L−1), and various concentration of heparin solutions were prepared in Tris buffer solution. The different amounts of PEI-CDs and 10 μL of CdTe QDs stock solution were transferred into a centrifuge tube, diluted to 200 μL with Tris buffer solution and cultivated for 2 min to investigate the fluorescent quenching effect. For the heparin assay, both 10 μL of PEI-CDs and CdTe QDs stock solutions and 20 μL of heparin or sample solution were transferred into a centrifuge tube, diluted to 200 μL with Tris buffer solution and cultivated for 5 min. Fluorescence spectra were recorded from 380 to 700 nm. Fluorescence intensities of PEI-CDs at 436 nm and CdTe QDs at 595 nm were adopted for heparin assay.
Results and discussion
Characterization of PEI-CDs and CdTe QDs
The transmission electron microscopy (TEM) images show that PEI-CDs keep well dispersed and have a diameter around 20 nm (Fig. S1), and the sizes of CdTe QDs have an average diameter of 4 nm roughly (Fig. S2). The light yellow solution of CDs turned into brown solution after reacting with PEI (Fig.S3A). In the FT-IR spectrum of PEI-CDs (Fig.S3B), the reduced carboxylic group bands at 3256, 1260 and 1089 cm−1 and enhanced N–H band at 3453 cm−1 compared with that of CDs confirmed the covalent attachment of PEI onto the CDs surface.
Detection mechanism of the PEI-CD/CdTe CD system for heparin
As illustrated in Fig. 1, the fluorescence of CDs at 436 nm remains almost constant in the presence of PEI, CdTe QDs and heparin. The fluorescence of CdTe QDs at 595 nm is quenched by PEI-CDs, but hardly quenched by CDs not modified with PEI. In the presence of heparin, the fluorescence intensity of PEI-CDs remains almost constant, but the quenched fluorescence of CdTe QDs is recovered. These phenomena may be explained from electrostatic interaction and hydrogen bonding action. The zeta potentials of the CDs, CdTe QDs, heparin and PEI-CDs were − 31.7, −27.7, −20.7 and 1.83 mV, respectively (Fig. S4). All with negative charge, CDs, CdTe QDs and heparin did not interact, while PEI-CDs with positive charge have electrostatic interaction with CdTe QDs to quench fluorescence, and banding affinity with heparin leading to itself desorption from the CdTe QDs surface and recovery of the quenched fluorescence. Apart from that, polyethyleneimine is rich in amino groups and heparin is a kind of polysaccharides which are rich in hydroxyl groups. The hydrogen bonding actions between polyethyleneimine modified carbon dots (PEI-CDs) and heparin may also contribute to the fluorescence recovery of CdTe. These results suggested that a novel “turn-off/on” dual-wavelength fluorescence method based on PEI-CD/CdTe CD system can be constructed for heparin detection.
The impact of cultivating pH and time
The cultivating pH and time were optimized to establish a sensitive heparin assay. Tris buffer solution was chosen and the pH value was adjusted with HCl or NaOH solution to avoid the possible perturbation of ionic strength. It can be seen from Fig. 2a that the fluorescence intensity is weak at low pH (pH < 5) for both CdTe QDs and PEI-CDs. The fluorescence intensity at 595 nm has greater recovery (ΔF) at the pH range of 7–11 in the presence of 0.15 μg·mL−1 heparin (Fig. 2b). Therefore, pH 7.5 was chosen as the heparin detection condition.
a The influence of pH values on fluorescence intensities of PEI-CDs (436 nm) and CdTe QDs (595 nm); b The fluorescence intensity difference (595 nm) of PEI-CD/CdTe CD system in the absence and presence of heparin (0.15 μg·mL−1) at different pH solutions; c The fluorescence intensities of PEI-CDs and CdTe QDs at different time after mixing; d The fluorescence intensity of PEI-CD/CdTe CD system at different time after addition heparin (1 μg·mL−1)
When PEI-CDs are added to the CdTe QDs solution, the fluorescence intensity of CdTe QDs at 595 nm decreases rapidly and reaches the bottom for about 2 min, while PEI-CDs fluorescence intensity at 436 nm remains stable (Fig. 2c). When heparin is added to the PEI-CD/CdTe CD system, the fluorescence intensity of CdTe QDs is recovered and maintains constant at least 15 min (Fig. 2d), and PEI-CDs fluorescence intensity at 436 nm remains stable. So, heparin incubation time can be chosen 5 min. These results indicate a promising application in fast heparin assay.
Fluorescence quenching study
The fluorescence spectra of PEI-CD/CdTe QDs system were measured in the presence of different concentrations of PEI-CDs. A remarkable fluorescence decrease was observed with the addition of PEI-CDs (Fig. S5A). The quenching constant (Ksv) was calculated according to following equation [35]:
In this equation, ΔF is the difference of emission intensities of CdTe QDs at 595 nm in the absence (F0) and presence of PEI-CDs, fa is the fraction of maximum accessible fluorescence, [Q] is the concentration of PEI-CDs, and KSV is the Stern-Volmer constant, which characterizes the quenching efficiency of the quencher. Results show that F0/ΔF is linearly proportional to PEI-CDs concentration from 0 to 1.13 nmol·L−1 (Fig. S4B), and KSV was calculated to be 1.549 × 106.
Ratiometric fluorometric assay for heparin
Figure 3a shows that the fluorescence intensity at 595 nm is gradually recovered with the increase of heparin concentration. Taking the fluorescence intensity at 436 nm as reference, the ratio value of I595/I436 fluorescence intensities displays a good linear relationship with the heparin concentration from 50 to 600 ng·mL−1 (around equal to 0.1–1.2 U·mL−1) (Fig. 3b). The regression equation is Y = 0.4117 + 0.7499X, with a correlation coefficient of 0.9974. The limit of detection (LOD) is 0.02 μg·mL−1 (0.04 U·mL−1) heparin, which is comparable to other methods (Table 1) and much lower than the heparin therapeutic level for cardiovascular surgery (2–8 U·mL−1) and postoperation and long-term therapy (0.2–1.2 U·mL−1). Therefore, the sensitivity of this method can meet the needs of the clinical detection. More importantly, the ratiometric fluorescence assay can provide a self-calibration and more reliable measuring result than single-wavelength assays.
Selectivity
The influence of three heparin analogues commonly existing in heparin samples, dextran (Dex), chondroitin sulfate (Chs) and hyaluronic acid (HA) was investigated. Other investigated relevant compounds and ions include BSA, ATP (each of 0.6 μg·mL−1), and Cl−, SO42−, HPO4−、HCO3−, Ac− and citrate (each of 10 mM). As shown in Fig. 4a, Dex, Chs and HA hardly produce obvious difference in signal of I595/I436 from blank even at 10-fold concentration of heparin. Similarly, the influences of other species also can be neglected. Further research demonstrates that coexisted interfering substances have little influences on detecting heparin (Fig. 4b). These results reveal that the ratiometric assay presents good selectivity.
a The signal (fluorescence ratio of I595/I436) response of PEI-CD/CdTe QDs system toward heparin or other interferents; b The signal response of PEI-CDs/CdTe QDs system toward heparin in the presence of various interferents (Heparin, 0.6 μg·mL−1; Dex、Chs、HA、BSA and ATP, each of 6 μg·mL−1; other irons, each of 10 mmol·L−1)
Detection of heparin in human serum samples
The feasibility of this system for the detection of heparin in human serum was validated. The human serum samples were collected from healthy volunteers, centrifuged at 13000 rpm for 3 min, then the supernatant liquor was diluted 10-fold with Tris buffer solution (pH 7.5, 20 mmol·L−1) to obtain the sample solution. None of heparin was found in sample solution. The average recoveries of spiked heparin in human serum samples reach 98.7–109.3% with a relative standard deviation (RSD) of less than 5% (Table 2), which is acceptable for quantitative assays performed in biological samples.
Conclusion
The PEI-CDs, CDs modified with PEI, possess strong fluorescent and contain positively charged amine groups, which provide effective quenching effect towards CdTe QDs due to electrostatic absorption and aggregating effect. The fluorescence of CdTe QDs being quenched contributes a relatively low background signal and further sensing toward heparin. The detection limit of constructed ratiometric assay based on PEI-CD/CdTe QDs for heparin is as low as 0.02 μg·mL−1 (0.04 U·mL−1). It is much lower than the clinical recommended heparin concentration. The selective can meet the requirement of biological sample assay. In sum, this method provides numerous advantages, such as simple operation process, high selectivity, rapid detection and reliable analytical result.
References
Walker C, Royston D (2002) Thrombin generation and its inhibition: a review of the scientific basis and mechanism of action of anticoagulant therapies. Br J Anaesth 88:848–863
Ding Y, Shi L, Wei H (2015) A “turn on” fluorescent probe for heparin and its over sulfated chondroitin sulfate contaminant. Chem Sci 6:6361–6366
Hung SY, Tseng WL (2014) A polyadenosine–coralyne complex as a novel fluorescent probe for the sensitive and selective detection of heparin in plasma. Biosens Bioelectron 57:186–191
Fu X, Chen L, Li J, Lin M, You H, Wang W (2012) Label-free colorimetric sensor for ultrasensitive detection of heparin based on color quenching of gold nanorods by graphene oxide. Biosens Bioelectron 34:227–231
Huang J, Li F, Guo R, Chen Y, Wang Z, Zhao C, Zheng Y, Weng S, Lin X (2018) A signal-on ratiometric fluorometric heparin assay based on the direct interaction between amino-modified carbon dots and DNA. Microchim Acta 185:260
Tawfik SM, Shim J, Biechele-Speziale D, Sharipov M, Lee YI (2018) Novel “turn off-on” sensors for highly selective and sensitive detection of spermine based on heparin-quenching of fluorescence CdTe QDs quantum dots-coated amphiphilic thiophene copolymers. Sensors Actuators B Chem 257:734–744
Murray DJ, Brosnahan WJ, Pennell B, Kapalanski D, Weiler JM, Olson J (1997) Heparin detection by the activated coagulation time: a comparison of the sensitivity of coagulation tests and heparin assays. J Cardiothorac Vasc Anesth 11:24–28
Raymond PD, Ray MJ, Callen SN, Marsh NA (2003) Heparin monitoring during cardiac surgery. Part 1: validation of whole-blood heparin concentration and activated clotting time. Perfusion 18:269–276
Qu G, Zhang G, Wu Z, Shen A, Wang J, Hu J (2014) A “turn-off” SERS assay of heparin with high selectivity based on heparin-peptide complex and raman labelled gold nanoparticles. Biosens Bioelectron 60:124–129
Yoshimi Y, Yagisawa Y, Yamaguchi R, Seki M (2018) Blood heparin sensor made from a paste electrode of graphite particles grafted with molecularly imprinted polymer. Sensors Actuators B Chem 259:455–462
Cai L, Zhan R, Pu KY, Qi X, Zhang H, Huang W, Liu B (2011) Butterfly-shaped conjugated oligoelectrolyte/graphene oxide integrated assay for light-up visual detection of heparin. Anal Chem 83:7849–7855
Bromfield SM, Barnard A, Posocco P, Fermeglia M, Pricl S, Smith DK (2013) Mallard blue: a high-affinity selective heparin sensor that operates in highly competitive media. J Am Chem Soc 135:2911–2914
You JG, Liu YW, Lu CY, Tseng WL, Yu CJ (2017) Colorimetric assay of heparin in plasma based on the inhibition of oxidase-like activity of citrate-capped platinum nanoparticles. Biosens Bioelectron 92:442–448
Kuo CY, Tseng WL (2013) Adenosine-based molecular beacons as light-up probes for sensing heparin in plasma. Chem Commun 49:4607–4609
Liu Z, Ma Q, Wang X, Lin Z, Zhang H, Liu L, Su X (2014) A novel fluorescent nanosensor for detection of heparin and heparinase based on CuInS2 quantum dots. Biosens Bioelectron 54:617–622
Cheng Q, He Y, Ge Y, Zhou J, Song G (2018) Ultrasensitive detection of heparin by exploiting the silver nanoparticle-enhanced fluorescence of graphitic carbon nitride (gC3N4) quantum dots. Microchim Acta 185:332
Wang R, Wang X, Sun Y (2017) Aminophenol-based carbon dots with dual wavelength fluorescence emission for determination of heparin. Microchim Acta 184:187–193
Ma SD, Chen YL, Feng J, Liu JJ, Zuo XW, Chen XG (2016) One-step synthesis of water-dispersible and biocompatible silicon nanoparticles for selective heparin sensing and cell imaging. Anal Chem 88:10474–10481
Rong M, Deng X, Chi S, Huang L, Zhou Y, Shen Y, Chen X (2018) Ratiometric fluorometric determination of the anthrax biomarker 2,6-dipicolinic acid by using europium(III)-doped carbon dots in a test stripe. Microchim Acta 185:201
Wang J, Peng X, Li D, Jiang X, Pan Z, Chen A, Huang L, Hu J (2018) Ratiometric ultrasensitive fluorometric detection of ascorbic acid using a dually emitting CdSe@SiO2@CdTe quantum dot hybrid. Microchim Acta 185:42
Yao J, Zhang K, Zhu H, Ma F, Sun M, Yu H, Sun J, Wang S (2013) Efficient ratiometric fluorescence probe based on dual-emission quantum dots hybrid for on-site determination of copper ions. Anal Chem 85:6461–6468
Wen T, Li NB, Luo HQ (2013) A turn-on fluorescent sensor for sensitive and selective detection of sodium dodecyl sulfate based on the eosin Y/polyethyleneimine system. Anal Chem 85:10863–10868
Ma Y, Liu WJ, Zhang N, Li YS, Jiang H, Sheng GP (2014) Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour Technol 169:403–408
Fan Y, Liu H, Zhang Y, Chen Y (2015) Adsorption of anionic MO or cationic MB from MO/MB mixture using polyacrylonitrile fiber hydrothermally treated with hyperbranched polyethylenimine. J Hazard Mater 283:321–328
Zhang H, Goeppert A, Prakash GK, Olah GA (2015) Applicability of linear polyethylenimine supported on nano-silica for the adsorption of CO2 from various sources including dry air. RSC Adv 5:52550–52562
Ling Y, Gao ZF, Zhou Q, Li NB, Luo HQ (2015) Multidimensional optical sensing platform for detection of heparin and reversible molecular logic gate operation based on the phloxine B/polyethyleneimine system. Anal Chem 87:1575–1581
Weng Y, Jiang B, Yang K, Sui Z, Zhang L, Zhang Y (2015) Polyethyleneimine-modified graphene oxide nanocomposites for effective protein functionalization. Nanoscale 7:14284–14291
Yan H, Wang HF (2011) Turn-on room temperature phosphorescence assay of heparin with tunable sensitivity and detection window based on target-induced self-assembly of polyethyleneimine capped Mn-doped ZnS quantum dots. Anal Chem 83:8589–8595
Liu J, Liu G, Liu W, Wang Y (2015) Turn-on fluorescence sensor for the detection of heparin based on rhodamine B-modified polyethyleneimine–graphene oxide complex. Biosens Bioelectron 64:300–305
Chen L, Xia N, Li T, BAi Y, Chen X (2016) Aptasensorfor visual andfluorometric determination of lysozyme based on the inner filter effect of gold nanoparticles on CdTe quantum dots. Microchim Acta 183:2917–2923
Luo PG, Sahu S, Yang ST, Sonkar SK, Wang J, Wang H, LeCroy G, Caoa L, Sun YP (2013) Carbon “quantum” dots for optical bioimaging. J Mater Chem B 1:2116–2127
Zhou XH, Chen ZX, Yan DH, Lu HB (2012) Deposition of Fe–Ni nanoparticles on polyethyleneimine-decorated graphene oxide and application in catalytic dehydrogenation of ammonia borane[J]. J Mater Chem 22(27):13506–13516
Lan M, Zhang J, Chui YS, Wang H, Yang Q, Zhu X, Wei H, Liu W, Wang P, Chen X, Lee C, Zhang W (2015) A recyclable carbon nanoparticle-based fluorescent probe for highly selective and sensitive detection of mercapto biomolecules. J Mater Chem B 3:127–134
Gu Z, Zou L, Fang Z, Zhu W, Zhong X (2008) One-pot synthesis of highly luminescent CdTe QDs/CdS core/shell nanocrystals in aqueous phase. Nanotechnology 19:135604–135611
Hu YJ, Liu Y, Xiao XH (2009) Investigation of the interaction between berberine and human serum albumin. Biomacromolecules 10:517–521
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21775100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1363 kb)
Rights and permissions
About this article
Cite this article
Liang, SS., Deng, X., Fan, YY. et al. A ratiometric fluorometric heparin assay based on the use of CdTe and polyethyleneimine-coated carbon quantum dots. Microchim Acta 185, 519 (2018). https://doi.org/10.1007/s00604-018-3061-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3061-6