Abstract
The authors describe a disposable non-enzymatic sensor for ascorbic acid (AA) that was obtained by modifying a screen printed electrode (SPE) with Cu(OH)2 nanorods (NRs). The NRs were synthesized by a wet chemical process which involves sequential addition of NH3 and NaOH to CuSO4 solution. NR formation was confirmed by thermogravimetric, spectroscopic, microscopic, and diffraction studies. The Cu(OH)2 NRs were mixed with carbon ink and printed onto an SPE. Electrochemical detection of AA was carried out at pH 7.4, at a typical voltage as low as 0 mV versus saturated calomel electrode with a scan rate of 100 mV/s, and is assumed to involve the chemical reduction of Cu(II) by AA followed by electrochemical oxidation of Cu(I). The sensor has a linear response in the 0.0125 to 10 mΜ AA concentration range. Response to AA is free from interference by urea, glucose, uric acid, dopamine, metal ions such as Fe2+, Zn2+ and Ni2+, NaCl, KCl and ethanol. It was applied to the determination of AA in a vitamin C tablet and in urine.

A disposable non-enzymatic sensor for ascorbic acid was fabricated using Cu(OH)2 nanorods. The assay is based on the chemical reduction of Cu(II) by AA followed by an electrochemical oxidation of Cu(I). The sensor electrode showed excellent linearity (0.0125–10 mM), sensitivity (268 μA/mM/cm2) and selectivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ascorbic acid (AA), commonly known as vitamin C, is used as an indicator of oxidative stress in human metabolism because of its excellent antioxidant properties [1]. High oxidative stress can lead to diseases like cancer, hepatic diseases and diabetes mellitus [2]. This increased the importance of clinical estimation of AA from body fluids. AA detection has also received great attention in food, beverages and pharmaceutical industries [3]. Spectroscopic, electrochemical and titrimetric methods are commonly used for the estimation of AA [4].
Non-enzymatic detection of AA on carbon based electrodes is studied in detail by many researchers. It is found that the oxidation potential of AA, uric acid (UA) and dopamine (DA) are very close to each other in a unmodified carbon electrode and hence very difficult to detect AA from a mixture of the three using an unmodified electrode [5]. Cresol red [6], polystyrene sulfonic acid [7], polyaniline [8], polypyrrole [9], polyglycine [10], ruthenium dioxide [11], tetrabromo-p-benzoquinone [12], N,N-dimetyl aniline [13] and polyvinyl chloride [14] are used to modify the electrode to reduce the overpotential for AA oxidation. Chemical or electrochemical pretreatments of carbon electrodes were performed to resolve oxidation peak of AA from other molecules. Preanodized screen printed carbon electrodes showed enhanced electrochemical response and reduced over potential for the oxidation of AA [5]. Graphene, graphene oxide and reduced graphene oxide modified with metal nanoparticles were used for the selective detection of AA [15,16,17]. Nanoparticles of metals such as platinum, gold, palladium, silver nanoparticles and their hybrid materials were used for the detection of AA [4, 18,19,20].
Metallic copper, oxides and complexes of copper are found to have enhanced electrocatalytic activity towards AA oxidation. Detection of AA was carried out on copper electrodeposited glassy carbon electrode [21, 22]. The testing was performed in weakly basic medium and at the anodic potential Cu(I), Cu(II) and Cu(III) were produced. During the cathodic scan reduction peak current of these ions were significantly reduced due to the chemical reduction of oxides of copper by AA. Similarly electrochemical oxidation of AA was analyzed on copper(I)oxide modified carbon paste electrode [23]. The detection was based on the formation of Cu(II)-AA complex and its reduction. Bis (2,2′’-bipyridyl)copper(II) complex modified gold electrode was used for the detection of AA [24]. The electrode showed good catalytic activity towards oxidation of AA. Electrochemical behaviors of copper germanate nanowires and CuO nanowires modified carbon based electrodes were also studied [25,26,27]. Mohamad Ali Taher et al. used Cu(II) loaded in zeolite to oxidize AA [28]. Cu4(OH)6SO4 which is an undesirable oxidation product of copper-bearing sulfides was used for highly selective and sensitive detection of AA in the presence of UA and DA [29]. The mechanism of oxidation of AA on Cu4(OH)6SO4 involves Cu(II)/Cu(I) redox species. Copper(II) phosphate modified carbon paste electrode was used for the detection of AA [30]. Principle of the detection was the oxidation of AA by Cu(II) ions and the resulting in cuprous ions electrochemically oxidized to Cu(II). Similarly copper vanadate nanobelts modified GCE was used for the electrochemical determination of ascorbic acid [31].
This paper describes electrochemical detection of AA on copper hydroxide NRs modified electrode. The detection is based on the chemical reduction of copper hydroxide by AA followed by an electrochemical oxidation of copper(I) at the applied potential. The potential required for the detection was much less than that required for direct electrooxidation of AA on modified electrode. The sensor electrode showed excellent linearity, sensitivity and selectivity. Best of our knowledge, this is the first report on Cu(OH)2 NRs modified screen printed electrode for the detection of AA.
Experimental
Chemicals and Instrumentation
L-Ascorbic acid, D-(+)-glucose, urea, uric acid and dopamine were purchased from Sigma-Aldrich (www.sigmaaldrich.com). All the other chemicals were of analytical grade and used as received. 0.1 M phosphate buffer saline (PBS) of pH 7.4 was prepared by dissolving Na2HPO4, KH2PO4, KCl and NaCl salts. Ionic solutions of Fe, Zn and Ni were prepared by dissolving FeSO4(H2O)7, ZnSO4, NiSO4(H2O)7 salts respectively. Inks for screen printing, silver (5874) and carbon (BQ242) were obtained from DuPont Ltd. (www.dupont.co.in). All solutions used in this study were prepared with Millipore water (15.2 MΩ·cm). Electrochemical experiments were carried out using CHI660C electrochemical workstation (CH Instruments, TX, USA (www.chinstruments.com)). The electrochemical cell consisted of a three-electrode system with screen printed electrode (SPE) as working electrode, a platinum wire as counter electrode and saturated calomel (Hg/Hg2Cl2 (sat. KCl)) as reference electrode. All potentials used in this work are in reference to calomel electrode. Surface morphology was studied with scanning electron microscope (SEM) (JEOL Model JSM-6390LV)(www.jeol.co.jp). The IR spectroscopic studies were carried out on JASCO 460 Plus FTIR spectrophotometer (Pike Technologies, USA)(www.piketech.com). Thermogravimetric analyses (TGA) were carried out using a Seiko Instruments TG/DTA6200, EXSTAR 6000 (www.sii.co.jp) under a 100 mL/min N2 flow and a heating rate of 10 °C/min at a temperature range of 10 to 1000 °C. Wide angle X-ray diffraction measurements were performed at room temperature (25 °C) on a Bruker D8 Focus X-ray diffractometer (www.bruker.com), in 2θ range of 10°–90°.
Preparation of Cu(OH)2 nanorods
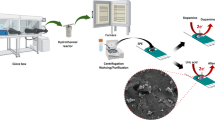
Cupric hydroxide NRs were synthesized by a wet chemical method as follows. 26 mL of ammonia was added dropwise to 700 mL of CuSO4 solution (0.05 M) under constant stirring. Following this, 140 mL of NaOH (1 M) was added dropwise, resulted in a blue colored precipitate. The precipitate was filtered and washed with distilled water several times to remove the impurities and dried at 60 °C for 12 h.
Fabrication of the sensor electrode
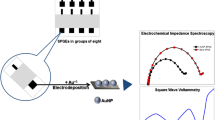
SPEs were indigenously fabricated by printing silver followed by carbon ink on poly (ethylene terephtalate) (PET) substrate. Both the layers were cured for 2 h at 60 °C in a hot air oven. The area of the working electrode is 3.14 mm2 (r = 1 mm). Cu(OH)2 nanorods (NRs) were thoroughly mixed with carbon ink (50 w/w%) to obtain an printable slurry. This was printed on the working electrode of SPE and cured at 45 °C for 1 h.
Electrochemical detection of AA
Electrochemical detection of AA was carried out by voltammetric and amperometric techniques. Cyclic voltammograms (CVs) were recorded at a potential window of −0.4 to 0 V at a scan rate of 100 mV/s. Chronoamperometry was performed in an unstirred solution at a potential of 0 V for 25 s. AA solution was injected into the PBS so that the resultant concentration in the test solution varies from 12.5 μM to 10 mM. The interference of glucose, urea, uric acid, dopamine, Fe2+, Zn2+, Ni2+, NaCl, KCl and ethanol was studied by injecting the respective solutions into the PBS.
Commercially available vitamin C tablet (Celin 500 mg) was dissolved in deionised water. Testing was performed with different dilutions and recovery studies were also carried out. Urine sample was collected from healthy volunteer and 1 mL sample was added to 7 mL PBS. Recovery analysis was carried out by spiking these samples with 300 μM AA.
Result and discussion
Choice of materials
The ascorbic acid detection on copper based nanostructures was well studied [21, 23]. Copper nanoparticles, cuprous oxide and cupric oxide nanoparticles had show a narrow detection range for AA. Detection with metallic copper in PBS is not desirable due to copper dissolution and electrode instability during the voltammetric experiments. Copper hydroxide sulphates and copper phosphates show better sensor characteristics than copper oxides. These are based on the chemical reduction of Cu2+ followed by an electrochemical oxidation of Cu+. In this work copper hydroxide nanorods modified on the electrode is quantitatively reduced to Cu+. The sensor has showed superior characteristics compared to other copper based materials as catalysts.
Characterization of Cu(OH)2 NRs
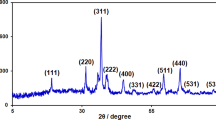
The mechanism of formation of cupric hydroxide NRs from copper precursors has been extensively discussed in literatures [32,33,34]. Initially the Cu2+ ions complex with ammonia and form complex cation [Cu(NH3)4]2+. Upon addition of NaOH, OH− replaces NH3 in the complex to form a square-planar [Cu(OH)4]2− complex anion. Coordination self-assembly of the >Cu(OH)2Cu < results in the formation of cupric hydroxide NRs. TGA curve (Fig. S1a) of the NRs show a weight loss at 160 °C indicates the characteristic decomposition of Cu(OH)2 to copper oxide. The percentage weight loss (19.34%) was matched with the theoretical weight loss of 18.45%. The FTIR spectrum obtained for copper hydroxide NRs was found matching with the literatures (Fig. S1b) [35]. XRD patterns (Fig. S1c) confirmed that the synthesized nanomaterial is orthorhombic, copper hydroxide in end-centered lattice (JCPDS data card 80–0656). Different lattice planes were identified using JCPDS data and the sharp diffraction patterns indicate that the material is highly crystalline. From the SEM image of copper hydroxide (Fig. S1d) it is clear that the material exists as thin, rods of width about 100 nm.
Morphological Characterization of SPE and modified SPE
Fig. 1 shows the SEM images of bare and modified electrodes. The characteristic granular structures of carbon were observed on SPE (Fig. 1a). On the modified SPE, Cu(OH)2 NRs were found embedded within the granular structures (Fig. 1b). SEM images confirmed the presence of exposed Cu(OH)2 NRs on the surface of modified electrode.
Determination of ascorbic acid
The electrochemical studies on the bare and modified electrodes were carried out using CV in PBS of pH 7.4. The electrochemical oxidation of AA occurs on the carbon printed electrode around 0.4 V. It is difficult to distinguish the oxidation potentials of AA and UA on a carbon printed electrode. On the Cu(OH)2 nanorods modified electrode, the oxidation peak appeared at around −0.1 V (Fig. 2a). The unmodified electrode was not giving any peak at this potential range. It was also found that UA is not giving any peak at this potential window on the modified electrode (Fig. S2). This peak can be attributed to the electrochemical oxidation of copper(I) formed by the chemical reduction of Cu(OH)2 by AA. Chemical reduction of Cu(II) to Cu(I) by AA is well established [29, 31]. This strategy enabled the detection of AA without direct electrooxidation. The potential required for the detection was much less than that required for direct electrooxidation of AA on modified electrode.
Fig. 2b shows cyclic voltammograms obtained on the modified SPE for different concentrations of AA. Cyclic voltammograms obtained for lower ascorbic acid concentrations were presented in supporting information (Fig. S3). The sensor current response increased linearly with the increase in concentration of AA. This can be attributed to the greater chemical reduction of copper hydroxide at higher concentration of AA, and subsequent oxidation of Cu(I) at the applied potential. Hence the increase in faradic current is directly proportional to AA concentration in the solution. The sensor showed a linear response in the range 12.5 μM to 10 mM. A calibration plot of peak current versus concentration was plotted (Fig. 2c) and the regression equation obtained was IP (μA) = 0.00842C (μM) + 0.26316 (R2 = 0.9986, σ = 1.51, n = 29, RSD = 107.86%). The sensitivity of the electrode was calculated as 268 μA/mM/cm2.
The relationship between scan rate and peak current can be used to explore valuable information regarding chemical behavior of AA on the modified electrode in PBS. CVs were recorded on the modified electrode in PBS pH 7.4 with 400 μM AA at different scan rates from 10 to 250 mV/s (Fig. S4). The peak currents are linearly increasing with the increase in scan rate with a regression coefficient of R2 = 0.990. This indicates that this nernstian process is adsorption controlled and obeys the relation,
Where ip, n, F, R,T, ν, A and Гo are the peak current, number of electrons involved in the reaction, Faraday’s constant, universal gas constant, absolute temperature, scan rate, area of the electrode and amount of adsorbed species at time respectively.
The results obtained for the steady state amperometric analysis at a constant potential of 0 V for duration of 25 s is shown in Fig. 3. The current response was found to increase linearly with the AA concentration. The current response at 25 s was used to plot the calibration plot. The regression coefficient and standard deviation obtained were 0.998 and 0.136 respectively (with RSD = 63.57% and n = 13).
The sensor showed high operational stability and reproducibility towards the detection of AA (RSD = 3.91% & 4.15% respectively with n = 10). The details of these studies are provided in the supplementary material.
Interference studies
Selectivity of the sensor was studied by performing chronoamperometry at a constant potential of 0 V in the presence of different molecules in PBS. Glucose, urea, uric acid and dopamine (glucose: 6 mM, urea: 10 mM, dopamine: 125 μM, uric acid: 125 μM) were tested and the modified electrode was shown negligible interference by these species. Similarly metal ions such as Fe2+, Zn2+ and Ni2+ (each 25 μM), common salts (NaCl: 5 mM, KCl: 5 mM) and ethanol (21 mM) were tested with the sensor. Fig. 4 shows chronoamperograms on modified electrode in the presence of these interfering molecules. Percentage responses of these molecules on the modified electrode is given in Table. S1. From the Table it is clear that on the modified electrode the response of species other than AA is minimal.
Sensor validation with pharmaceutical formulations and spiked urine
The sensor was tested with commercially available vitamin C tablet (Celin 500, Glaxo Smithkline Pharmaceuticals Ltd.). The tablet was dissolved in distilled water and diluted to different concentrations. The estimation of AA in these samples was carried out by the sensor electrode. The error was calculated and was found minimal (Table. S2). Recovery analysis was also carried out by adding AA of 125 μM to vitamin C tablet solution of concentration 353 μM. The concentration of AA after spiking was estimated using the sensor (Table. S3). Recovery was found 99.14%. From the data, it is evident that the sensor fabricated can be used as an efficient tool for the analysis of AA in various systems including commercial formulations. The sensor was used for the estimation of AA in urine samples also. The AA concentration in the spiked sample was estimated as 279 μM. The good recovery (93%) indicates the applicability of the sensor in real samples.
Comparison with other sensors
The sensor was compared with the reported AA sensors based on copper (Table 1) and other materials as catalyst [Table. S4]. The superiority of the sensor compared to other sensors is its wide detection range (0.0125–10 mM). Next sensor with wide detection range had an upper detection limit of 6 mM which is much below when compared with this sensor. Wide detection range makes the sensor ideal for direct application of sample without dilution. The working potential of the sensor is 0 V and at this potential other biomolecules will not undergo oxidation/reduction.
Conclusion
A disposable non-enzymatic electrochemical sensor for AA was fabricated using copper hydroxide NRs. The catalyst, copper hydroxide nanorods, was synthesized by a one pot wet chemical method using inexpensive chemicals. The detection was based on the chemical reduction of cupric hydroxide by AA and followed by the electrochemical oxidation of Cu(I) formed on the electrode surface. The sensor showed excellent linearity (0.0125–10 mM), sensitivity (268 μA/mM/cm2) and selectivity at 0 V. Interference from other species was found to be negligible or minimal. The sensor was used for estimating the concentration of AA in commercially available vitamin C tablets and spiked urine samples. Simple and one step catalyst synthesis, cost effective sensor fabrication, flexibility of incorporating disposable sensors into microfluidic platforms are the advantages of this work compared to other reported methods.
References
Bijur GN, Ariza ME, Hitchcock CL, Williams MV (1997) Antimutagenic and promutagenic activity of ascorbic acid during oxidative stress. Environ Mol Mutagen 30(3):339–345
Ambrosi A, Morrin A, Smyth MR, Killard AJ (2008) The application of conducting polymer nanoparticle electrodes to the sensing of ascorbic acid. Anal Chim Acta 609(1):37–43
Farrington AM, Jagota N, Slater JM (1994) Simple solid wire microdisc electrodes for the determination of vitamin C in fruit juices. Analyst 119(2):233–238
Satheesh Babu T, Suneesh P, Ramachandran T, Nair B (2010) Gold nanoparticles modified titania nanotube arrays for amperometric determination of ascorbic acid. Anal Lett 43(18):2809–2822
Prasad KS, Muthuraman G, Zen J-M (2008) The role of oxygen functionalities and edge plane sites on screen-printed carbon electrodes for simultaneous determination of dopamine, uric acid and ascorbic acid. Electrochem Commun 10(4):559–563
Chen W, Lin X, Huang L, Luo H (2005) Electrochemical characterization of polymerized cresol red film modified glassy carbon electrode and separation of electrocatalytic responses for ascorbic acid and dopamine oxidation. Microchim Acta 151(1–2):101–107
Zhang Y, Cai Y, Su S (2006) Determination of dopamine in the presence of ascorbic acid by poly (styrene sulfonic acid) sodium salt/single-wall carbon nanotube film modified glassy carbon electrode. Anal Biochem 350(2):285–291
Kit-Anan W, Olarnwanich A, Sriprachuabwong C, Karuwan C, Tuantranont A, Wisitsoraat A, Srituravanich W, Pimpin A (2012) Disposable paper-based electrochemical sensor utilizing inkjet-printed Polyaniline modified screen-printed carbon electrode for Ascorbic acid detection. J Electroanal Chem 685:72–78
Babu TS, Varadarajan D, Murugan G, Ramachandran T, Nair BG (2012) Gold nanoparticle–polypyrrole composite modified TiO2 nanotube array electrode for the amperometric sensing of ascorbic acid. J Appl Electrochem 42(6):427–434
Yu AM, Zhang HL, Chen HY (1997) Fabrication of a polyglycine chemically modified electrode and its electrocatalytic oxidation to ascorbic acid. Electroanalysis 9(10):788–790
Wu J, Suls J, Sansen W (2000) Amperometric determination of ascorbic acid on screen-printing ruthenium dioxide electrode. Electrochem Commun 2(2):90–93
Zare H, Nasirizadeh N, Ardakani MM (2005) Electrochemical properties of a tetrabromo-p-benzoquinone modified carbon paste electrode. Application to the simultaneous determination of ascorbic acid, dopamine and uric acid. J Electroanal Chem 577(1):25–33
Roy PR, Okajima T, Ohsaka T (2003) Simultaneous electroanalysis of dopamine and ascorbic acid using poly (N, N-dimethylaniline)-modified electrodes. Bioelectrochemistry 59(1):11–19
Aguilar R, Dávila M, Elizalde M, Mattusch J, Wennrich R (2004) Capability of a carbon–polyvinylchloride composite electrode for the detection of dopamine, ascorbic acid and uric acid. Electrochim Acta 49(6):851–859
da Silva RP, Lima AWO, Serrano SH (2008) Simultaneous voltammetric detection of ascorbic acid, dopamine and uric acid using a pyrolytic graphite electrode modified into dopamine solution. Anal Chim Acta 612(1):89–98
Li S-M, Wang Y-S, Hsiao S-T, Liao W-H, Lin C-W, Yang S-Y, Tien H-W, Ma C-CM HC-C (2015) Fabrication of a silver nanowire-reduced graphene oxide-based electrochemical biosensor and its enhanced sensitivity in the simultaneous determination of ascorbic acid, dopamine, and uric acid. J Mater Chem C 3(36):9444–9453
Ruan H, Liu B, Li H (2015) Controlled synthesis of graphene–Gd (OH) 3 nanocomposites and their application for detection of ascorbic acid. RSC Adv 5(27):21242–21248
Chen L-X, Zheng J-N, Wang A-J, Wu L-J, Chen J-R, Feng J-J (2015) Facile synthesis of porous bimetallic alloyed PdAg nanoflowers supported on reduced graphene oxide for simultaneous detection of ascorbic acid, dopamine, and uric acid. Analyst 140(9):3183–3192
Thiagarajan S, Chen S-M (2007) Preparation and characterization of PtAu hybrid film modified electrodes and their use in simultaneous determination of dopamine, ascorbic acid and uric acid. Talanta 74(2):212–222
Zhao D, Fan D, Wang J, Xu C (2015) Hierarchical nanoporous platinum-copper alloy for simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 182(7–8):1345–1352
Selvaraju T, Ramaraj R (2007) Simultaneous detection of ascorbic acid, uric acid and homovanillic acid at copper modified electrode. Electrochim Acta 52(9):2998–3005
Xi L, Ren D, Luo J, Zhu Y (2010) Electrochemical analysis of ascorbic acid using copper nanoparticles/polyaniline modified glassy carbon electrode. J Electroanal Chem 650(1):127–134
Dursun Z, Nişli G (2004) Voltammetric behavior of copper (I) oxide modified carbon paste electrode in the presence of cysteine and ascorbic acid. Talanta 63(4):873–878
Freire RS, Kubota LT (2002) Electrochemical behavior of the bis (2, 2′-bipyridyl) copper (ii) complex immobilized on a self-assembled monolayer modified electrode for l-ascorbic acid detection. Analyst 127(11):1502–1506
Pei L, Cai Z, Xie Y, Pei Y, Fan C, Fu D (2012) Electrochemical behaviors of ascorbic acid at CuGeO3/polyaniline nanowire modified glassy carbon electrode. J Electrochem Soc 159(10):G107–G111
Pei L, Xie Y, Cai Z, Yang Y, Pei Y, Fan C, Fu D (2012) Electrochemical Behavior of Ascorbic Acid at Copper Germanate Nanowire Modified Electrode. J Electrochem Soc 159(3):K55–K60
Ma Y, Zhao M, Cai B, Wang W, Ye Z, Huang J (2014) 3D graphene foams decorated by CuO nanoflowers for ultrasensitive ascorbic acid detection. Biosens Bioelectron 59:384–388
Rohani T, Taher MA (2009) A new method for electrocatalytic oxidation of ascorbic acid at the Cu (II) zeolite-modified electrode. Talanta 78(3):743–747
Xia C, Ning W (2011) A novel bio-electrochemical ascorbic acid sensor modified with Cu4 (OH) 6SO4 nanorods. Analyst 136(2):288–292
Teixeira MF, Ramos LA, Fatibello-Filho O, Cavalheiro ÉT (2003) Carbon paste electrode modified with copper (II) phosphate immobilized in a polyester resin for voltammetric determination of L-ascorbic acid in pharmaceutical formulations. Anal Bioanal Chem 376(2):214–219
Pei L, Lin N, Wei T, Liu H, Yu H (2015) Formation of copper vanadate nanobelts and their electrochemical behaviors for the determination of ascorbic acid. J Mater Chem A 3(6):2690–2700
Gurav K, Patil U, Shin S, Agawane G, Suryawanshi M, Pawar S, Patil P, Lokhande C, Kim J (2013) Room temperature chemical synthesis of Cu (OH) 2 thin films for supercapacitor application. J Alloys Compd 573:27–31
Lu C, Qi L, Yang J, Zhang D, Wu N, Ma J (2004) Simple template-free solution route for the controlled synthesis of Cu (OH) 2 and CuO nanostructures. J Phys Chem B 108(46):17825–17831
Wang W, Lan C, Li Y, Hong K, Wang G (2002) A simple wet chemical route for large-scale synthesis of Cu (OH) 2 nanowires. Chem Phys Lett 366(3):220–223
Li C, Yin Y, Hou H, Fan N, Yuan F, Shi Y, Meng Q (2010) Preparation and characterization of Cu (OH) 2 and CuO nanowires by the coupling route of microemulsion with homogenous precipitation. Solid State Commun 150(13):585–589
Acknowledgements
Authors greatly acknowledge the Department of Biotechnology (DBT), Government of India for the financial support under the project (Project No BT/PR4076/MED/32/221/2011) and services rendered by PSGTechs COE Indutech, PSG College of Technology, Neelambur. Jeethu Raveendran express her sincere thanks to Council of Scientific and Industrial Research (CSIR) for the financial support under CSIR-SRF Direct scheme (Sanction No. 09/1034(0003)/2 K15-EMR-I).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 1431 kb)
Rights and permissions
About this article
Cite this article
Raveendran, J., Krishnan, R.G., Nair, B.G. et al. Voltammetric determination of ascorbic acid by using a disposable screen printed electrode modified with Cu(OH)2 nanorods. Microchim Acta 184, 3573–3579 (2017). https://doi.org/10.1007/s00604-017-2391-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2391-0