Abstract
Upconversion nanoparticles (UCNPs) of the type α-NaYF4:Yb3+, Er3+ and a typical diameter of 6–7 nm were synthesized by thermal decomposition of the respective rare-earth stearate. The oleic acid on the surface of the UCNPs was then replaced by aptamer DNA. The assay was performed in a microplate format with a capture probe immobilized in the wells. Following binding of the vascular endothelial growth factor (VEGF), an auxiliary probe DNA is added that is labeled with UCNPs and binds to the VEGF-loaded capture probe. The method enables highly sensitive and highly specific detection of the VEGF which is a marker for breast cancer. Under the optimum conditions, the intensity of the upconversion luminescence (at excitation/emission wavelengths of 980/541 nm) is linearly proportional to the VEGF concentration in the 50 pM to 2000 pM concentration range, with a 6 pM detection limit. The method was applied to the determination of VEGF in spiked serum, typically at a 500 pM level, and gave recoveries that ranged from 98 to 113 %, with RSDs between 2.9 and 3.6 %. This makes it a viable tool for early diagnosis of breast cancer.

An upconversion luminescence based assay is presented for trace analysis of vascular endothelial growth factor. It is based on a sequence fragment-linked technique of target-induced aptamer (AP) and upconversion nanoparticles (UCNPs) and has potential application in the early diagnosis of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the process of tumorigenesis or proliferation, tumor cells synthesize and release substances, which can be used as tumor markers. Tumor markers may also be produced by host cells in response to the tumor and are often present in form of metabolites, antigens, enzymes and hormones. The expression of the vascular endothelial growth factor (VEGF) is closely related to the occurrence and progression of breast cancer. Therefore, monitoring VEGF levels is significant for breast cancer treatment and prognosis [1]. VEGF can stimulate the growth, survival and proliferation of vascular endothelial cells and modulate angiogenesis, which is a basic requirement for tumor infiltration and metastasis [2, 3]. These characteristics and functions make VEGF one of the most important markers for breast cancer. Currently, enzyme-linked immunosorbent assay (ELISA) is the most commonly used method to detect VEGF [4], but this method has many shortcomings, such as high cost, and complicated protocols. Thus, there is a need for VEGF detection technique that is more convenient, accurate and sensitive than ELISA.
So far, biosensing has been dominated by antibody-based detection techniques. In 1990, a new bio-recognition element, referred to as nucleic acid aptamers, also known as chemical antibodies, was published [5]. Nucleic acid aptamers have many advantages over traditional antibodies, such as a wide scope of target molecules, good chemical stability, easy modification and labelling, and low molecular weight. Nucleic acid aptamer biosensors have shown good prospects for clinical diagnosis and as biomedicines [6]. Mohammad et al. screened a library of nucleic acid aptamers for application as VEGF biosensors and provided a way to detect VEGF with high sensitively [7].
Rare-earth (RE)-doped upconversion nanoparticles (UCNPs) were recently discovered to have important biomedical applications. One such UCNP, NaMF4:Yb3+, Ln3+ (M = Y or Gd, Ln = Er or Tm) can transform near-infrared (NIR) exciting light into tunable short wavelength fluorescence in the range of from deep ultraviolet (UV) to NIR [8]. Due to their unique properties, such as adjustable multi-color light emission, special light-stability, deep tissue penetration, auto-fluorescence inhibition and low toxicity in vivo and in vitro, these UCNPs have been used in preclinical imaging studies, clinical diagnosis and therapeutics, drug delivery and bioassays [9]. Recently, a two-step thermal decomposition method using RE trifluoroacetates [10, 11] or RE oleates [12] as precursors has been widely adopted to obtain NaYF4:Yb3+, Er3+ UCNPs. The drawbacks of these strategies mainly include that the UCNPs have a relatively lower upconversion quantum yield, and the commonly used precursors are expensive metallic trifluoroacetate salts, which readily produce toxic fluorinated and oxyfluorinated carbon byproducts during the process. Therefore, it is important to find a way to prepare the UCNPs without generating these pollutants. Moreover, most particles have a size between 10 and 100 nm, but the hydrodynamic size of UCNPs is required to be <10 nm (optimized surfaces included) in order to warrant renal clearance [13]. Preparing high quality UCNPs under 10 nm with strong luminescence remains a technical challenge. Furthermore, UCNPs are strongly hydrophobic and have poor biocompatibility because their surfaces are usually covered with inert lipophilic groups [14]. To overcome this limitation, a one-step solvothermal synthesis [15, 16], ligand exchange [17, 18], and silicon coating methods as well as the functional modification of UCNPs have been introduced [19]. Although these methods improved the solubility and functionality of UCNPs, the use of heterogeneous multifunctional cross linking in the biological coupling step make the process more complex [20]. Enormous challenges remain in preparation of biocompatible UCNPs that can recognize specific molecules. DNA is becoming the most attractive application platform of bionanotechnology because of its excellent stability and unique base-pairing rules [21]. In particular, DNA coating technology by chemical modifications [22] has allowed DNA bionanotechnology to extend to the field of high sensitivity biosensors [23, 24], self-assembled nanomaterials [25] and gene drug delivery.
Therefore, considering the great potentials of both DNA based bionanotechnology and UCNPs, development of a simple, general, and versatile method to produce DNA-modified UCNPs will advance the biomedical application of UCNPs significantly. Interestingly, Lu et al. studied a simple strategy to prepare a biological probe with uniformly DNA-modified UCNPs [26]. This method can directly transform hydrophobic UCNPs into hydrophilic DNA-UCNPs without any chemical modifications of the UCNPs or oligonucleotides. Moreover, DNA molecules on DNA-UCNPs retain their biometric capability. Most importantly, DNA-UCNPs can pass through the cell membrane, a property that confers great prospects for application in tumor cell imaging and target drug delivery.
Inspired by the above research, we adopted RE stearates as precursors for thermal decomposition in a high temperature system and developed a simple, cheap and pollution-free approach to obtain small NaYF4:Yb3+, Er3+ UCNPs. Then we applied a simple one-step exchange strategy to obtain soluble DNA-UCNPs. The DNA oligonucleotide aptamer probe was designed according to the structure of VEGF. Using these UCNPs as luminescence labels, we developed a highly sensitive and selective upconversion luminescence (UCL) assay based on sequence fragment-linked of target-induced aptamer for the detection of VEGF. After optimizing testing conditions, we studied the ability of the aptasensor to detect VEGF in patient samples, providing the preclinical basis for the use of this aptamer in the early diagnosis and treatment of breast cancer.
Experimental
Materials and apparatus
All chemicals were used as received without further purification. Yttrium oxide (99.999 %), ytterbium oxide (99.999 %) and erbium oxide (99.999 %) were provided from Institute of Applied Chemistry Chinese Academy Sciences (China, http://ciac.cas.cn). NaF, NaCl, Tris, EDTA and stearic acid were obtained from Sinopharm Chemical Reagent Co., Ltd. (China, http://shreagent.com). Oleic acid (OA) and 1-octadecene (ODE) were purchased from Aladdin Reagent co., Ltd. (China, http://www.aladdin-e.com). Bovine serum albumin (BSA) was purchased from New England Biolabs co., Ltd. (China, http://www.neb-china.com). VEGF, human serum albumin (HSA) were obtained from Sigma-Aldrich Chemical Co., Ltd. ( http://www.sigmaaldrich.com/china-mainland.html ). DNA sequences of the capture probe (CP, 5′-biotin-TGTGGGGGTGGAC-3′) and the auxiliary probe (AP, 5′-GGGCCGGGTAGA-3′) were purchased from Sangon Biotechnology co., Ltd. (China, http://www.sangon.com). The hybrid buffer (pH 7.4) of DNA was consisting of 1 M NaCl, 10 mM Tris, 1.0 mM EDTA. All aqueous solutions were prepared in ultrapure water (purified by Milli-Q biocel from Milli-pore China Ltd., http://www.merckmillipore.com).
X-ray powder diffraction (XRD) measurements were performed on a Mini Flex II X’ Pert Pro diffractometer (Riguka Co., Tokyo, Japan) with graphite monochromatized CuKα radiation (λ = 0.15406 nm). The morphology and size of the UCNPs were characterized by a JEM-1200EX transmission electron microscope (JEOL Ltd., Tokyo, Japan). The DNA was centrifuged by a Neofuge 18R high speed refrigerated centrifuge (Heal Force Development Ltd., Hong Kong, China). The UCL spectra were recorded with a Cary Eclipse fluorescence spectrophotometer (Varian Co., Palo Alto, CA, USA) attached an external 980 nm laser (CNI Optoelectronics Technology Co., Changchun, China). UCL images were acquired with a Canon 60 D digital camera (Tokyo, Japan).
Preparation of UCNPs
A fixed percentage of RE stearates (C17H35COO)3RE (RE = Y0.78Yb0.20Er0.02) were synthesized according to previous studies (See ESM, section 1) [27]. Then UCNPs were synthesized by thermal decomposition [28] of RE stearates in a high temperature system. In an optimal procedure, 6 mL oleic acid (OA) and 14 mL 1-octadecene (ODE) were pipetted into a 100 mL three-necked flask, and 1.0 mmol RE stearates were added later; finally, the mixture was heated to 100 °C with vigorously magnetic stirring in an argon atmosphere for 30 min to form a clear solution. After that, the solution was heated to 290 °C with a heating rate of 5 °C·min−1. Then, 10 mmol NaF was poured into the solution, which was quickly heated to 310 °C and kept for 50 min in an argon atmosphere under vigorous stirring. After the reaction naturally cooled to room temperature, the UCNPs were precipitated by adding cyclohexane/ethanol (1:1 v/v) and isolated via centrifugation. The UCNPs were further purified by using the mixture of cyclohexane/distilled water (1:3 v/v) five or six times and then dried under a vacuum before storage in 5 mL cyclohexane.
The functionalization of UCNPs by DNA
Functionalized DNA-UCNPs were obtained by applying DNA to directly replace OA according to previously published protocols [25]. The AP was pre-processed according to the following steps. The AP was centrifuged for 20 min at 4000 rpm (1395 g) and diluted to the required concentration with deionized water. Then the solution was stored at 4 °C after vortexing for several minutes. In the procedure, the 20 μmol OA-coated UCNPs in 1.0 mL of chloroform was slowly added into 2 mL of water (with 200 nmol AP), and the mixture was vigorously stirred overnight. Afterward, the UCNPs were transferred into the aqueous phase from the organic phase due to the attachment of DNA. After sonication, excess DNA in the aqueous phase was removed from the surface of AP-UCNPs by centrifugation and washing. The resultant AP-UCNPs were re-dispersed in the buffer and stored at 4 °C for further use.
The upconversion luminescence assay for VEGF
First, the CP was fixed to 96-well plates. Before fixation, the CP was heated to 95 °C for 10 min and naturally cooled to room temperature. Every well of the 96-well plates was coated with 100 μL of avidin diluted in carbonate buffer (pH, 9.0). After less than 12 h of the coating reaction at 4 °C, the plates were rinsed several times with phosphate buffer (pH, 7.4) and dried. Next, the plates were sealed with 3 % bovine serum albumin (BSA), and after 10 μM CP was added, the reaction was performed for 30 min near 25 °C. The plates were rinsed with phosphate buffer to clear away the residuals and dried. After adding hybrid buffer, the 96-well plates fixed with CP were incubated at room temperature for 100 min. After the residuals were cleared, the plates were rinsed several times by phosphate buffer and dried. The reaction was carried out for 1 h after adding 10 μL certain concentrations of VEGF, 50 μL AP-UCNPs (100 μg·mL−1) and 40 μL 3 % BSA. Finally, the residuals were cleared away after the mixture was incubated at room temperature for 120 min, and the plates were rinsed by phosphate buffer and dried. The UCL was investigated by Cary Eclipse fluorescence spectrophotometer using exciting light of 980 nm laser (power modulation, 500 mW·cm−2) after adding 100 μL buffer into each well. According to the analysis scheme, the UCL of the system was detected after adding different concentrations of VEGF. For the analysis of VEGF levels in serum from breast cancer patients, all experiments were performed in accordance with relevant guidelines and regulations and were approved by the Ethics Committee of Fujian Medical University Union Hospital. The preparation of artificial serum sample was shown in the section 2 of ESM.
Results and discussion
Characterization of the UCNPs
The high-quality growth of UCNPs, a specific metric of the UCL assay, is especially important for the sensitivity of VEGF testing. Therefore, the crystal phase, structural constituents, size, morphology and luminescent properties of the UCNPs were characterized by different approaches.
As shown in Fig. 1a, b, TEM images showed that the UCNPs were highly uniform and had a roughly spherical shape. The statistics of crystallite size showed that the UCNPs had an average size of 6–7 nm (n = 400, see ESM, section 3). UCNPs were of a fine single crystalline nature as shown in Fig. 1c. The lattice fringes were clearly distinguished in the high resolution TEM images, and the value of the interplanar spacing between the lattice fringes (0.316 nm) belongs to the (111) lattice plane. The selected-area electron diffraction pattern (Fig. 1d) shows that spotty polycrystalline diffraction rings can be indexed as the (111), (200), (220) and (311) planes of the UCNPs lattice, respectively. The typical XRD pattern (Fig. 1e) corresponds almost exactly with the standard pattern of the cubic phase (α) NaYF4 (JCPDS No.77–2042), suggesting that the nanocrystals belong to the pure cubic phase and have fine crystallinity. The chemical constituent of the lanthanides in UCNPs were analyzed by X-ray energy dispersive spectrum. As shown in Fig. 1f, the measured atomic ratio of the lanthanide elements (Yb: Er = 29.22: 3.38) is very close to the theoretical values (Yb: Er = 0.2: 0.02), indicating that the doping contents of lanthanides in the UCNPs can be controlled precisely during the growth process. The UCL spectrum of 1 wt% UCNPs solution in cyclohexane when excited with a 980 nm laser is shown in Fig. 1g. There were three emission centers at approximately 521 nm, 541 nm and 656 nm, which are attributed to the 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions of Er3+ ions, respectively. Thereby, the 521 nm and 541 nm emission centers belong to the typical green region. The inset in Fig. 1g illustrates that the strong green light can be easily observed by the bare eye or other imaging systems, which suggests that the UCNPs are excellent potential biomarkers for biomedical applications.
Characteristics of the UCNPs. (a, b) TEM images; c High resolution TEM images; d Selected-area electron diffraction pattern; e XRD patterns; f Analysis of X-ray energy dispersive spectrum; g The UCL spectrum. Inset: Photograph of the UCNPs dissolved in cyclohexane (1 %, w/V) when excited by 980 nm laser
The test mechanism of the upconversion luminescence assay for VEGF
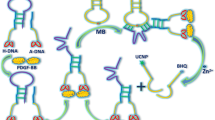
Based on fragment-linked technology of target-induced aptamer, a highly sensitive and selective UCL assay was designed for VEGF detection. The test mechanism of UCL assay for VEGF testing is shown in Fig. 2. First, the DNA aptamer probe for VEGF was designed according to the structure of VEGF. Then the DNA sequence of the VEGF aptamers was cut and transformed into two linear structures, one as a capture probe (CP), fixed on the microplate surface, and the other as a signal probe (AP), modified with UCNPs on its end (Characterization of AP- UCNPs was shown in ESM, section 4). When VEGF appears, the two probes combine with VEGF to form a stable aptamer complex on the surface of the microplate. Then the AP-UCNPs were fixed on the microplate surface and a strong UCL signal (541 nm, which peak is selected as research object in subsequent spectral analysis due to the strong luminescence intensity.) based on excitation of 980 nm exciting light was obtained. On the contrary, when no VEGF was present, AP was not fixed on the microplate surface and no UCL signal was detected. Thus, highly sensitive detection of VEGF can be realized through the identification of different UCL signals according to different concentrations of VEGF in the samples.
The sensitivity of the upconversion luminescence assay
The test results for UCL measurements of VEGF are shown in Fig. 3. Under optimal experimental conditions, the UCL intensity gradually increased with the concentration of VEGF from 0 to 10,000 pM. In the absence of VEGF, the UCL signal was hardly detectable because the two probes failed to combine without VEGF, confirming that CP alone failed to fix AP to the microplate surface. When VEGF concentration reaches 10,000 pM the UCL reaches a maximum, with an intensity 3.5 times higher than that of 2000 pM VEGF, and 7 times higher than that of 500 pM VEGF.
Increasing VEGF concentrations from 50 to 2000 pM produced linear increases in the UCL intensity (F). As shown in Fig. 4, the correlation equation can be expressed as: F = 0.1038c + 13.909 (r = 0.9987). The limit of detection (LOD) was 6 pM (S/N = 3). A VEGF concentration of 500 pM was chosen to investigate the precision of the method within each group of five repeated tests, and the relative standard deviation (RSD) was 2.56 %. The results showed that our assay exhibited good reproducibility and satisfactory stability, providing an excellent method for detecting low concentrations of VEGF in clinical samples. To further illustrate the increased sensitivity and other advantages of our UCL assay, we compared our assay to other methods of VEGF detection. For example, Wang et al. used aptamer sensors to detect VEGF based on fluorescence polarization (FP), and the resultant LOD was 0.32 nM [29]. Due to the different effects of protonation of DNA bases, such methods of VEGF detection are strongly affected by the pH of the solution. At the same time, the ionic strength of the solution can also seriously affect the sensitivity of detection. Freeman et al. reported several aptasensors based on quantum dots (QDs) and dye acceptors (Cy5) for VEGF detection; the typical LOD for these methods were 1 nM, 12 nM and 875 pM, respectively [30]. The previously reported aptamer sensors designed by QDs and organic dyes are more complicated, and the relative LOD only reaches nM levels, which leads to a sensitivity far below that of our UCL assay. Zhao et al. reported a method based on a folding electrochemical aptamer sensor to detect VEGF; the LOD of their sensor was 5 pM [31]. Here, we report the LOD of our UCL aptasensor to be 6 pM, which is similar to the electrochemical method. However, the electrochemical method has a higher rate of false positive and false negative results, poor stability and reproducibility and significant background interference complications. In summary, a comparison (see ESM, Table S1) of the LOD data and characteristics of previously designed sensors shows that our UCL assay shows better sensitivity, stability (see ESM, section 5), and reproducibility, no biological toxicity (see ESM, section 6), simpler design and a more concise operation.
The specificity of the upconversion luminescence assay
The specificity of the UCL assay was verified by the fluorescence method. The UCL signals of VEGF and different serum proteins were determined, respectively. Figure 5 shows the distinct contrast diagrams of the luminescence of blank, BSA, HSA and VEGF. One can clearly see that the luminescence of VEGF was stronger than that of the other samples, and this demonstrates that the UCL assay has good specificity.
To verify the selectivity of our assay, we further investigated normalized ratios of the luminescence response of the assay for VEGF and different serum proteins. As shown in Fig. 6, the ΔFx/ΔF0 values of the assay for VEGF, BSA and HSA were 95.1 %, 2.4 % and 4.3 %, respectively. These results suggest that our UCL assay can specifically recognize and detect VEGF. To evaluate the interference of other foreign species with the determination of 1 nM VEGF, a systematic study was also carried out. It was found that 1000-fold concentrations of glucose, lactose, L-cystine, DL-alanine, and cyclodextrin did not interfere with the luminescence intensity of VEGF (signal change below 5 %). In brief, the assay has good selectivity for VEGF.
VEGF detection in serum samples from breast cancer patients
VEGF detection in serum samples from breast cancer patients was carried out under optimal conditions. The results are presented in Table 1 and show that the recovery was distributed between 98 % and 113 %, and the RSD was distributed between 2.9 % and 3.6 % after five repeated measurements of the corresponding samples, which indicates that the reproducibility of the assay is suitable for clinical applications. Therefore, the UCL assay is expected to be used for ultrasensitive detection of VEGF in clinical serum samples, which would provide a credible basis for the early diagnosis and treatment of breast cancer.
Conclusions
In summary, we presented a special UCL assay based on the sequence fragment-linked technique of target-induced aptamer and UCNPs as a marker for VEGF detection. The ultra-small and high-quality α-NaYF4:Yb3+, Er3+ UCNPs were synthesized by using RE stearates as precursors for thermal decomposition in an OA-ODE system. Hydrophilic DNA-UCNPs had been successfully realized by the replacement method of DNA. The UCL assay shows good stability, high selectivity, and exhibits ultrasensitive detection with an LOD as low as 6 pM, and the determination of VEGF in spiked serum shows good reproducibility. Furthermore, facing the major limitations, we will probably improve the luminescence efficiency of UCNPs by advanced methods (such as surface modification, core-shell structures, etc.) and increase efficiency of DNA functionalization in the future. Therefore, the sensitivity of our assay will be further improved after appropriate improvement. Thus, this assay has the potential to be used for trace analysis of VEGF and provides a credible assay for use in the early diagnosis of breast cancer, which makes it a viable tool for early diagnosis in the future.
References
Ławicki S, Zajkowska M, Głażewska EK, Będkowska GE, Szmitkowski M (2016) Plasmalevels and diagnostic utility of VEGF, MMP-9, and TIMP-1 in the diagnosis of patients with breast cancer. Onco Targets Ther 9:911–919
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246(4935):1306–1309
Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG (2001) VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 7(2):186–191
Thompson DF, Eborall W, Dinsmore A, Smith CJ, Duckett CJ (2010) Development and validation of a NANOGold immunoassay for the detection of vascular endothelial growth factor (VEGF) in human serum using inductively coupled plasma mass spectrometry. Rapid Commun Mass Spectrom 24(7):927–932
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Cho EJ, Lee JW, Ellington AD (2009) Applications of aptamers as sensors. Annu Rev Anal Chem 2:241–264
Mohammad AA, Ghosh G (2013) Sensitive quantification of vascular endothelial growth factor (VEGF) using porosity induced hydrogel microspheres. Biosens Bioelectron 49:105–110
Haase M, Schäfer H (2011) Upconverting nanoparticles. Angew Chem Int Ed 50(26):5808–5829
Yang Y (2014) Upconversion nanophosphors for use in bioimaging, therapy, drug delivery and bioassays. Microchim Acta 181(3–4):263–294
Boyer JC, Cuccia LA, Capobianco JA (2007) Synthesis of colloidal upconverting NaYF4: Er3+/Yb3+ and Tm3+/Yb3+ monodisperse nanocrystals. Nano Lett 7(3):847–852
Zhang H, Xu D, Huang Y, Duan XF (2011) Highly spectral dependent enhancement of upconversion emission with sputtered gold island films. Chem Commun (Camb) 47(3):979–981
Liu CH, Wang H, Li X, Chen DP (2009) Monodisperse, size-tunable and highly efficient β-NaYF4:Yb, Er (Tm) up-conversion luminescent nanospheres: controllable synthesis and their surface modifications. J Mater Chem 19(21):3546–3553
Choi HS, Liu WH, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV (2007) Renal clearance of quantum dots. Nat Biotechnol 25(10):1165–1170
Feng W, Sun LD, Zhang YW, Yan CH (2010) Synthesis and assembly of rare earth nanostructures directed by the principle of coordination chemistry in solution-based process. Coord Chem Rev 254(9–10):1038–1053
Ju Q, Tu DT, Liu YS, Li RF, Zhu HM, Chen JC, Chen Z, Huang MD, Chen XY (2012) Amine-functionalized lanthanide-doped KGdF4 nanocrystals as potential optical/magnetic multimodal bioprobes. J Am Chem Soc 134(2):1323–1330
Zhou J, Yao LM, Li CY, Li FY (2010) A versatile fabrication of upconversion nanophosphors with functional-surface tunable ligands. J Mater Chem 20(37):8078–8085
Liu YS, Tu DT, Zhu HM, Li RF, Luo WQ, Chen XY (2010) A strategy to achieve efficient dual-mode luminescence of Eu3+ in lanthanides doped multifunctional NaGdF4 nanocrystals. Adv Mater 22(30):3266–3271
Liu YS, Zhou SY, Tu DT, Chen Z, Huang MD, Zhu HM, Ma E, Chen XY (2012) Amine-functionalized lanthanide-doped zirconia nanoparticles: optical spectroscopy, time-resolved fluorescence resonance energy transfer biodetection, and targeted imaging. J Am Chem Soc 134(36):15083–15090
Mader HS, Kele P, Saleh SM, Wolfbeis OS (2010) Upconverting luminescent nanoparticles for use in bioconjugation and bioimaging. Curr Opin Chem Biol 14(5):582–596
Li LL, Zhang RB, Yin LL, Zheng KZ, Qin WP, Selvin PR, Lu Y (2012) Biomimetic surface engineering of lanthanide-doped upconversion nanoparticles as versatile bioprobes. Angew Chem Int Ed 51(25):6121–6125
Pinheiro AV, Han DR, Shih WM, Yan H (2011) Challenges and opportunities for structural DNA nanotechnology. Nat Nanotechnol 6(12):763–772
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382(6592):607–609
Li D, Song SP, Fan CH (2010) Target-responsive structural switching for nucleic acid-based sensors. Acc Chem Res 43(5):631–641
Dai S, Wu S, Duan N, Wang Z (2016) A luminescence resonance energy transfer based aptasensor for the mycotoxin Ochratoxin a using upconversion nanoparticles and gold nanorods. Microchim Acta 183(6):1909–1916
He Y, Ye T, Ribbe AE, Mao CD (2011) DNA-templated fabrication of two-dimensional metallic nanostructures by thermal evaporation coating. J Am Chem Soc 133(6):1742–1744
Li LL, Wu PW, Hwang K, Lu Y (2013) An exceptionally simple strategy for DNA-functionalized up-conversion nanoparticles as biocompatible agents for nanoassembly, DNA delivery, and imaging. J Am Chem Soc 135(7):2411–2414
Wang M, Liu JL, Zhang YX, Hou W, Wu XL, Xu SK (2009) Two-phase solvothermal synthesis of rare-earth doped NaYF4 upconversion fluorescent nanocrystals. Mater Lett 63(2):325–327
Li CX, Lin J (2010) Rare earth fluoride nano−/microcrystals: synthesis, surface modification and application. J Mater Chem 20(33):6831–6847
Wang SE, Huang Y, Hu K, Tian JN, Zhao SL (2014) A highly sensitive and selective aptasensor based on fluorescence polarization for the rapid determination of oncoprotein vascular endothelial growth factor (VEGF). Anal Methods 6(1):62–66
Freeman R, Girsh J, Jou AF, Ho JA, Hug T, Dernedde J, Willner I (2012) Optical aptasensors for the analysis of the vascular endothelial growth factor (VEGF). Anal Chem 84(14):6192–6198
Zhao S, Yang WW, Lai RY (2011) A folding-based electrochemical aptasensor for detection of vascular endothelial growth factor in human whole blood. Biosens Bioelectron 26(5):2442–2447
Acknowledgments
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (21375017 and 21105012), the National Science Foundation for Distinguished Young Scholars of Fujian Province (2013 J06003), the Natural Science Foundation of Fujian Province (2015 J01597 and 2016 J01685), the Medical Elite Cultivation Program of Fujian, P.R. C (2014-ZQN-ZD-26), the Key Project of Fujian Science and Technology (2013Y0045), Program for Fujian University Outstanding Youth Scientific Research (JA11105 and JA10295), Program for New Century Excellent Talents of Colleges and Universities in Fujian Province (JA13130) and Academic Foundation for Professor of Fujian Medical University (JS14009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lan, J., Li, L., Liu, Y. et al. Upconversion luminescence assay for the detection of the vascular endothelial growth factor, a biomarker for breast cancer. Microchim Acta 183, 3201–3208 (2016). https://doi.org/10.1007/s00604-016-1965-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1965-6