Abstract
A photometric method is described for the determination of free and total thiamine (TH; vitamin B1) in food samples. It is based on the use of gold nanoparticles (AuNPs) which aggregate in the presence of TH owing to the interaction of its sulfur atom with the AuNPs. This is accompanied by a color change from wine red to purple-blue and an increase in absorbance at 590 nm. The effect is not observed for TH phosphates which, however, can be determined, as demonstrated for the example of TH pyrophosphate as a model ester, by treating them with alkaline phosphatase (ALP) which hydrolyses such esters. The use of two sample aliquots, one with and one without ALP, allows the determination of free and total TH, respectively. The dynamic range extends from 0.15 to 3.5 μM, and the detection limit is 54 nM of TH. The method has been applied to the analysis of spiked food samples and gave recoveries that ranged between 88.8 and 100.7 %.

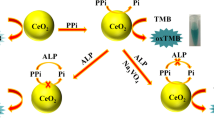
Free thiamine can be directly determined by photometry at 590 nm as a result of the aggregation of gold nanoparticles (1st aliquot), whereas total thiamine requires the previous hydrolysis of the phosphate esters using alkaline phosphatase (2nd aliquot). Photograph: Gold nanoparticles in the absence and in the presence of thiamine (in duplicate).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin B1, a water-soluble vitamin, can be present in foodstuff and animal tissue in four forms, viz. free thiamine (TH), thiamine mono- (THMP), pyro-(THPP), and tri-phosphate (THTP) [1]. Also, the presence of adenosine THTP has been described [2]. THPP is the best known ester that acts as an enzymatic cofactor, playing a vital role in carbohydrate metabolism. It is a coenzyme of three important enzymes, pyruvate dehydrogenase, α-ketoglutarate dehydrogenase and trans-ketolase, which have a fundamental function for intracellular glucose metabolism [3]. THMP is a product of the enzymatic hydrolysis of THPP and has no known physiological function [2], while THTP is involved in the regulation of ion channels in the nervous system [4]. The deficiency of TH causes deleterious changes in the cardiovascular and nervous systems that are effectively reversed by administration of the vitamin [5]. Also, the relationship between TH and diabetes has been widely discussed, finding low plasma TH in diabetic patients [6].
The different activities and stabilities of the different TH forms in food justify the interest in TH speciation. The classical method for TH determination is based on its oxidation to the fluorescent thiochrome by potassium hexacyanoferrate in an alkaline medium. Several liquid chromatographic (LC) methods have been reported for the determination of free TH and its phosphate esters by using either precolumn or postcolumn derivatization to thiochrome and fluorometric detection [3, 4, 7–10]. A wide variety of chromatographic modes, such as reversed-phase and ion pair chromatography, and isocratic and gradient elution techniques, have been employed in these fluorometric methods. A chromatographic method for total TH determination involves the dephosphorylation of TH esters using enzymatic hydrolysis and precolumn derivatization to thiochrome [10]. Total TH has been also determined using enzymatic hydrolysis and LC with photometric detection [11], which avoids the derivatization step, but the detection limit obtained is higher than those reached using luminescence detection. Another LC method is based on the enhancing effect of the photoproducts, generated on-line from the postcolumn photolysis of TH and its phosphate esters, on the luminol-permanganate chemiluminescence reaction [12].
Several methods for TH determination have been described using different nanoparticles as reagents, such as gold [13, 14], silica [15], quantum dots (QDs) [16, 17], and europium-doped yttrium oxide (Y2O3:Eu) [18]. The strong affinity of the sulfur present in TH toward gold nanoparticles (AuNPs), which gives rise to the aggregation of the NPs, has been used for the development of a method for TH determination using resonance Rayleigh scattering as detection system [13]. The use of AuNPs functionalized with 4-amino-6-hydroxy-2-mercaptopyridine has enabled the fluorometric detection of free TH [14]. Another method for TH determination is based the use of an iron phthalocyanine complex entrapped in silica NPs, which catalyzes the oxidation of TH to thiochrome by hydrogen peroxide [15]. The interaction of TH with QDs has given rise to the development of both quenching and enhancement luminescence methods, using CdSe QDs [16] and CdTe nanorods modified with thioglycolic acid and cysteine [17], respectively. The increase in the fluorescence of Y2O3:Eu NPs modified with captopril in the presence of TH has been applied to the determination of TH [18]. Table 1 summarizes some features of these methods, in which can be seen that they have been only devoted to free TH determination. Also, most of these methods have been only applied to the analysis of pharmaceutical samples, which have a very simple matrix, but their potential application in the presence of more complex matrices, such as food samples, has not been reported.
The method described here involves the use of AuNPs and alkaline phosphatase (ALP) for the fast and simple determination of both free and total TH. Although the use of resonance Rayleigh scattering as detection system reaches a low detection limit for free TH [13], its use was discarded because the presence of ALP to determine total TH caused a high increase in the blank signal. However, the development of the method has been possible using photometric detection. A microplate format using an automatic reader, which allows the consumption of very low sample and reagents volumes and reaches a high sample throughput, has been used for this purpose. The practical usefulness of the method has been shown by its application to the analysis of food samples.
Experimental
Instrumentation
A 1420 Multilabel counter Victor 3V microplate reader (Perkin Elmer and Analytical Sciences, Wallac Oy, Turku, Finland, www.perkinelmer.com) was used to perform absorbance measurements. A filter (nominal wavelength/passband) was used to select the absorption wavelength (590 nm/8 nm). A PerkinElmer Lambda 35 UV/VIS spectrometer (Perkin Elmer, Beaconsfield, UK, www.perkinelmer.com) was used to obtain absorbance spectra. A desktop laboratory MPW-350R centrifuge (MPW Med. Instrument, Warsaw, Poland, http://mpw.pl) with cooled chamber, and equipped with an HSL-11,199 angle rotor 45o, 12 × 12 × 1.5 mL, maximum speed =18,000 rpm, 30,065 RCF) was used. DELFIA microtitration plates from Wallac Oy (Turku, Finland) with a total volume of 350 μL were used to obtain the analytical measurements.
Reagents
All reagents were of analytical grade. Gold(III) chloride trihydrate was obtained from Aldrich (Aldrich, Milwaukee, USA), and trisodium citrate dihydrate and tris(hydroxymethyl) aminomethane (Tris) from Merck (Darmstadt, Germany, http://www.merck.de). TH was supplied by Fluka (Buchs, Switzerland, http://www.sigmaaldrich.com) and THPP, takadiastase from Aspergillus oryzae, magnesium chloride anhydrous and zinc chloride by Sigma (St. Louis, USA, https://www.sigmaaldrich.com). Copper sulphate pentahydrate, iron chloride and perchloric acid were obtained from Panreac (Castellar del Vallès, Spain www.panreac.es). Aqueous solutions were prepared using deionized water purified with a Milli-Q system (Millipore, Bedford, Ma, USA, www.merckmillipore.com/LabWater). ALP from bovine intestinal mucosa was supplied by Sigma. ALP stock solution (10,118 U mL−1) was prepared in 10 mM Tris buffer (pH 8) containing 5 mM magnesium chloride, 0.2 mM zinc chloride and 50 % glycerol. This ALP solution was kept at 4 °C. ALP working solutions (253 U mL−1) were daily prepared in 100 mM Tris buffer (pH 8) containing 5 mM magnesium chloride and 0.2 mM zinc chloride. The same Tris buffer was used to prepare the TH and THPP solutions.
Procedures
Synthesis of gold nanoparticles
The synthesis was performed according to the Turkevich method [19]: 5 mg of HAuCl4 trihydrate were dissolved in 47.5 mL of deionized water in a round-bottom flask. A volume of 2.5 mL of 3.4 × 10−2 M trisodium citrate dihydrate solution was added drop wise in two min and then was vigorously stirred under reflux at 100 °C for 30 min. The final aqueous solution, containing approximately 1 nM AuNPs, was kept stable in refrigerator at 4 °C until its use. The AuNPs concentration was calculated by dividing the initial concentration of HAuCl4 added over the average number of atoms per nanosphere, assuming a mean AuNP diameter of 20 nm [20].
Determination of free and total thiamine
Two sample aliquots were used for the determination of free and total TH. Free TH determination was carried out by mixing 50 μL of TH standard or sample solution, in a concentration range of 0.60–14.0 μM, with 100 μL of 1 nM AuNPs solution and 50 μL of Tris buffer (100 mM Tris, pH 8.0, 5 mM MgCl2, 0.2 mM ZnCl2). The mixture was incubated at 40 °C for 15 min in the multiplate reader and the absorbance was measured at 590 nm. The determination of total TH was carried out using a similar procedure but the Tris buffer was replaced by 50 μL of 253 U mL−1 ALP solution, which was prepared in the same Tris buffer.
Analysis of samples
Three food samples (pork meat, pork sausages and oat flakes), purchased from a local market, were analyzed. The determination required an acid hydrolysis step to release TH and its phosphate esters from the sample matrix. Each sample was weighed (1 g) and mixed with 3 mL of 0.4 M HClO4. The mixture was shaken using a magnetic stirrer at 500 rpm for 2 min and centrifuged at 20,670 RCF for 10 min. The pH of 2 mL of the supernatant was increased to about 8.0 using 2 M sodium hydroxide and the solution was diluted to 4 mL using the Tris buffer above described. Two aliquots (50 μL) of this solution were treated as described above.
Results and discussion
Photometric study of the system
The absorption spectra obtained for AuNPs alone and in the presence of ALP, TH, THPP, and THPP - ALP mixture are shown in Fig. 1. As can be seen, all the spectra show a maximum at 520–540 nm, corresponding to the typical plasmon band of the NPs. The presence of ALP or THPP does not modify the AuNPs spectrum, which demonstrates the absence of interaction of these species with the NPs. However, the spectra obtained in the presence of TH and THPP-ALP show a shoulder at about 590–600 nm, which indicates the aggregation of the AuNPs. Figure 1 also shows a photograph of AuNP solutions in the absence and in the presence of TH (in duplicate), where the change of color can be observed.
Optimization of the system
The experimental variables involved in the system were optimized using the univariate method and measuring the absorbance at 590 nm. The optimum values chosen were those providing the best absorbance values with the minimum standard deviation.
The following parameters were optimized: (a) choice of enzyme and its optimum activity; (b) temperature and time of enzyme incubation; (c) sample pH value; (d) choice of buffer and its optimum concentration; and (e) AuNPs concentration. Respective data and Figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) ALP was used at an activity value of 63.2 U mL−1 in the mixture; (b) 37 °C and 15 min were chosen as temperature and incubation time; (c) the sample pH was adjusted at 8.0 using (d) a 50 mM Tris buffer containing magnesium and zinc chlorides, in order to avoid the potential negative effect that phosphate buffer may have on AuNP aggregation and on ALP activity. Finally, (e) a 1 nM AuNP concentration was chosen for the development of the method.
Analytical features of the method
The calibration graph was obtained under the optimum experimental conditions and measuring the absorbance at 590 nm. Free TH was determined by interpolating in the calibration graph the absorbance of TH standard solutions in the absence of ALP, while total TH concentration was obtained using TH or THPP standard solutions in the presence of ALP. The difference between total and free TH is ascribed to the concentration of TH phosphate esters, expressed as THPP. The blank signal was subtracted from the absorbance values obtained for all the standards. The calibration curve was adjusted by linear regression, obtaining the calibration equation, A = 0.054 (± 0.003) + 0.134 (± 0.002) c, in which c is the TH concentration expressed as micromolar. The dynamic concentration range was of 0.15–3.5 μM (in the reaction mixture), and the correlation coefficient was 0.9996, which is indicative of the good linearity of the calibration curve. The detection limit, calculated according to IUPAC recommendations [21], was 54 nM. The precision of the method, expressed as the percentage of relative standard deviation (% RSD) and studied at two concentration levels of TH and THPP, 0.15 μM and 1.0 μM, was 2.3 % and 2.1 %, respectively, for TH, and 2.5 % and 2.3 %, respectively, for THPP.
The selectivity of the method was studied by assaying the potential effect of other water-soluble vitamins on the system. The presence of riboflavin, ascorbic acid, nicotinic acid and nicotinamide, at concentration levels 100-fold that of the analytes (1.5 μM), did not cause any effect on the method. Also, the system was not interfered by the presence of metal ions such as Cu(II) and Fe(III) at concentration levels 3-fold and 30-fold, respectively, that of the analytes.
Applications
Three food samples were chosen to study the applicability of the method: pork meat and sausage, which contain TH and TH phosphate esters, and oat flakes, which only contain TH. Several procedures have been described for the separation of TH and its phosphate esters from the food sample matrix, which involve a previous acid treatment. Hydrochloric acid is usually used for this purpose [1, 10–12, 22], including, in some instances, a trichloroacetic acid treatment for sample deproteinization [10, 12, 22]. However, a simpler and shorter treatment has been described using perchloric acid [7], which only requires a centrifugation step. Both treatments were assayed for the analysis of the samples, obtaining better recovery results when perchloric acid was used.
Table 2 shows the results obtained, in which can be seen that free TH and TH phosphate esters, expressed as THPP, were found in the two meat samples, but, as it was expected, only free TH was found in the sample of oat flakes. Although it has been described that the concentration of TH phosphate esters in fresh pork meat is relatively high, these esters undergo dephosphorylation during storage, being free TH the main fraction [9]. The TH contents found in the samples analyzed are in agreement with those described for the analysis of similar samples [10, 11, 22]. Table 2 also includes the recovery study, which was performed by adding two amounts of TH to each sample and two amounts of THPP to the meat samples. The recovery percentages obtained ranged from 88.8 % to 100.7 % and the mean recoveries for TH and THPP were 95.8 % and 91.2 %, respectively.
Conclusions
The results obtained show the usefulness of the combined use of AuNPs and ALP for the determination of free and total TH in food samples. This method can be seen as a fast and simple alternative to the chromatographic methods described for this purpose, although it cannot quantify the individual concentration of each TH phosphate ester. However, it allows the discrimination of the two main TH forms avoiding the chromatographic separation, with involves the use of organic solvents, and reducing the time required to obtain analytical results. The use of a multiplate reader allows a low consumption of sample and reagents and the automation of measurements, as well as the achievement of a high sample throughput since 32 samples or standards can be simultaneously analyzed after 15 min incubation. Although different nanoparticles have been previously used for TH determination (Table 1), these methods only allow the quantification of free TH, whereas the method described here shows the usefulness of nanotechnology for TH speciation in food samples. The change in the color of the AuNPs observed when TH or its esters are present in the sample would enable the use of this system for the development of a visual colorimetric method, similar to other approaches recently described using nanomaterials, such as AuNPs [23] or quantum dots [24].
References
Yoshida M, Hishiyama T, Ogawara M, Fuse K, Mori M, Igarashi T, Taniguchi M (2012) A novel method for determining vitamin B1 in a wide variety of foodstuffs with or without polyphenols. Food Chem 135:2387–2392
Bettendorff L, Wins P (2009) Thiamin diphosphate in biological chemistry: new aspects of thiamin metabolism, especially triphosphate derivatives acting other as cofactors. FEBS J 276:2917–2925
Pinto E, Pedersén M, Snoeijs P, Van Nieuwerburg L, Colepicolo P (2002) Simultaneous detection of thiamine and its phosphate esters from microalgae by HPLC. Biochem Biophys Res Commun 291:344–348
Lu J, Fran EL (2008) Rapid HPLC measurement of thiamine and its phosphate esters in whole blood. Clin Chem 54:901–908
Shenkin A, Baines M, Fell GS, Lyon TD (2006) Vitamins and trace elements. In: Burtis CA, Ashwood ER, Bruns DE (eds) Tietz textbook of clinical chemistry and molecular diagnostics, 4th edn. Elsevier Saunders, St. Louis, pp. 1075–1164
Luong KVQ, Nguyen LTH (2012) The impact of thiamine treatment in the diabetes mellitus. J Clin Med Res 4:153–160
Batifoulier F, Verny MA, Besson C, Demigné C, Rémésy C (2005) Determination of thiamine and its phosphate esters in rat tissues analyzed as thiochromes on a RP-amide C16 column. J Chromatogr B 816:67–72
Lynch PLM, Young IS (2000) Determination of thiamine by high-performance liquid chromatography. J Chromatogr A 881:267–284
Poel C, Bäckermann S, Ternes W (2009) Degradation and conversion of thiamin and thiamin phosphate esters in fresh stored pork and in raw sausages. Meat Sci 83:506–510
Viñas P, López-García I, Bravo-Bravo M, Briceño M, Hernández-Córdoba M (2012) Dispersive liquid-liquid microextraction coupled to liquid chromatography for thiamine determination in foods. Anal Bioanal Chem 403:1059–1066
Gratacós-Cubarsí M, Sárraga C, Clariana M, García Regueiro JA, Castellari M (2011) Analysis of vitamin B1 in dry-cured sausages by hydrophilic interaction liquid chromatography (HILIC) and diode array detection. Meat Sci 87:234–238
Pérez-Ruiz T, Martínez-Lozano C, García-Martínez MD (2009) Simultaneous determination of thiamine and its phosphate esters by a liquid chromatographic method based on post-column photolysis and chemiluminescence detection. J Pharm Biomed Anal 50:315–319
Liu S, Chen Y, Liu Z, Hu X, Wang F (2006) A highly sensitive Rayleigh scattering method for the determination of vitamin B1 with gold nanoparticles probe. Microchim Acta 154:87–93
Shankar S, John SA (2015) Sensitive and highly selective determination of B1 in the presence of other vitamin B complexes using functionalized gold nanoparticles as fluorophore. RSC Adv 5:49920–49925
Zou F, Chen X (2007) Using silica nanoparticles as a catalyst carrier to the highly sensitive determination of thiamine. Microchem J 86:42–47
Sun J, Liu L, Ren C (2008) A feasible method for the sensitive and selective determination of vitamin B1 with CdSe quantum dots. Microchim Acta 163:271–276
Li Y, Wang P, Cao M, Xia YS, Cao C, Liu MG, Zhu CQ (2010) An immediate luminescence enhancement method for determination of vitamin B1 using long-wavelength emitting water-soluble CdTe nanorods. Microchim Acta 169:65–71
Moghaddam AB, Gudarzy F, Ganjkhanlou Y (2014) A fluorescent probe for detecting thiamine using the luminescence intensity of nanoparticles. J Fluoresc 24:1025–1030
Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A (2006) Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B 110:15700–15707
Liu X, Atwater M, Wang J, Huo Q (2007) Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf B 58:3–7
Long GL, Winefordner JD (1983) Limit of detection. A closer look at the IUPAC definition. Anal Chem 55:712A–724A
Tang X, Cronin DA, Brunton NP (2006) A simplified approach to the determination of thiamine and riboflavin in meats using reverse phase HPLC. J Food Compos Anal 19:831–837
Liu W, Zhang D, Zhu W, Zhang S, Wang Y, Yu S, Liu T, Zhang X, Zhang W, Wang J (2015) Colorimetric and visual determination of total nereistoxin-related insecticides by exploiting a nereistoxin-driven aggregation of gold nanoparticles. Microchim Acta 182:401–408
Durán GM, Benavidez TE, Ríos A, García CD (2015) Quantum dot-modified paper-based assay for glucose screening, Microchim Acta, published online, 7 december doi:10.1007/s00604-015-1685-3.
Acknowledgments
Authors gratefully acknowledge financial support from the MICINN (Grant No. CTQ2012-32941), from the Junta of Andalucía (Grant No.P09-FQM4933) and from the FEDER Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 59 kb)
Rights and permissions
About this article
Cite this article
Molina-Delgado, M.Á., Aguilar-Caballos, M.P. & Gómez-Hens, A. Simultaneous photometric microplate assay for free and total thiamine using gold nanoparticles and alkaline phosphatase. Microchim Acta 183, 1385–1390 (2016). https://doi.org/10.1007/s00604-016-1767-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1767-x