Abstract
A multisyringe flow injection system with spectrophotometric detection is presented as a fast, robust and low-reagent consumption system for the determination of azinphos methyl (AzMe) in water samples. Determination is based on the Griess reaction. The analyte is hydrolyzed and the reaction product reacts with nitrite in acid medium to form the diazonium salt that reacts further with 1-naphtol reagent to produce an azo compound. The azo derivative is spectrophotometrically monitored at 485 nm. The influence of several chemical and flow variables has been investigated. Under the optimum analytical conditions, the linearity of the calibration curve for AzMe ranges from 1 to 32 μg mL−1. The detection limit is 0.17 µg mL−1, and recoveries between 95 to 109% have been obtained. The repeatability (RSD) is 0.8% for a 10 μg mL−1 solution, and the injection throughput is seven samples h−1. The system has been satisfactorily applied to the determination of AzMe in spiked river and dam water samples. The results were in agreement at the 95% confidence level with those obtained by HPLC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Azinphos methyl (AzMe, S-(3,4-dihydro-4-oxobenzo[1,2,3-d]-triazin-3-ylmethyl) is an organophosphate insecticide widely used in Mexico for pest control on a number of food crops [1, 2]. Its half-life is approximately 26 days at 30°C and pH 7, and it can reach watercourses via runoff and spray drift [2, 3]. AzMe has a relatively high water solubility of 28–29 μg mL−1 and its residues have been detected in ground or surface waters [2, 4–6]. The pesticide may also exhibit a high toxicity to non-target aquatic species. Several episodes of massive fish mortality have been reported in the last decade [2, 7, 8]. This pesticide is thus of significant environmental concern; therefore, it is necessary to develop simple, rapid and accurate analytical methods for routine screening purposes.
Several analytical methods have been published on the determination of AzMe in environmental and water samples and most of them are based on gas chromatographic techniques [9–13]. Other methods such as liquid chromatography with mass spectrometry [14], voltammetry [15], electrochemical methods [16], and enzyme immunoassay [17, 18] are often found in the published reports. Each of these approaches suffers from several disadvantages such as being costly, time consuming, and requiring complex sample preparation, especially when routine work has to be performed on large number of samples. These assays are also susceptible to operational errors (e.g., inadequate mixing of samples or reagents, or poor reproducibility regarding time events). Automation can overcome these limitations. In this context, multisyringe flow injection analysis (MSFIA) that is one of the most recent flow analysis techniques [19], allow the delivery of low (µL magnitude), precise volumes with reproducible flow rate and high versatility concerning liquid management. The versatility is conferred by the multi-channel capability of this liquid driver, where up to 4 channels can be operated simultaneously, and allowing selective introduction of solutions into the flow network. Compared to other flow techniques, MSFIA allows versatile flow management, including flexible sample manipulation, excellent precision on timing events and capacity to accommodate a variety of assays in the same manifold by changing the parameters using software control [19–21].
In this work, a coupled multisyringe flow injection/reactor tank system is presented for the spectrophotometric determination of AzMe in water samples. Determination is based on the Griess reaction [22, 23]. The multysyringe flow system relies on on-line hydrolysis of AzMe, followed by generation of a diazonium salt and diazo coupling to form an azo dye that is spectrophotometrically determined at 485 nm.
Experimental
Reagents and solutions
Analytical grade reagents and double-deionized water (DDI water, resistivity >18 MΩ cm) from Milli-Q system (Millipore, Bedford, MA, USA) was used to prepare all the solutions. A stock solution of AzMe was prepared by dissolving 10 mg of AzMe (Riedel-de Haën AG, Seeize, Germany) in 50 mL of methanol. Appropriate AzMe working standard solutions were prepared by direct dilution with DDI water. A 10−3 mol L−1 1-naphthol (E. Merck, Darmstadt, Germany) solution was prepared by dissolving 14 mg of 1-naphthol in 100 mL of 0.2 M NaOH. 0.4% (w/v) Ammonium sulfamate (Sigma-Aldrich, St. Louis, USA) and 0.4% (w/v) sodium nitrite (J.T. Baker, Xalostoc, Mexico) solutions were prepared in DDI water. 0.6 mol L−1 HCl solution (J.T. Baker, Xalostoc, Mexico) was used.
Flow system, optical detector and software
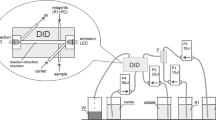
The coupled MSFIA/reactor tank system for the determination of AzMe by diazotation reaction is presented in Fig. 1. The manifold consisted of two multisyringe modules (BU-4-S, Crison, Alella, Barcelona), with a total of six syringes in use (S) (Hamilton Bonaduz AG, Switzerland), each connected at the head to a three-way solenoid valve (V) (N-Research, Caldway, NJ, USA). Additionally, two external solenoid valves (V7 and V8) were added. The valve module was equipped with an 8-port multi-position selection valve (MPSV). An open mini-reactor tank (RT, 3 mL, 1.5 cm internal diameter (i.d.)) operated at the atmospheric pressure under continuous agitation with a magnetic stirrer bar was incorporated in the manifold in order to allow a better mix between sample and reagents, and avoid the formation of gas bubbles during the formation of diazonium salt.
All tubes connecting the different components of the set up were made of polytetrafluoroethylene (PTFE, Omnifit, Cambridge, UK) with 0.8 mm and 1.5 mm i.d. and end fittings and connectors were also used (Gilson, Villiers-le-Bel, France). The two holding coil were 100 cm long. The remaining reagents were loaded and supplied directly with syringes. All connections were carried out by means of PVC connectors.
As detection system, an Ocean Optics SD2000 (Dunedin, FL., USA) spectrophotometer with a diode array LED as light source (LED, maximum intensity of 15 mA, Sciware, Palma de Mallorca, Spain), a pair of 400 µm of i.d. fiber optic cable (P400-2-UV-VIS, Ocean Optics, Dunedin, FL, USA), a flow-through cell (Hellma 178.711-QS, 18 µL inner volume, 1 cm optical path) was used. System control, data acquisition and processing were performed using the software package Autoanalysis v 5.0 (Sciware, Palma de Mallorca, Spain) [24]. The spectrophotometric measurement of azo-derivative compound was carried out at the wavelength of 485 nm. Reference wavelength for minimizing the schlieren effect was set at 750 nm.
MSFIA procedure for AzM monitoring
The flow system evaluated for AzMe determination in water samples is depicted in Fig. 1. Syringes S2, S3, S4 and S5, with their commutation valves V2, V3, V4 and V5, contained, respectively, hydrochloric acid (HCl), sodium nitrite (NaNO2), ammonium sulfamate (AS) and 1-naphthol (1-Nph) solutions. S6 and its commutation valve V6 contained the carrier (DI water), that was used to wash the manifold system and RT between samples. The six syringes had a capacity of 5 mL. The sequence adopted for the spectrophotometric determination of AzMe is illustrated in Table 1. Initially, the syringes were filled with solutions from their respective reservoirs at 5.0 mL min−1. S1 and its commutation valve V1 was used for loading and dispensing standard or sample, and sodium hydroxide (NaOH) through the MPSV (ports 3 and 5, respectively) into the holding coil 1 (HC-1) where the hydrolysis reaction of AzMe took place. The port 2 of the MPSV was used to deliver a know volume of the hydrolyzed AzMe from the HC-1 to the RT through the V7 (on position). In “off” position, the V7 allowed to dispense a certain volume of the dead volume of the previous sample directly into the waste reservoir, as well as to rinse the RT with carrier (DDI water). Afterwards, a total volume of 3.6, and 0.4 mL of HCl, NaNO2, AS and 1-Nph, respectively, were aspirated to the RT via V7 valve in the “on” position mode to generate the azo-derived compound. Then, the reaction product was propelled through the holding coil 2 (HC-2) via V8 valve in the “off” position. In the final step the resulting colored product was transported from HC-2 via S6 syringe and V8 valve in the “on” position to the detector and the analytical signal was registered at 485 nm. All the measurements were performed five times for each solution. The concentration of AzMe in the water samples was calculated from the peak height absorbance at 485 nm. Finally, the manifold system was rinsed with carrier (S6 syringe) using V6 valve in “on” position and V8 valve in “off” position. After this step, the flow system was ready for a new analytical cycle.
RP-HPLC comparison
The concentration of AzMe in the analyzed samples was also determined for comparative purposes using RP-HPLC, the official chromatographic standard technique used in AzMe analyses [25]. The apparatus used was an Agilent Model 1200 series LC instrument equipped with UV-Vis detector at 286 nm. Chromatographic separation was achieved with a Du Pont Zorbax ODS column (250 mm × 4 mm, 5 µm i.d.). The mobile phase was acetonitrile-water (65:35) and flow rate of 1 mL min−1. The sample injection volume was 5 μl. In all the cases, the analytical signal measured was peak area.
Sample conditions
Surface water samples from river and dams from Guanajuato state were analyzed. Samples were filtered due to high content of suspended solids present and then, were fortified with AzMe concentrations of 2, 16 and 32 µg mL−1. The amount of AzMe was determined according to aforesaid procedure.
Results and discussion
The proposed MSFIA-spectrophotometric method for the determination of AzMe was based on the Griess reaction [22, 23]. The antranilic acid (hydrolyzed AzMe compound) was converted to dizonium salt by reaction with HCl and NaNO2 followed by diazotation and coupling reaction with 1-Nph to give a colored product that was monitored at 485 nm. The MSFIA chemical and physical factors affecting color development, reproducibility; sensitivity and conformity with Beer’s law were investigated. The MSFA parameters used in the determination of AzMe were optimized by the univariated method with the purpose of maximizing the analytical frequency and reproducibility. The parameters studied were: sample and reagent concentrations and volumes, and reagent flow rates. Each parameter was measured in triplicate (n = 3).
Chemical parameters
The chemical parameters investigated were NaOH, HCl, AS and 1-Nph concentrations.
-
a)
Alkaline hydrolysis of AzMe. The anthranilic acid was generated during the alkaline hydrolysis of AzMe compound and the reaction can be carried out under pH controlled conditions at room temperature. The effect of NaOH concentration upon the analytical response of the flow system was examined in the concentration range from 3 to 10% (w/v). Maximum response was obtained at 5% (w/v).
-
b)
Reagent concentrations. The effect of reagent concentrations in the Griess reaction was studied. In Fig. 2 is illustrated the influence of the HCl, NaNO2 and AS concentrations. Firstly, the diazonium salt was formed when the antranilic acid reacted with NaNO2 in an acidic medium, so the effect of HCl concentration was examined in the range of 0.1–1.2 mol L−1. As the concentration of HCl was increased, analytical signal was increased up to 0.4 mol L−1 above which remained constant. Therefore, 0.4 mol L−1 HCl was chosen for subsequent studies. Concentrations of NaNO2 within the range 0.1–1.2% (w/v) were assayed. The peak height absorbance increased with increasing the reagent concentration up to 0.4% (w/v). Thus, a concentration of 0.4% (w/v) was chosen for subsequent experiments since it provided the highest signal and a good repeatability. However, during the generation of diazonium salt, bubble generation was observed due to the nitrogen dioxide production that could interfere with the spectrophotometric determination. For this reason, the use of a reactor tank was required. On the other hand, the excess of NaNO2 was removed by the addition of AS. The dependence of the AS concentration was evaluated within the range 0.4–3.0% (w/v). The analytical signal at 485 nm remained constant; therefore 1.0% (w/v) AS was adopted for further experiments. The second step of the Griess reaction was the diazotation/coupling reaction of the diazonium salt with 1-Nph to generate the azo compound. Thus, the effect of 1-Nph was evaluated in the range from 10−3 to 10−2 mol L−1. The peak height remained constant with increase of 1-Nph concentration. Therefore, a 10−3 mol L−1 1-Nph was chosen. Reaction time to generate the azo-compound was assayed within the range 0.0–210 s in order to investigate its effect on signal response. Peak height absorbance was constant in the range 150–200 s and decreased at reaction times below and above these values. The optimum reaction time was chosen as 180 s since it gave the best intensity together with a good repeatability.
-
c)
Hydrodynamic parameters. It is known that low flow rates can originate small and enlarged peaks due to a high dispersion of the sample. On the other hand, at very high flow rates the reaction may not progress enough to give an adequate signal-to-noise ratio. Obviously, an optimum flow rate will depend on the sample volume. An optimized value for each variable must be taken to be that resulting in a good compromise among peak high, calibration slope, reproducibility and baseline stability. The effect of injection flow rates of the reagents HCl, NaNO2, AS, 1-Nph and sample was studied in the range of 1–20 mL min−1. The flow rates of sample and reagents had not significant effect in the analytical signal. The RSD was less than 1.5% for the studied parameters. This could be attributed to the use of the stirred reactor tank operated in semi batch mode during the coupling reaction. Thus, the flow rates of 15, 10, 10, 10 and 8 mL min−1 were chosen for HCl, NaNO2, AS, 1-Nph and sample, respectively.
The influence of volumes of reagents (HCl, NaNO2, AS and 1-Nph) on the formation of the azo-derived compound were studied (Fig. 3). Different sample volumes within the range 0.1–0.5 mL were tried for HCl, NaNO2 and AS in order to investigate their effect on analytical signal. The peak height absorbance increased with increasing volume. Nevertheless, it was noticed that the effect of HCl volume was not significant above 0.2 mL. Therefore, a sample volume of 0.2 mL was chosen for subsequent experiments. NaNO2 and AS volumes were found to be optimum at 0.15 and 0.3 mL, respectively. On the other hand, the required volume of 1-Nph was higher compared to the volume used for the other reagents. 1-Nph varied between 0.4 and 1.2 mL. The signal intensity increased with increasing 1-Nph volume. Maximum signal intensity was observed between 0.6 and 1.0 mL. 1 mL 1-Nph was used for further experiments. The optimal operation conditions are summarized in Table 2.
Analytical performance
Under the optimized chemical and physical variables, the dynamic linear range of the MSFIA method was established over the range 1–32 μg mL−1. The calibration curve equation was A = (0.0222 ± 0.0007) [AzMe] + (0.0183 ± 0.0080) with a coefficient of regression (r) of 0.9997. The limits of detection (LOD) and quantitation (LOQ) were estimated as 3 and 10 times the standard deviation of the absorbance for 10 injections of the blank and found to be 0.17 and 0.57 μg mL−1, respectively. The repeatability (RSD) calculated from ten replicates using 1 mL injections of 2, 16 and 32 μg mL−1 concentrations were 3.7, 1.8 and 0.6 %, respectively. A sample throughput of seven samples per hour for a sampling volume of 1 mL was achieved.
Analysis of water samples
For the evaluation of matrix influences, river and dam water samples were spiked each with 2, 16 and 32 µg mL−1 AzMe, afterwards filtered through 0.45-μm nylon membrane filters and directly analyzed. The blank concentrations of all samples were below the detection limit. Analytical results and recovery values, calculated using peak height data compared to aqueous standards are given in Table 3. Recoveries ranging from 95 to 109% were obtained for the entire set of samples analyzed indicating the nonexistence of multiplicative matrix interferences. As an alternative methodology, a liquid chromatographic method [25] was chosen. Recoveries and RSDs for in HPLC ranged between 94–99% and 0.3–2.4%, respectively. No statistical differences were observed between the results obtained by the developed MSFIA system and the standard HPLC method using a t-test comparison of the means [26] at a significance level of 0.05 since the t critical value was higher than that of t experimental for the entire set of samples analyzed.
Conclusions
The above experiments demonstrated the viability of the determination of AzMe in water samples using a MSFIA assembly with spectrometric detection. The Griess reaction was carried out in a continuous stirred tank reactor in order to avoid undesirable bubble formation. The simplicity, low cost and sample throughput (seven samples per hour in a triplicate analysis) with good precision (RSD of 0.8 %, n = 20) and accuracy, ranked the proposed method competitive for routine analysis of AzMe in wastewater samples and drainage water samples from production plant. The main advantages of the developed method compared to the HPLC method were high sample throughput, low cost analysis and automatization of the entire procedure.
References
Flocco CG, Carranza MP, Carvajal LG, Loewy RM, Pechen de D’Angelo AM, Giulietti AM (2004) Removal of azinphos methyl by alfalfa plants (Medicago sativa L.) in a soil-free system, Sci. Total Environ 327:31–39
USEPA (2001) Interim registration eligibility decision for azinphos-methyl. Case No. 0235. <http://www.epa.gov./REDs/azinphosmethyl_ired.pdf>
Schulz R, Peall SKC, Dabrowski JM, Reinecke AJ (2001) Spray deposition of two insecticides into surface waters in a South African orchard area. J Environ Qual 30:814–822
Loewy RM, Carvajal LG, de D’Angelo AM Pechen (2003) Residuos de plaguicidas en efluentes de industrias agroalimentarias y aguas superficiales. First book of Latinoamerican Society of Environmental Toxicology and Chemistry, SETAC, Buenos Aires, pp 193–195
Loewy RM, Carvajal LG, Novelli M, Pechen de D’Angelo AM (2003) Effect of pesticide use in fruit production orchards on shallow ground water. J Environ Sci Health Part B 238:317–325
Ragnarsdottir VK (2000) Environmental fate and toxicology of organophosphate pesticides. J Geol Soc Lond 157:859–876
Gormley KL, Teather KL, Guignion DL (2005) Changes in salmonid communities associated with pesticide runoff events. Ecotoxicology 14:671–678
Schulz R, Hahn C, Bennett ER, Dabrowski JM, Thiere G, Peall SKC (2003) Fate and effects of azinphos-methyl in a flow-through wetland in South Africa. Environ Sci Technol 37:2139–2144
Liapis KS, Aplada-Sarlis P, Kyriakidis NV (2003) Rapid multi-residue method for the determination of azinphos methyl, bromopropylate, chlorpyrifos, dimethoate, parathion methyl and phosalone in apricots and peaches by using negative chemical ionization ion trap technology. J Chromatogr A 996:181–187
Avramides EJ, Gkatsos S (2007) A multiresidue method for the determination of insecticides and triazine herbicides in fresh and processed olives. J Agric Food Chem 55:561–565
Sanchez-Brunete C, Albero B, Miguel E, Tadeo JL (2002) Determination of insecticides in honey by matrix solid-phase dispersion and gas chromatography with nitrogen-phosphorus detection and mass spectrometric confirmation. J AOAC Int 85:128–133
Lehotay SJ (2000) Analysis of pesticide residues in mixed fruit and vegetable extracts by direct sample introduction/gas chromatography/tandem mass spectrometry. J AOAC Int 83:680–697
Sabik H, Fouquet A, Proulx S (1997) Ultratrace determination of organophosphorus and organonitrogen pesticides in surface water. Analusis 25:267–273
Lacorte S, Barcelo D (1996) Determination of parts per trillion levels of organophosphorus pesticides in groundwater by automated on-line liquid-solid extraction followed by liquid chromatography atmospheric pressure chemical ionization mass spectrometry using positive and negative ion modes of operation. Anal Chem 68:2464–2470
Erdogdu G (2008) Determination of azinphos-ethyl insecticide by adsorptive stripping voltammetry on the hanging mercury-drop electrode. J Anal Chem 63:762–767
Ivanov A, Evtugyn G, Budnikov H, Girotti S, Ghini S (2008) Amperometric immunoassay of azinphos-methyl in water and honeybees based on indirect competitive ELISA. Anal Lett 41:392–405
Tang JS, Zhang M, Cheng GG, Lu YT (2008) Development of fluorescence polarization immunoassay for the detection of organophosphorus pesticides parathion and azinphos-methyl. J Immunoassay Immunochem 29:356–369
Sardinha JPM, Gil MH, Mercader JV, Montoya A (2002) Enzyme-linked immunofiltration assay used in the screening of solid supports and immunoreagents for the development of an azinphos-methyl flow immunosensor. J Immunol Methods 260(1–2):173–182
Cerdà V, Estela JM, Forteza R, Cladera A, Becerra E, Altimira P, Sitjar P (1999) Flow techniques in water analysis. Talanta 50:695–705
Miró M, Cerdà V, Estela JM (2002) Multisyringe flow injection analysis: characterisation and applications. Trends Anal Chem 21:199–210
Segundo MA, Magalhães LM (2006) Multisyringe flow injection analysis: state-of-the-art and perspectives. Anal Sci 22:3–8
APHA/AWWA/WPCF (1989) Standard methods for the examination of water and wastewater, 17th edition, American Public Health Association/American Water Works Association/Water Pollution Controlling Federation, Washington, DC
Feres MA, Reis BF (2005) A downsized flow set up based on multicommutation for the sequential photometric determination of iron(II)/iron(III) and nitrite/nitrate in surface water. Talanta 68:422–428
Becerra E, Cladera A, Cerda V (1999) Design of a very versatile software program for automating analytical methods. Lab Rob Autom 11:131–140
Helrich K (1990) Official methods of analysis, association of official analytical chemists, 15th Ed., Arlington, VA. Vol. 1, pp. 198
Miller JC, Miller JN (1993) Statistics for analytical chemistry, 3rd edn. Ellis Horwood, Chichester, p 55
Acknowledgements
This work was supported by the National Council of Science of Technology of Mexico (CONACyT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ornelas-Soto, N.E., Guzmán-Mar, J.L., López de Alba, P.L. et al. Coupled multisyringe flow injection/reactor tank for the spectrophotometric detection of azinphos methyl in water samples. Microchim Acta 167, 273–280 (2009). https://doi.org/10.1007/s00604-009-0233-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-009-0233-4