Abstract
The authors describe a method for the extraction of the herbicide 2-methyl-4-chlorophenoxyacetic acid (MCPA) from agricultural products. The metal organic framework (MOF) HKUST-1 (a copper(II) benzene-1,3,5-tricarboxylate) was used as a sorbent for efficient clean-up and preconcentration of MCPA. The effects of pH value, stirring time, amount of sorbent on extraction were optimized by central composite design. Ultrasonic waves were used for desorption procedure and its advantage was demonstrated for an increase in extraction recovery. Corona discharge ion mobility spectrometry (IMS) was then applied for fast and sensitive determination of MCPA. The method was validated in terms of sensitivity, recovery and reproducibility. Under the optimum conditions the calibration plot is linear between 0.035–0.200 μg. L−1. The detection limit is 10 ng L−1, with relative standard deviations of <5%. Real samples (water, soil and agricultural product) were spiked and then analyzed by this method, and the results revealed efficient solid phase extraction and recovery.

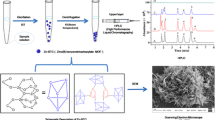
Schematic presentation of a procedure for extraction of an organochlorine pesticide (2-methyl-4-chlorophenoxyacetic acid) from agriculture products using the HKUST-1 metal-organic framework prior to determination by ion mobility spectrometry based on its ionization in drift cell.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The widespread utilization of herbicides in agriculture can change the nature of surface and ground waters, and of soil. In addition, residuals of these components in food samples are toxicity and have harmful effects on human and animal health [1]. These concerns are the most important reason of the researcher for studying and developing different analytical methods for the analysis of residues of these compounds in the plant, animal, environmental matrices and drinking water in order to periodically monitor food and water safety.
2-Methyl-4-chlorophenoxyacetic acid (MCPA), one of the organochlorine herbicide, is widely used in agriculture to control weeds in cereals, grasses and orchards [2]. It is a Restricted Use Pesticide used extensively in agriculture due to its relatively low cost and high efficiency even at low concentrations [3]. High solubility of this compound in water causes its entrance in surface water and leads to increase water pollutant and toxicity; therefore, monitoring possible herbicide contamination is an essential task in environmental protection.
Only few papers reported the residue determination of MCPA in water and food samples [2, 4, 5] using HPLC and LC–MS–MS performed after carrying out a solid phase extraction step (SPE) by an efficient sorbent as a solid phase. These methods required skilled operators and are, generally, time consuming; so, a reduced number of samples can be analyzed in a working day. Thus, it would be desirable to develop a novel analytical tool to perform rapid and selective screening of MCPA in food samples to increase the number of analyzed samples in a day. Ion mobility spectrometry (IMS) is a fast technique based on the ionization of analytes under an electric field at ambient pressure and the gas-phase separation by their ion mobility into the drift gas [6]. This technique has significant advantages, including low detection limit, fast response (within milliseconds and the whole analysis time is usually less than 1 min per sample), simplicity and relative low cost. In addition, sample pretreatment and preconcentration such as solid phase microextraction (SPME) [7] or liquid-liquid microextraction (LLME) [8] can improve the capability of IMS for determination of toxins [9,10,11], fungicides [12] and pharmaceuticals [13, 14] in very complex matrices.
Various sorbents have been investigated for MCPA adsorption, removing and extraction, including silica-bonded sorbent C18 [15], polymeric resins [16, 17] and divinylbenzene polymers (SDB and Oasis HLB) [18, 19]. Among these, metal organic frameworks (MOFs) have been investigated because of their high surface area, porosity and thermal and moisture stability that is used in this study for the first time.
Cu-BTC framework, i.e. copper benzene-1,3,5-tricarboxylate or HKUST-1 [20] is one of the most studied MOFs as sorbent in a variety of extraction methods to adsorb organic compounds due to its high surface area (normally in a range of 200–7000 m2. g−1) [21], large pore volume (ca. 0.70 cm3. g−1) [22] and good thermal stability (thermally stable up to 350 °C) [23]. These features are very important aspect for a good sorbent to use in different media and especially in thermal desorption based techniques such as IMS. However, there is little information about HKUST-1 as adsorbent for herbicide preconcentration [24,25,26].
An efficient method for the extraction and preconcentration of MCPA from water, soil and agriculture products using HKUST-1 prior to their rapid determination by IMS was introduced.
Experimental
Materials and instruments
Chemicals were purchased from Merck (Darmstadt, Germany, www.merck-chemicals.com) and Sigma-Aldrich (Missouri, United States, www.sigmaaldrich.com) chemical companies. The stock solution of MCPA (at a concentration of 100 μg. L−1) was prepared in double distilled water (DDW) with specific conductivity of 1 μS.cm−1 and was stored in a fridge at 4 °C. Working solutions were prepared by diluting with DDW at different concentrations.
The mobility measurement was carried out by ion mobility spectrometer (IMS-300) designed and constructed by TOF Tech. Pars (Iran, www.toftech.ir). A universal ultrasonic cleaner water bath with the heating system (Transonic TI-H-5, Elma, Singen, Germany, www.elma-ultrasonic.com) was utilized for the ultrasound-assisted desorption procedure. A Metrohm Model 827 pH lab (Herisau, Switzerland, www.metrohm.com) pH-meter with a combined glass electrode was utilized for pH measurements. The phase separation was assisted with PIT320R centrifuge machine (Pole Ideal Tajhiz, Tehran, Iran, www.medpit.com). For characterization of the prepared MOF, X-ray powder diffraction (XRD) measurements were performed using a Philips X’pert diffractometer with mono chromated Cu-Kα radiation (Malvern Panalytical, United Kingdom, www.malvernpanalytical.com). FT-IR spectra (4000–400 cm−1) in KBr pellet were obtained on Equinox55 BRUKER model infrared spectrophotometer (Bruker optics, Karlsruhe, Germany, www.bruker.com).
Dispersive solid phase extraction experiments
The following procedure was applied for the dispersive solid phase extraction (d-SPE) and preconcentration of MCPA from real samples: an aliquant of aqueous solution of MCPA (with concentration of 0.10 μg. L−1) in 50 mL of DDW was prepared and 10 mL of the solution was transferred into a 25 mL beaker. The initial pH of the solutions was adjusted to 4.0–10.0 by the drop wise addition of 1.0 mol L−1 sodium hydroxide and 1.0 mol L−1 hydrochloric acid. 0.10–1.0 mg of sorbent were added to the solution. The mixture was stirred for 2 min and HKUST-1 containing extracted MCPA settled down for 2 min. The solution was transferred into a conical centrifuge tube for separation of sorbent. The supernatant aqueous phase was separated and discarded. In the next step, the residue solid phase was evaporated and then the dry extract exposed by an ultrasound bath at different time intervals (30.0–180.0 S) in 25 °C and subsequently eluted by 0.5 mL methanol.
Afterwards, the eluent was separated by centrifuging for 5 min and the supernatant filtered through a 0.45 m Mem (Whatman, Maidstone, UK) to introduce to IMS injection port. Solutions with different concentrations were prepared based on this procedure and finally, 1 μL of each sample was directly injected into the IMS system in order to determine the amount of MCPA. The dispersive SPE procedure is schematically shown in Scheme 1.
The remaining sorbent was washed with 5 mL distilled water, reconditioned by 5 mL methanol and reused for the remaining experiments.
IMS analysis
Identification and quantification of MCPA were carried out by the IMS. The IMS cell is comprised of two parts, an ionization region followed by a drift tube. The ionization region is equipped with a corona discharge ionization source operating in both negative and positive modes. Zero air is used as both the carrier and the drift gas to transfer the sample into the ionization region and to clean the cell. H3O+ and NH4+ are the main reactant ions in the positive mode which can ionize the analytes via proton transfer reactions [27]. The generated ions separate in the electric field and have different mobility due to their size, shape and electrical properties. The intensity of formed product ions is depended on analyte concentration.
Direct determination of MCPA was done by injecting of 1 μL of the sample to the IMS cell. Carrier gas transfer and carry the analyte into the drift cell for ionization. To achieve the best ion mobility signal, different parameters such as voltage, flow rate, temperature and pulse width were optimized. The optimized operating conditions of IMS are summarized in Table S1 (electronic supporting information).
Preparation of real samples
Well water samples were collected from Alzahra University in plastic flasks and filtered in the lab.
5 g of soil sample was weighed into a 250 mL beaker, and 10 mL of 0.01 M sodium hydroxide was added. After 30 min the alkaline extracts were centrifuged at 2000 rpm (380 rcf) for 20 min to separate the soil supernatant. The separated solution was filtered through 0.45 mm filters. Filtered extract was diluted up to 100 mL with DDW. The solution was spiked with MCPA of different concentration levels and the pH of solution was adjusted to optimized value.
Agriculture products (rice and tomato) were purchased from a local supermarket. The samples were chopped and homogenized by a blender. 2.0 g of the homogenized sample was spiked with MCPA. 4 mL of water was added into the samples and placed in a water bath at 45 °C for 20 min, and finally centrifuged for 10 min at 4000 rpm (1520 rcf). The clear supernatant was collected and diluted (1:2) with water to reduce the matrix effect and kept in a refrigerator before dispersive solid phase extraction [28].
Experimental design methodology
In order to investigate the effect of the experimental variables on the extraction efficiency, the central composite design (CCD) approach was used. In this work the Design-Expert statistical software program (8.0.6 trial version, Stat-Ease Minneapolis, USA, www.statease.com) was used for the analysis of the experimental design data and calculating the predicted responses.
Result and discussion
Ion mobility spectra and MCPA determination
MCPA ion mobility spectra and background were obtained under optimum conditions of the instrument (Table S1) and are shown in Fig. 1. MCPA shows one peak at 8.02 ms with respect to the ammonium peak at 4.04 ms at 200 °C.
In IMS, ion drift times are often reported as reduced mobility constants. This parameter is used for comparison of results in different environmental and instrumental conditions. Reduced mobility is normalized to a standard pressure (760 Torr) and temperature (273 K) and calculated using Eq. 1:
where K0 is the reduced mobility with the units of (cm2 V−1 s−1), and td is the drift time for external standard and MCPA. Ammonium (NH4+) was used as the external standard to calibrate the reduced mobility scale [29]. Based on ammonium reduced mobility (K0) equal to 3.24 cm2 V−1 s−1 at 200 °C and eq. 1, the K0 value of MCPA was calculated as 1.63 cm2 V−1 s−1. The relative drift times and reduced mobility values for the MCPA are given in Table S2 (electronic supporting information).
However, based on the structure of MCPA and its functional group (Fig. 1 inset) it can be concluded that the most typical ion is characterized by the proton transfer between the MCPA and reactant ions and might be due to the formation of protonated form of MCPA (MCPA…H+) in drift cell.
Based on Fig. 1, the height of the peak with a drift time of 8.02 ms increase by addition of MCPA concentration, therefore this signal was employed as an analytical response for determination of concentration.
Choice of HKUST-1 as a sorbent
Generally, porous materials are good candidates to host other molecules due to porous characteristics. Among these materials, MOFs attracts great attention due to flexible, tunable and highly permanent nanoscale pore structure. The shape and size of the pores lead to selectivity towards the guest molecules that may be adsorbed. These properties make HKUST-1 as one of the ideal sorbent to host herbicides [26]. The structural diversity of MOFs can expand the application scope to related detection schemes.
Characterization of HKUST-1
Porous HKUST-1 was prepared according to the previously reported procedure using a hydrothermal method [30] (the detailed procedure and HKUST-1 structure in ESM). HKUST-1 was characterized by FT-IR and XRD. FTIR was used to assess the quality of HKUST-1 before and after using as solid phase (Fig. 2a). In the mid-IR frequency range, the spectra of the prepared sample are dominated by the vibrational mode of BTC ligand. The wide band attributed to O–H stretching modes appeared in the region of 3550–3200 cm−1. The IR absorption bands in the 1700–1500 and 1500–1300 cm−1 ranges are due to υasym (C − O2) and υsym (C − O2) stretching modes, respectively which is in good agreement with published sources [31]. IR bands around 700 cm−1 are due to υ (C − H) bending mode (Fig. 2a).
In order to investigate the phase purity of the prepared HKUST-1, powder X-ray diffraction (PXRD) was carried out. Figure 2b shows acceptable matches between the simulated XRD pattern (blue) and as the prepared sample (red). The intensive peaks shown in Fig. 2 appeared at small 2θ angles are characteristics of micro-porous materials, which possess numerous tiny pores or cavities that are in accordance with published data [30, 32].
To explore the reusability of the solid phase XRD pattern was recorded after extraction. The result shows that HKUST-1 retains its structure after extraction and thus can be proposed as a stable solid phase.
HKUST-1, a Cu-BTC framework was used as a sorbent for dispersive solid phase extraction of the MCPA from complex real samples. A standard solution of MCPA was prepared and after addition of sorbent, the SPE procedure was carried out based on section “Dispersive solid phase extraction experiments”. After elution of HKUST-1 by methanol, the extracted MCPA was injected into the IMS drift cell and its mobility spectra were recorded. The same procedure was employed for real samples such as agriculture products and the results were evaluated. The use of HKUST-1 extraction provided a high sensitivity for MCPA due to preconcentration procedure and selectivity because of the analyte extraction from the complex matrix of real samples. Thus, the use of a MOF-SPE was proposed in order to: i) preconcentrate the MCPA for trace analysis, and ii). increase the selectivity that led to IMS signal which is free of matrix interferent compounds.
Optimization of method
The following parameters were optimized: (a) sample pH value; (b) sorbent dosage; (c) sorption time; (d) desorption conditions (elution solvent, desorption time and ultrasonic irradiation). Respective data and Figures are given in the Electronic Supporting Material (Table S3 and S4, Fig. S2 and S3). The following experimental conditions were found to give the best results: (a) sample pH value: 9; (b) sorbent amount: 0.61 mg; (c) sorption time: 2 min; and (d) desorption conditions: elution solvent: 0.5 mL methanol; desorption time, 3 min by ultrasonic irradiation.
Analytical parameters and performance of the method (evaluation of SPE procedure)
The figures of merits, including limit of detection (LOD), limit of quantification (LOQ), dynamic range, and precision were calculated under optimized conditions for evaluating the current SPE method. The dynamic range was linear in the range of 0.035 to 0.20 μg. L−1 with good correlation coefficient, 0.997. The LOD and LOQ were calculated based on signal-to-noise ratio of 3:1 and 10:1, respectively. The LOD and LOQ were 0.01 and 0.035. μg. L−1, respectively. The pre-concentration factor which was obtained by comparing the slopes of the calibration plots before and after the preconcentration, was 20. Extraction recoveries (ER%) were calculated by the following equation and were in the range 98.0–104.0%.
Where ni,initial is the initial analyte moles present in the sample solution and ni,final is the analyte moles in the eluting solution at the end of the extraction process. Ve and Vi are the volumes of the eluting solution and the sample solution volume, respectively. Ci,initial is the initial concentration of the analyte in the sample solution; and Ce,final is the final concentration of the analyte in the eluting solution that was calculated from the calibration plot of the analyte after the extraction. For the intra and inter day precision, the relative standard deviations were calculated for 0.1 μg. L−1 of analyte (n = 3) and obtained 1.38 and 5.04%, respectively, (Table 1).
Effect of the potentially interfering species
Selectivity of the method was studied by performing the procedure in the presence of different co-existing compounds that may be present in the real samples. The tolerance limit defined as the largest concentration of the interfering species that decrease the recovery of the analyte to less than 95%. 1000-fold excess of K+, Na+, Ca2+, Mg2+, Ba2+, Cd2+, Ni2+, Al3+, Cl−, F−, S2−, CO32−, HCO3− and Cu2+, NO3−, Pb2+, Hg2+ did not interfere with the determination of MCPA.
In addition, the interference of some other pesticides such as N,N′-dimethyl-4,4′-bipyridinium dichloride, 2,4-dichlorophenoxy acetic acid, lindane, permethrin and cypermethrine on the IMS measurements was examined. The obtained results indicate that a 50-fold excess of them had no effect on the signal intensity of MCPA. However, the applicability of this method for other pesticides is still understudy.
The results demonstrated that the presence of large amounts of species commonly present in water and vegetable samples has no significant effect on the SPE of MCPA not only due to high adsorption capacity of the sorbent but also because of the selectivity of the IMS.
Application of the method for real samples
In order to validate the applicability of the method in real samples with different matrices such as water, soil and agriculture product samples (rice and tomato) were spiked by two different concentrations of MCPA and were analyzed under optimum conditions. To validate the accuracy of the method, the spiking recoveries were calculated and are listed in Table 2.
The recovery values more than 90% show a non-significant matrix effect in the real samples analysis by the method. Good recoveries and low RSDs demonstrate the capability of the method for the fast analysis of MCPA in real samples.
Comparison of the method with previously reported methods
Table 3 provides a comparison between the characteristics of this method with other SPE methods that were reported in the literature for determination of MCPA. It is shown that along with its simplicity, this method demonstrated a good linearity range and an acceptable reproducibility which is comparable with existing methods. Moreover, LOD of this method is better than those of other works presented in Table 3. The speed of preconcentration and determination steps ares the most important advantage of this method. In addition, the consumption of organic solvent in this method is minimized compared with conventional SPE. Moreover, the proposed sorbent is successfully synthesized via hydrothermal synthesis as a convenient, rapid, low cost and green process showing high stability in experimental conditions.
Conclusion
Dispersive solid phase extraction and preconcetration step (using an efficient sorbent) followed by IMS provides fast detection of MCPA at ultra-trace level (ng. L−1). HKUST-1 with high surface area has been utilized as a proficient sorbent in d-SPE of MCPA from different samples, including water, soil and agriculture products for the first time. The sorbent showed high stability and reusability and can be used for several times without measurable performance loss.
The important advantages of this method are low sample amount, environmentally friendly method, due to low amount of HKUST-1 required and organic solvent, adequate timing (10 min for the overall extraction step and 1 min for the IMS determination, more less than chromatographic methods such as LC and GC), low operational costs, ease of use, and not required skilled operators, etc. However, the current method has some limitations for relatively large molecular structure due to their difficult ionization in the IMS drift cell.
References
Ikeura H, Kobayashi F, Tamaki M (2011) Removal of residual pesticides in vegetables using ozone microbubbles. J Hazard Mater 186:956–959. https://doi.org/10.1016/j.jhazmat.2010.11.094
Hu J, Yang T, Yin S, Cao D (2012) Dissipation and residue of MCPA (4-chloro-2-ethylphenoxyacetate) in wheat and soil. Environ Monit Assess 184:5017–5024. https://doi.org/10.1007/s10661-011-2317-y
Torres-Cartas S, Gómez-Benito C, Meseguer-Lloret S (2012) FI on-line chemiluminescence reaction for determination of MCPA in water samples. Anal Bioanal Chem 402:1289–1296. https://doi.org/10.1007/s00216-011-5567-1
Steinborn A, Alder L, Spitzke M, Dörk D, Anastassiades M (2017) Development of a QuEChERS-based method for the simultaneous determination of acidic pesticides, their esters, and conjugates following alkaline hydrolysis. J Agric Food Chem 65:1296–1305. https://doi.org/10.1021/acs.jafc.6b05407
Chen X, Dong B, Zhong M, Hu J (2015) Dissipation kinetics and residues of amidosulfuron and MCPA in wheat ecosystems based on a modified QuEChERS and low-temperature cleanup method using the RRLC-QqQ-MS/MS technique. Anal Methods 7:10299–10305. https://doi.org/10.1039/c5ay02392k
Creaser CS, Griffiths JR, Bramwell CJ, Noreen S, Hill CA, Thomas CLP (2004) Ion mobility spectrometry: a review. Part 1. Structural analysis by mobility measurement. Analyst 129:984. https://doi.org/10.1039/b404531a
Saraji M, Jafari MT, Mossaddegh M (2016) Halloysite nanotubes-titanium dioxide as a solid-phase microextraction coating combined with negative corona discharge-ion mobility spectrometry for the determination of parathion. Anal Chim Acta 926:55–62. https://doi.org/10.1016/j.aca.2016.04.034
Peñuela-Pinto O, Armenta S, Esteve-Turrillas FA, de la Guardia M (2017) Selective determination of clenbuterol residues in urine by molecular imprinted polymer—ion mobility spectrometry. Microchem J 134:62–67. https://doi.org/10.1016/j.microc.2017.05.008
Sheibani A, Tabrizchi M, Ghaziaskar HS (2008) Determination of aflatoxins B1 and B2 using ion mobility spectrometry. Talanta 75:233–238. https://doi.org/10.1016/j.talanta.2007.11.006
Khalesi M, Sheikh-Zeinoddin M, Tabrizchi M (2011) Determination of ochratoxin a in licorice root using inverse ion mobility spectrometry. Talanta 83:988–993. https://doi.org/10.1016/j.talanta.2010.11.004
Mohammadnejad M, Farhadpour M, Mahdavi V, Tabrizchi M (2017) Rapid monitoring and sensitive determination of DDT and its metabolites in water sample using solid-phase extraction followed by ion mobility spectrometry. Int J Ion Mobil Spectrom 20:23–30. https://doi.org/10.1007/s12127-016-0211-6
Armenta S, de la Guardia M, Abad-Fuentes A, Abad-Somovilla A, Esteve-Turrillas FA (2015) Off-line coupling of multidimensional immunoaffinity chromatography and ion mobility spectrometry: a promising partnership. J Chromatogr A 1426:110–117. https://doi.org/10.1016/j.chroma.2015.11.050
Jafari MT, Badihi Z, Jazan E (2012) A new approach to determine salicylic acid in human urine and blood plasma based on negative electrospray ion mobility spectrometry after selective separation using a molecular imprinted polymer. Talanta 99:520–526. https://doi.org/10.1016/j.talanta.2012.06.023
Jafari MT, Kamfirozi M, Jazan E, Ghoreishi SM (2014) Selective extraction and analysis of pioglitazone in cow plasma using a molecularly imprinted polymer combined with ESI ion mobility spectrometry. J Sep Sci 37:573–579. https://doi.org/10.1002/jssc.201301206
Al Housari F, Höhener P, Chiron S (2011) Factors responsible for rapid dissipation of acidic herbicides in the coastal lagoons of the Camargue (Rhône River Delta, France). Sci Total Environ 409:582–587. https://doi.org/10.1016/j.scitotenv.2010.10.036
Moret S, Sánchez JM, Salvadó V, Hidalgo M (2005) The evaluation of different sorbents for the preconcentration of phenoxyacetic acid herbicides and their metabolites from soils. J Chromatogr A 1099:55–63. https://doi.org/10.1016/j.chroma.2005.08.078
Tran ATK, Hyne RV, Doble P (2007) Determination of commonly used polar herbicides in agricultural drainage waters in Australia by HPLC. Chemosphere 67:944–953. https://doi.org/10.1016/j.chemosphere.2006.11.002
Comoretto L, Arfib B, Chiron S (2007) Pesticides in the Rh?? Ne river delta (France): basic data for a field-based exposure assessment. Sci Total Environ 380:124–132. https://doi.org/10.1016/j.scitotenv.2006.11.046
Gervais G, Brosillon S, Laplanche A, Helen C (2008) Ultra-pressure liquid chromatography-electrospray tandem mass spectrometry for multiresidue determination of pesticides in water. J Chromatogr A 1202:163–172. https://doi.org/10.1016/j.chroma.2008.07.006
Chui SSY, Lo SMF, Charmant JPH, Orpen AG, Williams ID (1999) A chemically Functionalizable Nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283:1148–1150. https://doi.org/10.1126/science.283.5405.1148
Farha OK, Eryazici I, Jeong NC, Hauser BG, Wilmer CE, Sarjeant AA, Snurr RQ, Nguyen SBT, Yazaydın AÖ, Hupp JT (2012) Metal–organic framework materials with ultrahigh surface areas: is the sky the limit? J Am Chem Soc 134:15016–15021. https://doi.org/10.1021/ja3055639
Schlesinger M, Schulze S, Hietschold M, Mehring M (2010) Evaluation of synthetic methods for microporous metal-organic frameworks exemplified by the competitive formation of [Cu2(btc)3(H2O)3] and [Cu2(btc)(OH)(H2O)]. Microporous Mesoporous Mater 132:121–127. https://doi.org/10.1016/j.micromeso.2010.02.008
Lee YR, Kim J, Ahn WS (2013) Synthesis of metal-organic frameworks: a mini review. Korean J Chem Eng 30:1667–1680. https://doi.org/10.1007/s11814-013-0140-6
Ghorbani-Kalhor E (2016) A metal-organic framework nanocomposite made from functionalized magnetite nanoparticles and HKUST-1 (MOF-199) for preconcentration of Cd(II), Pb(II), and Ni(II). Microchim Acta 183:2639–2647. https://doi.org/10.1007/s00604-016-1896-2
Deng Y, Zhang R, Li D, Sun P, Su P, Yang Y (2018) Preparation of iron-based MIL-101 functionalized polydopamine@Fe3O4 magnetic composites for extracting sulfonylurea herbicides from environmental water and vegetable samples. J Sep Sci 41:2046–2055. https://doi.org/10.1002/jssc.201701391
Tadjarodi A, Abbaszadeh A (2016) A magnetic nanocomposite prepared from chelator-modified magnetite (Fe3O4) and HKUST-1 (MOF-199) for separation and preconcentration of mercury(II). Microchim Acta 183:1391–1399. https://doi.org/10.1007/s00604-016-1770-2
Tabrizchi M, Khayamian T, Taj N (2000) Design and optimization of a corona discharge ionization source for ion mobility spectrometry. Rev Sci Instrum 71:2321–2328. https://doi.org/10.1063/1.1150618
Saraji M, Rezaei B, Boroujeni MK, Bidgoli AAH (2013) Polypyrrole/sol-gel composite as a solid-phase microextraction fiber coating for the determination of organophosphorus pesticides in water and vegetable samples. J Chromatogr A 1279:20–26. https://doi.org/10.1016/j.chroma.2013.01.017
Tabrizchi M (2001) Temperature corrections for ion mobility spectrometry. Appl Spectrosc 55:1653–1659
Panella B, Hirscher M, Pütter H, Müller U (2006) Hydrogen adsorption in metal-organic frameworks: Cu-MOFs and Zn-MOFs compared. Adv Funct Mater 16:520–524. https://doi.org/10.1002/adfm.200500561
Borfecchia E, Maurelli S, Gianolio D, Groppo E, Chiesa M, Bonino F, Lamberti C (2012) Insights into adsorption of NH3 on HKUST-1 metal − organic framework : a multitechnique approach. J Phys Chem C 116:19839–19850. https://doi.org/10.1021/jp305756k
Lin KS, Adhikari AK, Su YH, Shu CW, Chan HY (2012) Synthesis, characterization, and hydrogen storage study by hydrogen spillover of MIL-101 metal organic frameworks. Adsorption 18:483–491. https://doi.org/10.1007/s10450-012-9438-7
Tennakoon DASS, Perera KAPB, Hathurusinghe LS (2014) An unusual case of non-fatal poisoning due to herbicide 4-chloro-2-methyl phenoxyacetic acid (MCPA). Forensic Sci Int 243:90–94. https://doi.org/10.1016/j.forsciint.2014.04.039
Rahemi V, Garrido JMPJ, Borges F, Brett CMA, Garrido EMPJ (2015) Electrochemical sensor for simultaneous determination of herbicide MCPA and its metabolite 4-chloro-2-methylphenol. Application to photodegradation environmental monitoring. Environ Sci Pollut Res 22:4491–4499. https://doi.org/10.1007/s11356-014-3693-y
Hogenboom AC, Hofman MP, Jolly DA, Niessen WMA, Brinkman UAT (2000) On-line dual-precolumn-based trace enrichment for the determination of polar and acidic microcontaminants in river water by liquid chromatography with diode-array UV and tandem mass spectrometric detection. J Chromatogr A 885:377–388. https://doi.org/10.1016/S0021-9673(00)00388-5
Gao N, Cai K, Guo X, Zhang Y, Yang S, Hu D (2014) Analysis of MCPA and TCP in water by liquid chromatography-ion trap-electrospray tandem mass spectrometry. Int J Environ Anal Chem 94:594–605. https://doi.org/10.1080/03067319.2013.831411
Acknowledgements
The authors acknowledge the Research Council of Alzahra University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 691 kb)
Rights and permissions
About this article
Cite this article
Mohammadnejad, M., Gudarzi, Z., Geranmayeh, S. et al. HKUST-1 metal-organic framework for dispersive solid phase extraction of 2-methyl-4-chlorophenoxyacetic acid (MCPA) prior to its determination by ion mobility spectrometry. Microchim Acta 185, 495 (2018). https://doi.org/10.1007/s00604-018-3014-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3014-0