Abstract
Electrochemical sandwich immunoassay strategies involving the use of carboxyl-functionalized magnetic microbeads (cMBs) and magnetic nanoparticles (cMNPs) have been evaluated and compared. The proteolytically cleaved soluble tyrosine kinase receptor sAXL was used as the target analyte. Antibodies against AXL were covalently immobilized on cMBs or cMNPs. Immunobinding of AXL was detected by means of a secondary biotinylated antibody and a streptavidin-horseradish peroxidase conjugate. The electrochemical transduction was accomplished by capturing the cMBs or cMNPs bearing the immunoconjugates onto screen-printed carbon electrodes (SPCEs) by using a small magnet. The amperometric response was measured at −0.20 V (vs the silver pseudo-reference electrode of the SPCE) upon the addition of H2O2 in the presence of hydroquinone as the redox mediator. The calibration plots for AXL extended up to 7.5 ng mL−1 when cMBs were used for the preparation of the immunosensor and up to 40 ng mL−1 in the case of using cMNPs. The respective slope values were 158 (cMBs) and 43 nA mL ng−1 (cMNPs), while the achieved LODs were 74 (cMBs) and 75 pg mL−1 (cMNPs). Although the immunosensors prepared with cMBs provided a shorter range of linearity, they exhibited a 3.7-times larger sensitivity than those constructed with cMNPs. The successful application of the new strategies was demonstrated for the determination of the endogenous content of sAXL in real human serum samples (a cut-off value of 71 ng mL−1 have been established for patients with risk of heart failure). The immunosensors constructed using cMBs or cMNPs can be advanta geously compared, in terms of sensitivity and fabrication time, with the only immunosensor for AXL previously reported. In addition, these new immunosensors took approximately half time than ELISA to perform the assay.

Comparative evaluation of the performance of amperometric immunosensors for tyrosine kinase receptor AXL determination using carboxyl-modified magnetic microparticles (cMBs) and nanoparticles (cMNPs) and application to the determination of the endogeneous concentration in real human serum samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The attractive features offered by magnetic microbeads (MBs) in the development of electrochemical immunosensors with improved performance [1,2,3] have made the number of applications grow considerably [4]. On the contrary, far fewer reports involving the use of magnetic nanoparticles (MNPs) have been found so far in the literature [5]. Immobilization of immunoreagents onto functionalized magnetic particles instead of the electrode surface minimizes some drawbacks related to practical application of immunosensors such as the long time required for immobilization, the steric hindrance of the electron transfer, or their reusability [4]. Moreover, the easy capturing of magneto-conjugates by simple application of a magnetic field diminishes nonspecific adsorptions and makes possible the analysis of complex samples by improving the selectivity and avoiding in a great extent matrix effects. Other interesting advantages of the use of MBs include the possibility to achieve high loadings of immobilized biomolecules due to the large surface area of these particles, which contributes to decrease the limits of detection, and faster assay kinetics because the beads are under continuous stirring in suspension thus reducing largely the assay time. The efficient magnetic capture of MBs on the electrode surface also implies that detectable products, usually generated through an enzyme reaction, are formed very close to the electrode surface, thus allowing the electrochemical response to be reached rapidly. Pividori’s group compared the application of magnetic particles with diameters of 1 μm and 300 nm for the construction of electrochemical immunosensors using magneto graphite-epoxy composite electrodes for the determination of Plasmodium falciparum [6]. An improved reactivity with enhanced efficiency in coupling the capture antibody and higher sensitivity were observed when the smaller diameter particles were employed. Same authors prepared immunomagnetic sensors for the determination of Salmonella Typhimurium in whole milk, where the smaller magnetic particles provided lower limits of detection but also suffered from higher matrix effects in the analysis of samples [7].
With the double objective to develop immunosensors using magnetic particles as solid supports where the immunoreactions are carried out and to give useful insights about the influence of the magnetic particle size on the immunosensor performance, in this work, we have compared two different electrochemical sandwich immunoassay strategies using magnetic particles similarly functionalized but with significant differences in size. Commercial carboxylated magnetic microbeads (cMBs) and magnetic nanoparticles (cMNPs), with diameters of 2.8 μm and 20 nm, respectively, were employed. It is worth to highlight at this point the differences between this study and those mentioned above [6, 7]. Apart from the use of disposable SPCEs instead of graphite-epoxy composite electrodes to perform the electrochemical transduction, the size of the used cMNPs is more than 10 times smaller than that tested before.
As the target protein for this study, we selected the tyrosine phosphorylated protein AXL [8]. The proteolytically processed extracellular fraction of this protein (sAXL) is considered a relevant biomarker in cancer [9], inflammatory processes [10] and pathophysiology of heart failure (HF) [11]. In fact, elevated serum levels of sAXL have been found in HF patients with an established cut-off value of 71 ng mL−1 with respect to healthy individuals [12], and they are also associated to inflammatory biomarkers in cardiovascular disease [12, 13].
Although various ELISA kits allow the detection of sAXL concentrations as low as 2 pg mL−1, the reproducibility of the measurements yields coefficients of variation around 10% and require long assay times (more than 4 h) and expensive and non-portable instrumentation. In this context, electrochemical immunosensors are considered as a very attractive alternative due to inherent high sensitivity and selectivity, precision and accuracy, relatively low cost, minimum sample requirement, simplicity of operation and possible integration in compact analytical devices [14, 15]. It is worth to mention at this point that the only one electrochemical immunosensor for sAXL reported so far was developed by our group [16] and involved a sandwich assay at screen-printed carbon electrodes modified with electropolymerized poly(pyrrole-propionic acid).
The comparison of the electrochemical sandwich immunoassay strategies involving the use of cMBs and cMNPs carried out in this work relied on the amperometric transduction, upon magnetic capture of the magnetoimmunocomplexes, on SPCEs at −0.20 V (vs Ag pseudoreference electrode) using the H2O2/HQ system. Once their analytical characteristics were critically compared, both immunosensors were applied to the analysis of human serum samples.
Experimental
Apparatus and electrodes
Amperometric measurements were performed with a CHI 812 B potentiostat (CH Instruments, www.chinstruments.com) controlled by CHI 812 B software. Cyclic voltammograms and electrochemical impedance spectroscopy (IES) measurements were made using a PGSTAT 101 potentiostat from Autolab controlled by Nova 1.6 electrochemical software, and an Antolab type III controlled by FRA2 software (EcoChemie, www.ecochemie.nl), respectively. The screen-printed carbon electrodes (SPCEs) (DRP-110, 4 mm ∅ DropSens, S.L., www.dropsens.com) included a carbon counter electrode and an Ag pseudo-reference electrode. A specific cable connector (DRP-CAC, DropSens, S.L.) acted as interface between the SPCEs and the potentiostat. All measurements were carried out at room temperature. A P-Selecta (Scharlab, http://scharlab.com) ultrasonic bath and a Thermomixer MT100 constant temperature incubator shaker (Universal Labortechmik, www.treffpunkt-labor.de/index.php?impressum) were also used. Magnetic separation during the incubation/washing steps was performed using a DynaMag™-2 (Dynal Biotech ASA, Thermo Fisher, www.thermofisher.com) magnetic particle concentrator. A neodymium magnet (AIMAN GZ, www.aimangz.es) was used to control the attraction of the modified cMBs and cMNPs to the SPCE surface. A Bunsen AGT-9 Vortex was also used for the homogenization of the solutions.
Reagents and solutions
All reagents used were of the highest available grade. Tween®20, hydroquinone (HQ), hydrogen peroxide (30%, w/v), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC), N-hydroxysulfosuccinimide (Sulfo-NHS) and ethanolamine were purchased from Sigma-Aldrich (www.sigmaaldrich.com). Sodium di-hydrogen phosphate, di-sodium hydrogen phosphate and sodium chloride were purchased from Scharlau. 2-(N-morpholino) ethane sulfonic acid (MES), from Gerbu Biotechnik, Gmbh (www.gerbu.de), was also used. Blocker casein solutions (blocking buffer) consisted of a ready-to-use 1% w/v purified casein prepared in phosphate-buffered saline (PBS 1×, pH 7.5) (Thermo Scientific). Deionized water was obtained from a Millipore Milli-Q purification system (18.2 MΩ cm).
Carboxyl-functionalized magnetic microbeads (cMBs, 2.8 μm, 10 mg mL−1, 2×109 particles mL−1, Dynabeads® M-270 Carboxylic Acid) were purchased from Dynal Biotech ASA (Thermo Scientific). Carboxyl-modified magnetic nanoparticles (cMNPs, 20 nm, 5 mg mL−1, 8.6×1014 particles mL−1, 79–02-201 nanomag®Dspio) were from Micromod Partikeltechnologie GmbH (www.micromod.de). Buffers used were 0.05 M sodium phosphate buffer of pH 7.5 supplemented with 0.1 M NaCl, PBS consisting of 0.01 M phosphate buffer containing 137 mM NaCl and 2.7 mM KCl (pH 7.5), 0.05 M phosphate buffer of pH 6.0, 0.1 M Tris-HCl (pH 7.2) and 0.025 M MES buffer of pH 5.0.
Mouse anti-human AXL antibody (anti-AXL), biotinylated goat anti-human AXL antibody (Biotin-anti-AXL), and recombinant human AXL were used. These bioreagents are components of the Human Total AXL DuoSet®IC ELISA kit from R&D Systems, Inc. (www.rndsystems.com) Catalog Number DYC 1643–2. This commercial ELISA kit was also used for comparison purposes. A high sensitivity streptavidin-horseradish peroxidase (HRP-Strept) conjugate (Ref 000000011089153001) from Sigma-Aldrich was also used. Solutions of anti-AXL antibodies were prepared in 25 mM MES buffer of pH 5.0, while blocking buffer was used to prepare Biotin-anti-AXL, AXL and HRP-Strept solutions.
Human cardiac troponins I and T (cTnI and cTnT), and N-terminal pro b-type natriuretic peptide (NT-proBNP), from HyTest Ltd. (www.hytest.fi); lipoprotein (a) (Lp(a)) and human C-reactive protein (CRP) from Audit Diagnostics; human tumor necrosis factor (TNF) (R&D Systems), and human interleukin 8 (IL-8) (Abcam) were tested as potential interferents.
Procedures
Preparation of HRP-Strept-Biotin-anti-AXL-AXL-anti-AXL-cMBs (or -cMNPs) immunoconjugates
All the incubation steps described below were performed at 25 °C and 950 rpm. After each incubation step, cMBs were isolated by placing the Eppendorf tube in the magnetic particle concentrator during 3 min while cMNPs were centrifuged at 14,000 rpm during 10 min before removing the corresponding liquid phase.
Three μL of cMBs (or 1 μL of cMNPs) commercial suspension were washed twice with 50 μL of MES buffer for 10 min and isolated. Thereafter, cMBs (or cMNPs) were incubated in 25 μL of a 100 mM EDC and 100 mM Sulfo-NHS solution (prepared in MES buffer) for 35 min. Next, activated particles were washed twice with 50 μL MES buffer and after isolation they were re-suspended in 25 μL of a solution of 10.0 μg mL−1 (cMBs) or 5.0 μg mL −1 (cMNPs) anti-AXL (prepared also in MES buffer) and incubated for 15 min (cMBs) or 30 min (cMNPs). Thereafter, two washing steps with 50 μL MES buffer were performed and the modified magnetic particles were incubated with 25 μL of a 1 M ethanolamine solution prepared in 0.1 M phosphate buffer of pH 8.0, used as blocking solution, for 1 h. The resulting anti-AXL-cMBs or anti-AXL-cMNPs were washed once with 50 μL of Tris-HCl of pH 7.2, and twice with blocking buffer. The so prepared anti-AXL-cMBs or anti-AXL-cMNPs were stored (in filtered PBS pH 7.5 at 4 °C) until they were used for performing the immunoassay. The magnetic conjugates were re-suspended in 25 μL of standard AXL or sample solutions (prepared in blocking buffer) and incubated for 30 min. The resulting AXL-anti-AXL-magnetoconjugates were washed twice with 50 μL of blocking buffer and sandwiched by incubation in 25 μL of 0.5 μg mL−1 (cMBs) or 3.0 μg mL−1 (cMNPs) Biotin-anti-AXL solution (prepared in blocking buffer) for 30 or 15 min, respectively. After washing twice with 50 μL of blocking buffer, incubation was performed in 25 μL of a 1/1000 (cMBs) or 1/5000 (cMNPs) diluted HRP-Strept prepared in the same buffer for 15 or 30 min, respectively. Finally, HRP-Strept-Biotin-anti-AXL-AXL-anti-AXL-cMBs or HRP-Strept-Biotin-anti-AXL-AXL-anti-AXL-cMNPs were washed twice with 50 μL of phosphate buffer of pH 7.5.
Amperometric measurements
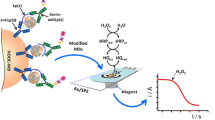
Magnetic immunoconjugates were re-suspended in 50 μL of phosphate buffer (pH 6.0) and captured on the working electrode surface of the SPCE by keeping the SPCE in a horizontal position after placing it in the homemade magnet holding block with an encapsulated neodymium magnet. The ensemble magnet holding block/SPCE with the captured cMBs or cMNPs immunoconjugates was immersed into an electrochemical cell containing 10 mL of 0.05 M phosphate buffer of pH 6.0 and 1 mM HQ (freshly prepared). The amperometric measurements in stirred solutions were performed at a potential value of −0.20 V vs. Ag pseudo-reference electrode upon addition of 50 μL of 0.1 M H2O2 solution until the steady-state current was reached (approx. 1 min).
Analysis of serum samples
Human serum samples were provided from Hospital Clinic of Barcelona. These samples were analyzed with the HRP-Strept-Biotin-anti-AXL-AXL-anti-AXL-cMBs or HRP-Strept-Biotin-anti-AXL-AXL-anti-AXL-cMNPs immunosensors after just a ten-fold dilution with blocking buffer. Validation of the method was performed by comparison of the results with those provided by the commercial ELISA kit involving the same immunoreagents.
Results and discussion
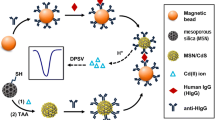
The immunoassays involved the covalent immobilization of capture antibodies onto cMBs or cMNPs and implementation of sandwich-type configurations using Biotin-anti-AXL and HRP-Strept as the detection enzyme conjugate (Fig. 1). The protocols used for the preparation of the immunosensors are detailed in the Experimental section. In brief, once cMBs or cMNPs were activated by carbodiimide chemistry, anti-AXL antibodies were covalently immobilized and a blocking step of the remaining free active sites on the particles surface was carried out with ethanolamine. Sandwich-type assays involved the target protein and biotinylated detection antibodies. A further conjugation with HRP-Strept and the subsequent capture of magnetoimmunoconjugates onto SPCEs allowed the amperometric determination of sAXL to be performed using H2O2 as HRP substrate and hydroquinone (HQ) as the redox mediator.
Optimization of the experimental variables
The different variables affecting the preparation of HRP-Strept-Biotin-anti-AXL-AXL-anti-AXL-cMBs (or -cMNPs) conjugates were optimized taking as a criterion the largest ratio between the currents measured with the immunosensors in the presence (S) or in the absence (N) of the target protein. These optimization studies involved evaluation of: a) the anti-AXL loading and the corresponding incubation time on cMBs or cMNPs; b) the incubation time of AXL onto anti-AXL-cMBs (or -cMNPs); c) the Biotin-anti-AXL loading and incubation time on AXL-anti-AXL-cMBs (or -cMNPs); d) the HRP-Strept loading and incubation time on Biotin-anti-AXL-AXL-anti-AXL-cMBs (or -cMNPs). Details about these optimization studies are provided in the Supporting Information and in Figs. S1–S4. Table 1 summarizes the tested ranges and the selected values. Other variables such as the composition of the H2O2/HQ system or the detection potential used were optimized in previous works [17]. It is remarkable that, under the optimal conditions, the immunosensors prepared with cMBs and cMNPs using 5.0 or 25.0 ng mL−1 AXL standard solutions, respectively, yielded specific-to-unspecific ratios (S/N) slightly higher than 9 and 6, respectively, with unspecific cathodic currents (obtained in the absence of AXL) lower than 200 nA in both cases.
Analytical performance of the immunosensors
Calibration plots for AXL standard solutions were constructed with the HRP-Strept-Biotin-anti-AXL-AXL-anti-AXL-cMBs (or -cMNPs)/SPCE immunosensors under the optimized working conditions (Fig. 2). The corresponding analytical characteristics are summarized in Table 2.
Interestingly, both linear ranges are suitable for the determination of sAXL in human serum after an adequate dilution, where the established cut-off value is 71 ng mL−1 [12]. As it is observed, the immunosensor prepared with cMBs exhibited a shorter range of linearity but provided a 3.7-times higher sensitivity. This observation contradicts the few reported works where the performance of electrochemical immunoassays using different sized magnetic particles is compared [6, 7]. This discrepancy may be attributed to the use of much smaller nanomagnetic particles (20 vs 300 nm) than those employed in the previous works which may cause the cMNPs agglomerate more and be captured less efficiently on the surface of the SPCE. This poorer capture efficiency would justify the slightly worse behavior of these immunosensors with respect to those prepared with the cMBs. These results, taken together with those reported previously by other authors, show that while magnetic particles of hundred nanometers seem to allow a better sensitivity with the immunosensors than that obtained using conventional microbeads, sensitivity is worsened when particles of tens of nanometers are involved.
The immunosensors constructed with cMBs or cMNPs allow the determination of 4.5 times lower AXL concentrations than that achieved with the only immunosensor reported so far for AXL [16]. Moreover, the assay time is significantly reduced (2 h 15 min) as well as with respect to commercial ELISA kits (even more than 4 h counting from coating of the of capture antibody).
The reproducibility of the amperometric measurements for 1.0 ng mL−1 AXL was tested with ten different immunosensors fabricated using cMBs or cMNPs on the same day and different days. Relative standard deviation (RSD) values of 3.8 (cMBs) and 3.6% (cMNPs) were calculated for measurements made in the same day, whereas RSD values of 3.8 (cMBs) and 6.5% (cMNPs) were obtained when measuring in different days.
The storage stability of the anti-AXL-cMBs and anti-AXL-cMNPs conjugates was checked by keeping them at 4 °C in eppendorf tubes containing 50 μL of filtered PBS (pH 7.5). Control charts constructed by taking the mean value of ten measurements made in the presence of 1.0 ng mL−1 AXL on the first day of the sets of experiments as the central value, and ±3 × s of this value as the upper and lower control limits, respectively, showed as that the amperometric responses obtained with the immunosensors prepared using the stored conjugates remained within the control limits for at least 25 days (no longer times were assayed) using both types of magnetic particles.
The selectivity of the immunosensors toward AXL was tested using various non-target proteins (cTnI, cTnT, NT-proBNP, Lp(a), CRP, TNF, IL-8) as negative controls. The amperometric responses obtained with the immunosensors for 0.0 and 5.0 ng mL−1 AXL were compared with those measured both in the absence and in the presence of the potential interfering compound at the concentrations expected in serum from healthy people [18, 19]. As an example, the results obtained with the immunosensor prepared using cMBs are displayed in Fig. 3. As it is shown, similar S/N ratios were measured for AXL and in the presence of cTnI, cTnT or CRP (at 500 ng mL−1), NT-proBNP (7.5 ng mL−1), Lp(a) (2 ng mL−1), TNF (20 pg mL−1), or IL-8 (30 pg mL−1), indicating the absence of significant interference in the presence of all these proteins at the concentrations assayed. Interestingly, this ratio is also similar in the presence of 30 pg mL−1 IL-8, although the amperometric currents for AXL were slightly higher in the presence of IL-8, probably because of a slight cross-reactivity of the used antibodies used toward this protein. As expected, and due to the same immunoreagents were employed when the immunosensors were prepared with cMNPs, similar results were obtained with these immunosensors since the selectivity depends mainly on the antibodies used.
Selectivity tests for HRP-Strept-Biotin-anti-AXL-AXL-anti-AXL-cMBs immunosensors. Currents measured in the absence (white bars) and in the presence (grey bars) of 5 ng mL−1 AXL in blocking buffer, pH 7.5, and in the presence of cTnI, cTnT and CRP (500 ng mL−1), NT-proBNP (7.5 ng mL−1), Lp(a) (2 ng mL−1), TNF (20 pg mL−1) and IL-8 (30 pg mL−1)
Analysis of human serum
The practical usefulness of the methods implemented with immunosensors prepared with both types of magnetic particles was evaluated by determining the endogenous content of sAXL in serum collected from 5 different HF patients. The possible existence of a matrix effect was evaluated by constructing a calibration plot from the samples, ten-times diluted with blocking buffer, and spiked with growing concentrations of a sAXL standard solution up to 7.5 ng mL−1 (cMBs) or 10 ng mL−1 (cMNPs). The slope values of the corresponding linear calibration plots obtained were 127 ± 9 and 43 ± 1 nA mL ng−1 with the immunosensors prepared with cMBs and cMNPs, respectively, which indeed are not statistically different than the slope values of the calibration plots constructed with sAXL standard solutions: 158 ± 6 nA mL ng−1, and 51.0 ± 0.2 nA mL ng −1 (texp = 1.299 and 2.870, respectively, ttab = 3.355). Accordingly, no significant matrix effect was apparent upon the above- mentioned samples dilution, and the sAXL endogenous concentration was determined in a straightforward mode by interpolating the amperometric responses measured in the ten-times diluted samples into the calibration plot prepared with sAXL standards.
The results obtained by triplicate with the immunosensors for the serum samples collected from the five patients are summarized in Table 3. In addition, and for validation purposes, these samples were also analyzed with a commercial ELISA kit that used the same immunoreagents. A paired samples t-test demonstrated that no significant differences (α = 0.05) existed for the results provided by the two methods (p-value = 0.35). These results demonstrated that, on the contrary to that reported previously [6, 7] describing higher matrix effect using nanomagnetic particles, there is not a significant difference in the potential matrix effect when the determination of sAXL is carried out in ten-times diluted human serum samples with immunosensors prepared with both types of particles used in this work.
Conclusions
Amperometric sandwich immunoassay strategies using SPCEs as electrodes and micro- and nano- magnetic beads as solid supports to perform the immunological reactions were implemented and compared for the determination of sAXL. The analytical characteristics obtained from the calibration plots for sAXL showed a sensitivity 3 times higher and a shorter linear range for the immunosensors prepared using cMBs when compared with those prepared with cMNPs. However, both strategies provided very similar LOD values which were three orders of magnitude lower than the clinical cut-off value established in serum for patients at risk of heart failure. In addition, both strategies demonstrated to be useful for the accurate determination of the target protein in serum samples from HF patients providing results in good agreement with those obtained by applying a conventional ELISA.
Taking together the results presented in this paper and those reported previously, it can be concluded that the sensitivity, linear range and agglomeration and matrix effects observed with immunosensors prepared with magnetic beads are dependent on the size of the particles.
Therefore, since the tendency to suffer particle agglomeration and the potential matrix effects are strongly dependent both on the size of the magnetic beads and the sample complexity and dilution applied, it is difficult to provide in advance general rules about the performance of the resulting immunosensors. Accordingly, comparative experiments should be carried out for particular applications, especially those involving analysis in scarcely pretreated complex samples.
References
Centi S, Laschi S, Frànek M, Mascini M (2005) A disposable immunomagnetic electrochemical sensor based on functionalised magnetic beads and carbon-based screen-printed electrodes (SPCEs) for the detection of polychlorinated biphenyls (PCBs). Anal Chim Acta 538:205–212
Zacco E, Adrian J, Galve R, Marco MP, Alegret S, Pividori MI (2007) Electrochemical magneto immunosensing of antibiotic residues in milk. Biosens Bioelectron 22:2184–2191
Ricci F, Volpe G, Micheli L, Palleschi G (2007) A review on novel developments and applications of immunosensors in food analysis. Anal Chim Acta 605:111–129
Xu Y, Wang E (2012) Electrochemical biosensors based on magnetic micro/nano particles. Electrochim Acta 84:62–73
Yáñez-Sedeño P, Campuzano S, Pingarrón JM (2016) Magnetic particles coupled to disposable screen printed transducers for electrochemical biosensing. Sensors 16:1585. doi:10.3390/s16101585
de Souza CM, Laube T, Yamanaka H, Alegret S, Pividori MI (2011) Magneto immunoassays for Plasmodium falciparum histidine-rich protein 2 related to malaria based on magnetic nanoparticles. Anal Chem 83:5570–5577
Brandão D, Liébana S, Campoy S, Alegret S, Pividori MI (2015) Immunomagnetic separation of Salmonella with tailored magnetic micro and nanocarriers. A comparative study. Talanta 143:198–204
O’Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R 3rd, Le Beau MM, Earp HS, Liu ET (1991) Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol 11:5016–5031
Neubauer A, Fiebeler A, Graham DK, O’Bryan JP, Schmidt CA, Barckow P, Serke S, Siegert W, Snodgrass HR, Huhn D (1994) Expression of AXL, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood 84:1931–1941
Paccez JD, Vogelsang M, Parker MI, Zerbin LF (2014) The receptor tyrosine kinase Axl in cancer: biological functions and therapeutic implications. Int J Cancer 134:1024–1033
Batchu SN, Hughson A, Gerloff J, Fowell DJ, Korshunov VA (2013) Role of Axl in early kidney inflammation and progression of salt-dependent hypertension. Hypertension 62:302–309
Batlle M, Recarte-Pelz P, Roig E, Castel MA, Cardona M, Farrero M, Campos B, Pulgarín MJ, Ramírez J, Pérez-Villa F, García de Frutos P (2014) AXL receptor tyrosine kinase is increased in patients with heart failure. Int J Cardiol 173:402–409
Liu YW, Yang QF, Zuo PY, Xiao CL, Chen XL, Liu CY (2015) Elevated serum levels of soluble Axl in acute coronary syndrome. Am J Med Sci 349:124–129
Batlle M, Campos B, Farrero M, Cardona M, González B, Castel MA, Ortiz J, Roig E, Pulgarín MJ, Ramírez J, Bedini JL, Sabaté M, García de Frutos P, Pérez-Villa F (2016) Use of serum levels of high sensitivity troponin T, galectin-3 and C-terminal propeptide of type I procollagen at long term follow-up in heart failure patients with reduced ejection fraction: comparison with soluble AXL and BNP. Int J Cardiol 225:113–119
Martínez-Mancera FD, García-López P, Hernández-López JL (2015) Pre-clinical validation study of a miniaturized electrochemical immunoassay based on square wave voltammetry for early detection of carcinoembryonic antigen in human serum. Clin Chim Acta 444:199–205
Serafín V, Torrente-Rodríguez RM, Batlle M, García de Frutos P, Campuzano S, Yáñez-Sedeño P, Pingarrón JM (2017) Electrochemical immunosensor for receptor tyrosine kinase AXL using poly(pyrrolepropionic acid)-modified disposable electrodes. Sensors Actuators B Chem 240:1251–1256
Eguílaz M, Moreno-Guzmán M, Campuzano S, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM (2010) An electrochemical immunosensor for testosterone using functionalized magnetic beads and screen-printed carbon electrodes. Biosens Bioelectron 26:517–522
Arican O, Aral M, Sasmaz S, Ciragil P (2005) Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat Inflamm 5:273–279
Esteban-Fernández de Ávila B, Escamilla-Gómez V, Campuzano S, Pedrero M, Salvador JP, Marco MP, Pingarrón JM (2013) Ultrasensitive amperometric magnetoimmunosensor for humanC-reactive protein quantification in serum. Sensors Actuators B Chem 188:212–220
Acknowledgements
The financial support of projects: Retos Colaboración RTC-2015-4184-1 (cofinanced by the Ministry of Economy and Competitivity and FEDER “una manera de hacer Europa”), CTQ2015-70023-R and CTQ2015-64402-C2-1-R (Spanish Ministry of Economy and Competitivity Research Projects) and S2013/MT-3029 (NANOAVANSENS Program from the Comunidad de Madrid) are gratefully acknowledged. R.M. Torrente-Rodríguez acknowledges a predoctoral contract from the Spanish Ministry of Economy and Competitivity.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 156 kb)
Rights and permissions
About this article
Cite this article
Serafín, V., Torrente-Rodríguez, R.M., Batlle, M. et al. Comparative evaluation of the performance of electrochemical immunosensors using magnetic microparticles and nanoparticles. Application to the determination of tyrosine kinase receptor AXL. Microchim Acta 184, 4251–4258 (2017). https://doi.org/10.1007/s00604-017-2455-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2455-1